Freezing–Thawing Procedures Remodel the Proteome of Ram Sperm before and after In Vitro Capacitation
Abstract
:1. Introduction
2. Results
2.1. Influence of Cryopreservation and Capacitation on Sperm Functionality
2.2. Protein Profile of Fresh and Cryopreserved Ram Sperm Incubated under Capacitating and Non-capacitating Conditions
2.3. Effect of Cryopreservation and Capacitation on Protein Changes
2.4. ELISA Analysis
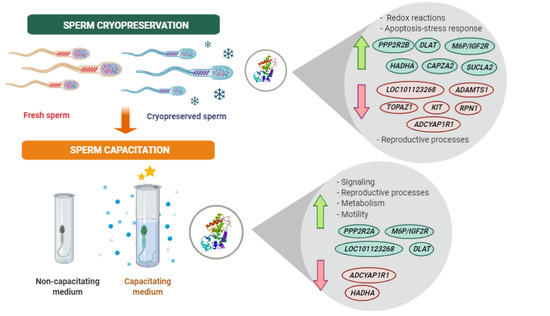
2.5. Functional Analysis of Identified Proteins in Fresh and Cryopreserved Sperm Incubated under Capacitating and Non-capacitating Conditions
2.6. Different Representation of Those Biological Processes Directly or Indirectly Involved in Reproduction between Fresh and Cryopreserved Sperm Incubated under Capacitating and Non-Capacitating Conditions
3. Discussion
4. Materials and Methods
4.1. Semen Collection and Initial Evaluation
4.2. Sperm Cryopreservation and Thawing
4.3. In Vitro Sperm Capacitation
4.4. Sperm Quality Assessment
4.5. Protein Extraction
4.6. Proteomics Data Acquisition and Analysis
4.7. ELISA Validation
4.8. Gene Ontology Analysis
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Austin, C.R. The capacitation of the mammalian sperm. Nature 1952, 170, 326. [Google Scholar] [CrossRef] [PubMed]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Leclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121, 1139–1150. [Google Scholar] [PubMed]
- Grasa, P.; Cebrián-Pérez, J.A.; Muiño-Blanco, T. Signal transduction mechanisms involved in in vitro ram sperm capacitation. Reproduction 2006, 132, 721–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ficarro, S.; Chertihin, O.; Westbrook, V.A.; White, F.; Jayes, F.; Kalab, P.; Marto, J.A.; Shabanowitz, J.; Herr, J.C.; Hunt, D.F.; et al. Phosphoproteome analysis of capacitated human sperm: Evidence of tyrosine phosphorylation of a kinase-anchoring protein 3 and valosin-containing protein/p97 during capacitation. J. Biol. Chem. 2003, 278, 11579–11589. [Google Scholar] [PubMed]
- Secciani, F.; Bianchi, L.; Ermini, L.; Cianti, R.; Armini, A.; La Sala, G.B.; Focarelli, R.; Bini, L.; Rosati, F. Protein profile of capacitated versus ejaculated human sperm. J. Proteome Res. 2009, 8, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Arcelay, E.; Salicioni, A.M.; Wertheimer, E.; Visconti, P.E. Identification of proteins undergoing tyrosine phosphorylation during mouse sperm capacitation. Int. J. Dev. Biol. 2008, 52, 463–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, M.A.; Reeves, G.; Hetherington, L.; Aitken, R.J. Analysis of proteomic changes associated with sperm capacitation through the combined use of IPG-strip prefractionation followed by RP chromatography LC-MS/MS analysis. Proteomics 2010, 10, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Tardif, S.; Dubé, C.; Beaulieu, M.; Reyes-Moreno, C.; Lefièvre, L.; Leclerc, P. Use of phosphoproteomics to study tyrosine kinase activity in capacitating boar sperm: Kinase activity and capacitation. Theriogenology 2005, 63, 599–614. [Google Scholar] [CrossRef]
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Kim, J.; Yoon, S.J.; Park, Y.J.; You, Y.A.; Hwang, S.; Pang, M.G. A comprehensive proteomic approach to identifying capacitation related proteins in boar spermatozoa. BMC Genom. 2014, 15, 897. [Google Scholar] [CrossRef]
- Hou, Z.; Fu, Q.; Huang, Y.; Zhang, P.; Chen, F.; Li, M.; Xu, Z.; Yao, S.; Chen, D.; Zhang, M. Comparative proteomic identification buffalo spermatozoa during in vitro capacitation. Theriogenology 2019, 126, 303–309. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Pang, M.G. Prediction of male fertility using capacitation-associated proteins in spermatozoa. Mol. Reprod. Dev. 2017, 84, 749–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aitken, R.J.; Baker, M. The role of proteomics in understanding sperm cell biology. Int. J. Androl. 2007, 31, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Oliva, R.; De Mateo, S.; Estanyol, J.M. Sperm cell proteomics. Proteomics 2009, 9, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Gur, Y.; Breitbart, H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006, 20, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Guo, X.J.; Shi, Z.H.; Wang, F.Q.; Huang, X.Y.; Huo, R.; Zhu, H.; Wang, X.R.; Liu, J.Y.; Zhou, Z.M.; et al. Role of translation by mitochondrial-type ribosomes during sperm capacitation: An analysis based on a proteomic approach. Proteomics 2009, 9, 1385–1399. [Google Scholar] [CrossRef]
- Zhu, Z.; Umehara, T.; Okazaki, T.; Goto, M.; Fujita, Y.; Hoque, S.A.M.; Kawai, T.; Zeng, W.; Shimada, M. Gene expression and protein synthesis in mitochondria enhance the duration of high-speed linear motility in boar sperm. Front. Physiol. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Samanta, L.; Swain, N.; Ayaz, A.; Venugopal, V.; Agarwal, A. Post-Translational Modifications in sperm Proteome: The chemistry of proteome diversifications in the pathophysiology of male factor infertility. Biochim. Biophys. Acta 2016, 1860, 1450–1465. [Google Scholar] [CrossRef]
- Hernández-Silva, G.; Chirinos, M. Proteins from male and female reproductive tracts involved in sperm function regulation. Zygote 2018, 27, 5–16. [Google Scholar] [CrossRef]
- Gur, Y.; Breitbart, H. Protein synthesis in sperm: Dialog between mitochondria and cytoplasm. Mol. Cell. Endocrinol. 2008, 282, 45–55. [Google Scholar] [CrossRef]
- Watson, P.F. Recent Developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod. Fertil. Dev. 1995, 7, 871–891. [Google Scholar] [CrossRef]
- Satorre, M.M.; Breininger, E.; Beconi, M.T.; Beorlegui, N.B. Protein tyrosine phosphorylation under capacitating conditions in porcine fresh spermatozoa and sperm cryopreserved with and without alpha tocopherol. Andrologia 2009, 41, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Pérez, L.J.; Valcárcel, A.; De Las Heras, M.A.; Moses, D.; Baldassarre, H. Evidence that frozen/thawed ram spermatozoa show accelerated capacitation in vitro as assessed by chlortetracycline assay. Theriogenology 1996, 46, 131–140. [Google Scholar] [CrossRef]
- Wang, S.; Wang, W.; Xu, Y.; Tang, M.; Fang, J.; Sun, H.; Yangyang, S.; Gu, M.; Liu, Z.; Zhang, Z.; et al. Proteomic characteristics of human sperm cryopreservation. Proteomics 2014, 14, 298–310. [Google Scholar] [CrossRef]
- Mostek, A.; Dietrich, M.A.; Słowińska, M.; Ciereszko, A. Cryopreservation of bull semen is associated with carbonylation of sperm proteins. Theriogenology 2017, 92, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bogle, O.A.; Kumar, K.; Attardo-Parrinello, C.; Lewis, S.E.M.; Estanyol, J.M.; Ballescà, J.L.; Oliva, R. Identification of protein changes in human spermatozoa throughout the cryopreservation process. Andrology 2016, 5, 1–13. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Hu, C.; Hao, H.; Zhang, J.; Li, K.; Zhao, X.; Qin, T.; Zhao, K.; Zhu, H.; et al. Identification of differentially expressed proteins in fresh and frozen-thawed boar spermatozoa by iTRAQ-coupled 2D LC-MS/MS. Reproduction 2014, 147, 321–330. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, K.; Zhao, X.; Zhang, Y.; Ma, Y.; Hu, J. Differential proteome association study of freeze-thaw damage in ram sperm. Cryobiology 2016, 72, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Pini, T.; Rickard, J.; Leahy, T.; Crossett, B.; Druart, X.; De Graaf, S. Cryopreservation and egg yolk medium alter the proteome of ram spermatozoa. J. Proteom. 2018, 181, 73–82. [Google Scholar] [CrossRef]
- Westfalewicz, B.; Dietrich, M.A.; Ciereszko, A. Impact of cryopreservation on bull(Bos taurus) semen proteome. J. Anim. Sci. 2015, 93, 5240–5253. [Google Scholar] [CrossRef]
- Nynca, J.; Arnold, G.J.; Fröhlich, T.; Ciereszko, A. Cryopreservation-induced alterations in protein composition of rainbow trout semen. Proteomics 2015, 15, 2643–2654. [Google Scholar] [CrossRef]
- Bailey, J.; Morrier, A.; Cormier, N. Semen cryopreservation: Successes and persistent problems in farm species. Can. J. Anim. Sci. 2003, 83, 393–401. [Google Scholar] [CrossRef]
- Bilodeau, J.F.; Chatterjee, S.; Sirard, M.A.; Gagnon, C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing. Mol. Hum. Reprod. 2000, 55, 282–288. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino. Acids 2003, 25, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–750. [Google Scholar] [PubMed]
- Connell, M.O.; Mcclure, N.; Lewis, S.E.M. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. 2002, 17, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Kim, M.; Song, H.; Kang, E.; Ock, S.; Kumar, B.M.; Balasubramanian, S.; Rho, G. Effect of a-tocopherol supplementation during boar semen cryopreservation on sperm characteristics and expression of apoptosis related genes. Cryobiology 2009, 58, 181–189. [Google Scholar] [CrossRef] [PubMed]
- García-Álvarez, O.; Maroto-Morales, A.; Jiménez-Rabadán, P.; Ramón, M.; Del Olmo, E.; Iniesta-Cuerda, M.; Anel-López, L.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of different media additives on capacitation of frozen-thawed ram spermatozoa as a potential replacement for estrous sheep serum. Theriogenology 2015, 84, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Huneau, D.; Crozet, N.; Ahmed-Ali, M. Estrous sheep serum as a potent agent for ovine IVF: Effect on cholesterol efflux from spermatozoa and the acrosome reaction. Theriogenology 1994, 42, 1017–1028. [Google Scholar] [CrossRef]
- Aguilar, J.; Reyley, M. The uterine tubal fluid: Secretion, composition and biological effects. Anim. Reprod. 2005, 91–105. [Google Scholar]
- Perez-Patiño, C.; Barranco, I.; Li, J.; Padilla, L.; Martinez, E.A.; Rodriguez-Martinez, H.; Roca, J.; Parrilla, I. Cryopreservation differentially alters the proteome of epididymal and ejaculated pig spermatozoa. Int. J. Mol. Sci. 2019, 20, 1791. [Google Scholar] [CrossRef]
- Belmonte, S.A.; Romano, P.S.; Sosa, M.A. Mannose-6-phosphate receptors as a molecular indicator of maturation of epididymal sperm. Arch. Androl. 2002, 48, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon. J. Androl. 2000, 21, 1–7. [Google Scholar] [PubMed]
- Wang, G.; Wu, Y.; Zhou, T.; Guo, Y.; Zheng, B.; Wang, J.; Bi, Y.; Liu, F.; Zhou, Z.; Guo, X.; et al. Mapping of the N-linked glycoproteome of human spermatozoa. J. Proteome Res. 2013, 12, 5750–5759. [Google Scholar] [CrossRef] [PubMed]
- Stein, K.K.; Go, J.C.; Lane, W.S.; Primakoff, P.; Myles, D.G. Proteomic analysis of sperm regions that mediate sperm-egg interactions. Proteomics 2006, 6, 3533–3543. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.; Li, Y.; Zhang, Y.; Liang, K.; Ren, Y.; Zhang, M.; Qazi, I.H.; Zhang, H.; Zeng, C. Transcriptome sequencing reveals the differentially expressed lncRNAs and mRNAs involved in cryoinjuries in frozen-thawed giant panda (Ailuropoda melanoleuca) Sperm. Int. J. Mol. Sci. 2018, 19, 3066. [Google Scholar] [CrossRef] [PubMed]
- Brubel, R.; Kiss, P.; Vincze, A.; Varga, A.; Varnagy, A.; Bodis, J.; Mark, L.; Jambor, E.; Maasz, G.; Hashimoto, H.; et al. Effects of pituitary adenylate cyclase activating polypeptide on human sperm motility. J. Mol. Neurosci. 2012, 48, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Cseh, S.; Somoskoi, B.; Fulop, B.D.; Szentleleky, E.; Szegezcki, V.; Kovacs, A.; Varga, A.; Kiss, P.; Hashimoto, H.; et al. Disturbed spermatogenic signaling in pituitary adenylate cyclase activating polypeptide-deficient mice. Reproduction 2018, 155, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Kwon, W.S.; Rahman, M.S.; Pang, M.G. Diagnosis and prognosis of male infertility in mammal: The focusing of tyrosine phosphorylation and phosphotyrosine proteins. J. Proteome Res. 2014, 13, 4505–4517. [Google Scholar] [CrossRef]
- Visconti, P.E. Understanding the molecular basis of sperm capacitation through kinase design. Proc. Natl. Acad. Sci. USA 2009, 106, 667–668. [Google Scholar] [CrossRef] [Green Version]
- Battistone, M.A.; Da Ros, V.G.; Salicioni, A.M.; Navarrete, F.A.; Krapf, D.; Visconti, P.E.; Cuasnicú, P.S. Functional human sperm capacitation requires both bicarbonate-dependent PKA activation and down-regulation of Ser/Thr phosphatases by Src family kinases. Mol. Hum. Reprod. 2013, 19, 570–580. [Google Scholar] [CrossRef] [Green Version]
- Nolan, M.A.; Babcock, D.F.; Wennemuth, G.; Brown, W.; Burton, K.A.; McKnight, G.S. Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc. Natl. Acad. Sci. USA 2004, 101, 13483–13488. [Google Scholar] [CrossRef]
- Losano, J.; Angrimani, D.; Dalmazzo, A.; Rui, B.; Brito, M.; Mendes, C.; Kawai, G.; Vannucchi, C.; Assumpção, M.; Barnabe, V.; et al. Effect of mitochondrial uncoupling and glycolysis inhibition on ram sperm functionality. Reprod. Domest. Anim. 2017, 52, 289–297. [Google Scholar] [CrossRef]
- Moscatelli, N.; Lunetti, P.; Braccia, C.; Armirotti, A.; Pisanello, F.; De Vittorio, M.; Zara, V.; Ferramosca, A. Comparative proteomic analysis of proteins involved in bioenergetics pathways associated with human sperm motility. Int. J. Mol. Sci. 2019, 20, 3000. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Bioenergetics of mammalian sperm capacitation. Biomed. Res. Int. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the actin cytoskeleton during mammalian sperm capacitation and acrosome reaction. Biol. Reprod. 2003, 68, 837–845. [Google Scholar] [CrossRef]
- Kumar, V.; Kota, V.; Shivaji, S. Hamster sperm capacitation: Role of pyruvate dehydrogenase a and dihydrolipoamide dehydrogenase. Biol. Reprod. 2008, 79, 190–199. [Google Scholar] [CrossRef]
- Aitken, R.J.; Harkiss, D.; Knox, W.; Paterson, M.; Irvine, D.S. A novel signal transduction cascade in capacitating human spermatozoa characterised by a redox-regulated, cAMP-mediated induction of tyrosine phosphorylation. J. Cell Sci. 1998, 111, 645–656. [Google Scholar]
- Visconti, P.E.; Bailey, J.L.; Moore, G.D.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 1995, 121, 1129–1137. [Google Scholar]
- Grasa, P.; Colas, C.; Gallego, M.; Monteagudo, L.; Muiño-Blanco, T.; Cebrián-Pérez, J.Á. Changes in content and localization of proteins phosphorylated at tyrosine, serine and threonine residues during ram sperm capacitation and acrosome reaction. Reproduction 2009, 137, 655–667. [Google Scholar] [CrossRef] [Green Version]
- Signorelli, J.; Diaz, E.S.; Morales, P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res. 2012, 349, 765–782. [Google Scholar] [CrossRef]
- Ashizawa, K.; Wishart, G.J.; Katayama, S.; Takano, D.; Ranasinghe, A.R.A.H.; Narumi, K.; Tsuzuki, Y. Regulation of acrosome reaction of fowl spermatozoa: Evidence for the involvement of protein kinase C and protein phosphatase-type 1 and/or -type 2A. Reproduction 2006, 131, 1017–1024. [Google Scholar] [CrossRef]
- Gadella, B.M.; Tsai, P.; Boerke, A.; Brewis, I.A. Sperm head membrane reorganisation during capacitation. Int. J. Dev. Biol. 2008, 52, 473–480. [Google Scholar] [CrossRef]
- Tecle, E.; Gagneux, P. Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol. Reprod. Dev. 2015, 82, 635–650. [Google Scholar] [CrossRef]
- Benoff, S.; Cooper, G.W.; Hurley, I.; Napolitano, B.; Rosenfeld, D.L.; Scholl, G.M.; Hershlag, A. Human sperm fertilizing potential in vitro is correlated with differential expression of a head-specific mannose-ligand receptor. Fertil. Steril. 1993, 59, 854–862. [Google Scholar] [CrossRef]
- Garde, J.J.; Gutiérrez-Adán, A.; Artiga, C.G.; Vázquez, I. Influence of freezing process on “in vitro” capacitation of ram semen. Theriogenology 1993, 39, 225. [Google Scholar] [CrossRef]
- Maciel, V.L.; Caldas-Bussiere, M.C.; Silveira, V.; Reis, R.S.; Rios, A.F.L.; Paes de Carvalho, C.S. L-arginine alters the proteome of frozen-thawed bovine sperm during in vitro capacitation. Theriogenology 2018, 119, 1–9. [Google Scholar] [CrossRef]
- Amaral, A.; Castillo, J.; Ramalho-Santos, J.; Oliva, R. The combined human sperm proteome: Cellular pathways and implications for basic and clinical science. Hum. Reprod. Update 2013, 20, 1–23. [Google Scholar] [CrossRef]
- Ferraz, M.A.M.M.; Carothers, A.; Dahal, R.; Noonan, M.J.; Songsasen, N. Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 2019, 9, 9484. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef]
- Amaral, A.; Castillo, J.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Human sperm tail proteome suggests new endogenous metabolic pathways. Mol. Cell. Proteom. 2013, 12, 330–342. [Google Scholar] [CrossRef]
- Swegen, A.; Curry, B.J.; Gibb, Z.; Lambourne, S.R.; Smith, N.D.; Aitken, R.J. Investigation of the stallion sperm proteome by mass spectrometry. Reproduction 2015, 149, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Amaral, A.; Paiva, C.; Attardo-Parrinello, C.; Estanyol, J.M.; Ballescà, J.L.; Ramalho-Santos, J.; Oliva, R. Identification of proteins involved in human sperm motility using high-throughput differential proteomics. J. Proteome Res. 2014, 13, 5670–5684. [Google Scholar] [CrossRef]
- Asghari, A.; Marashi, S.; Ansari-Pour, N. A sperm-specific proteome-scale metabolic network model identifies non-glycolytic genes for energy deficiency in asthenozoospermia. Syst. Biol. Reprod. Med. 2017, 63, 100–112. [Google Scholar] [CrossRef]
- Kwon, W.S.; Rahman, M.S.; Lee, J.S.; Yoon, S.J.; Park, Y.J.; Pang, M.G. Discovery of predictive biomarkers for litter size in boar spermatozoa. Mol. Cell. Proteom. 2015, 14, 1230–1240. [Google Scholar] [CrossRef]
- Takahashi, Y.; First, N.L. In vitro development of bovine one-cell embryos: Influence of glucose, lactate, pyruvate, amino acids and vitamins. Theriogenology 1992, 37, 963–978. [Google Scholar] [CrossRef]
- Cognié, A.; Poulin, N.; Locatelli, Y.; Pascal, M. State-of-the-art production, conservation and transfer of in-vitr produced embryos in small ruminants. Reprod. Fertil. Dev. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Wessel, D.; Flügge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Villar, M.; Ayllón, N.; Alberdi, P.; Moreno, A.; Moreno, M.; Tobes, R.; Mateos-Hernández, L.; Weisheit, S.; Bell-Sakyi, L.; De la Fuente, J. Integrated metabolomics, transcriptomics and proteomics identifies metabolic pathways affected by Anaplasma phagocytophilum infection in tick cells. Mol. Cell. Proteom. 2015, 14, 3154–3172. [Google Scholar] [CrossRef]






| Sperm Parameters | Fresh Sperm | Cryopreserved Sperm |
|---|---|---|
| Total motility (%) | 44.16 ± 4.17 | 22.48 ± 3.89 * |
| Progressive motility (%) | 29.48 ± 3.40 | 17.16 ± 3.25 * |
| Apoptosis (%) | 12.05 ± 2.21 | 20.16 ± 1.35 * |
| Mitochondrial activity (%) | 32.44 ± 2.45 | 18.64 ± 2.45 * |
| ROS levels (mean fluorescence intensity) | 58.83 ± 4.32 | 86.82 ± 4.01 * |
| Tyrosine phosphorylation (%) | 46.68 ± 5.07 | 57.11 ± 5.07 |
| Sperm Parameters | CAP | NC |
|---|---|---|
| Total motility (%) | 41.69 ± 4.50 | 24.92 ± 4.50 * |
| Progressive motility (%) | 28.81 ± 3.48 | 17.85 ± 3.92 * |
| Apoptosis (%) | 14.84 ± 1.54 | 16.37 ± 1.54 |
| Mitochondrial activity (%) | 31.68 ± 2.54 | 19.40 ± 2.67 * |
| ROS levels (mean fluorescence intensity) | 56.29 ± 2.54 | 89.36 ± 3.10 * |
| Tyrosine phosphorylation (%) | 64.73 ± 4.49 | 39.05 ± 5.01 * |
| Accession Number | Protein Name | Gene ID | Protein Representation | Reproductive Process | Subcellular Location | |
|---|---|---|---|---|---|---|
| In Cryopreserved Sperm a (Fresh vs. Cryopreserved Sperm) | In CAP Conditions over Time b (0–240 min) | |||||
| E5FYH0 | Testis- and ovary-specific PAZ domain containing protein 1 | TOPAZ1 | ↓ at 0 min in NC | Spermatogenesis | Cytoplasm | |
| Q8SQ25 | Mannose-6-phosphate/insulin-like growth factor II receptor | M6P/IGF2R | ↑ at 0 min in NC | ↑ after 240 min in F | Sperm-oocyte interaction | Plasma membrane |
| B6UV59 | Hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit alpha | HADHA | ↑ at 0 min in NC | ↓ after 1 min in C | Metabolic process | Mitochondria |
| W5PEA2 | Succinate-CoA ligase (ADP-forming) subunit beta, mitochondrial | SUCLA2 | ↑ at 15 min in CAP | Metabolic process | Mitochondria | |
| W5P1S6 | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 1 | RPN1 | ↓ at 15 min in CAP | Sperm-oocyte interaction | Plasma membrane | |
| W5PJ95 | Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit A, alpha isoform | PPP2R2A | ↑ after 240 min in F | Signal transduction | Cytoplasm | |
| W5PEC5 | Dolichyl-diphosphooligosaccharide-protein glycosyltransferase | LOC101123268 | ↓ at 0 min in NC | ↑ after 15 min in C | Sperm-oocyte interaction | Plasma membrane |
| W5QCD4 | Ankyrin repeat-SAM-basic leucine zipper domain-containing protein 1 | ASZ1 | ↓ after 240 min in C | Spermatogenesis | Cytoplasm | |
| D5K281 | ADAM metallopeptidase with thrombospondin type 1 motif 1 | ADAMTS1 | ↓ at 15 min in CAP | Spermatogenesis | Plasma membrane | |
| W5QBN6 | Dihydrolipoamide cetyltransferase component of pyruvate dehydrogenase complex | DLAT | ↑ at 15 min in CAP | ↑ after 15 min in C; ↑ after 240 min in F | Metabolic process | Mitochondria |
| Q8WMQ9 | Pituitary adenylate cyclase-activating polypeptide type 1 receptor hop 1 splice variant | ADCYAP1R1 | ↓ at 15 min in CAP | ↓ after 15 min in C | Sperm motility | Plasma membrane |
| W5NZH7 | Serine/threonine-protein phosphatase 2A 55 kDa regulatory subunit B | PPP2R2B | ↑ at 15 min in CAP | Cell cycle-apoptosis-stress | Mitochondria | |
| Q09YI7 | Capping protein (actin filament) muscle Z-line, alpha 2, 3 prime | CAPZA2 | ↑ at 15 min in CAP | Sperm motility | Cytoskeleton | |
| A0A0C5GE36 | V-kit Hardy–Zuckerman 4 feline sarcoma viral oncoprotein | KIT | ↓ at 15 min in NC | Signal transduction | Plasma membrane | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peris-Frau, P.; Martín-Maestro, A.; Iniesta-Cuerda, M.; Sánchez-Ajofrín, I.; Mateos-Hernández, L.; Garde, J.J.; Villar, M.; Soler, A.J. Freezing–Thawing Procedures Remodel the Proteome of Ram Sperm before and after In Vitro Capacitation. Int. J. Mol. Sci. 2019, 20, 4596. https://doi.org/10.3390/ijms20184596
Peris-Frau P, Martín-Maestro A, Iniesta-Cuerda M, Sánchez-Ajofrín I, Mateos-Hernández L, Garde JJ, Villar M, Soler AJ. Freezing–Thawing Procedures Remodel the Proteome of Ram Sperm before and after In Vitro Capacitation. International Journal of Molecular Sciences. 2019; 20(18):4596. https://doi.org/10.3390/ijms20184596
Chicago/Turabian StylePeris-Frau, Patricia, Alicia Martín-Maestro, María Iniesta-Cuerda, Irene Sánchez-Ajofrín, Lourdes Mateos-Hernández, J. Julián Garde, Margarita Villar, and Ana Josefa Soler. 2019. "Freezing–Thawing Procedures Remodel the Proteome of Ram Sperm before and after In Vitro Capacitation" International Journal of Molecular Sciences 20, no. 18: 4596. https://doi.org/10.3390/ijms20184596