Zerumbone Suppresses Enterotoxigenic Bacteroides fragilis Infection-Induced Colonic Inflammation through Inhibition of NF-κΒ
Abstract
:1. Introduction
2. Results
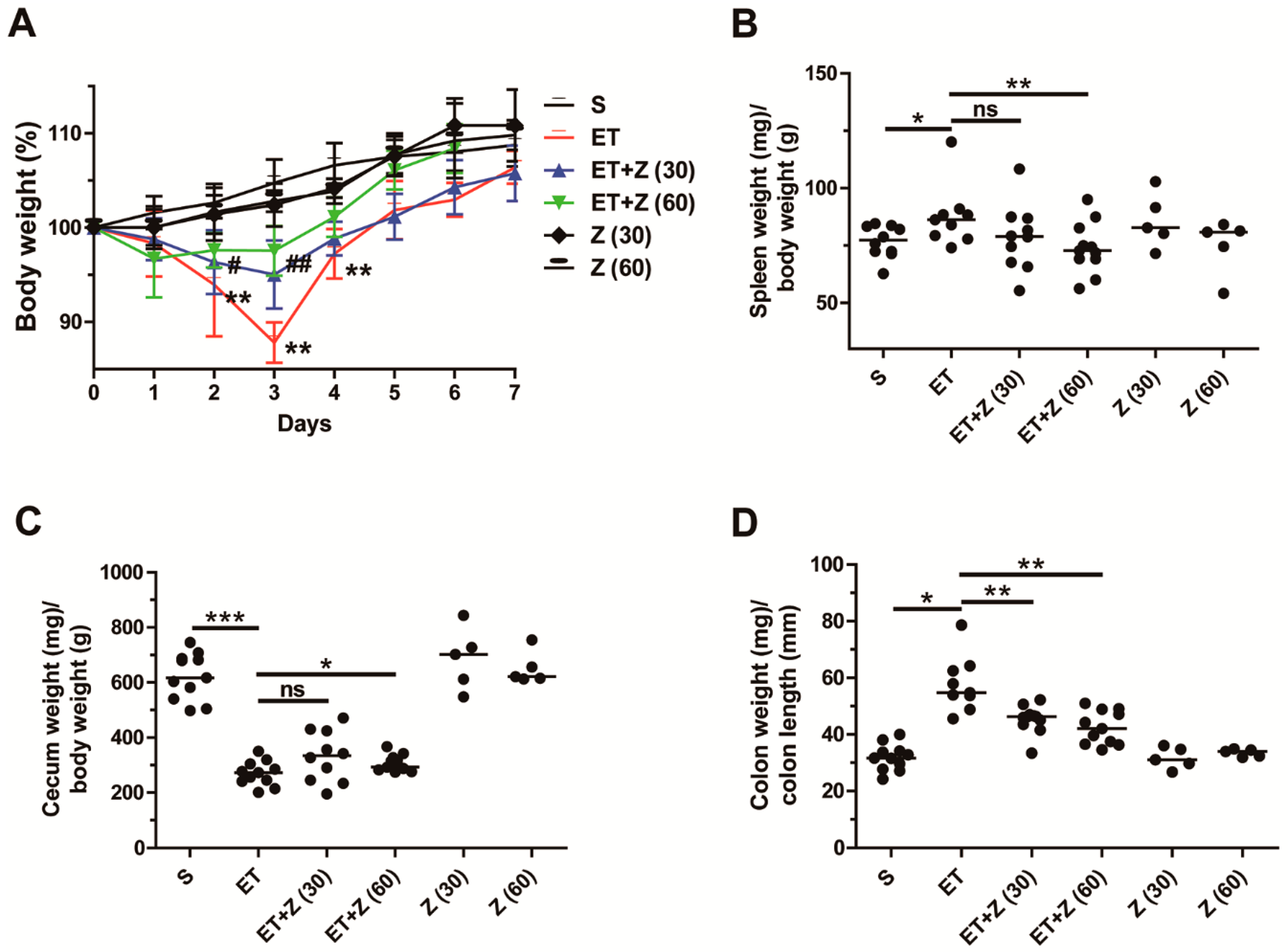
2.1. Zerumbone Reduced Indirect Parameters of ETBF-Induced Colonic Inflammation in Mice
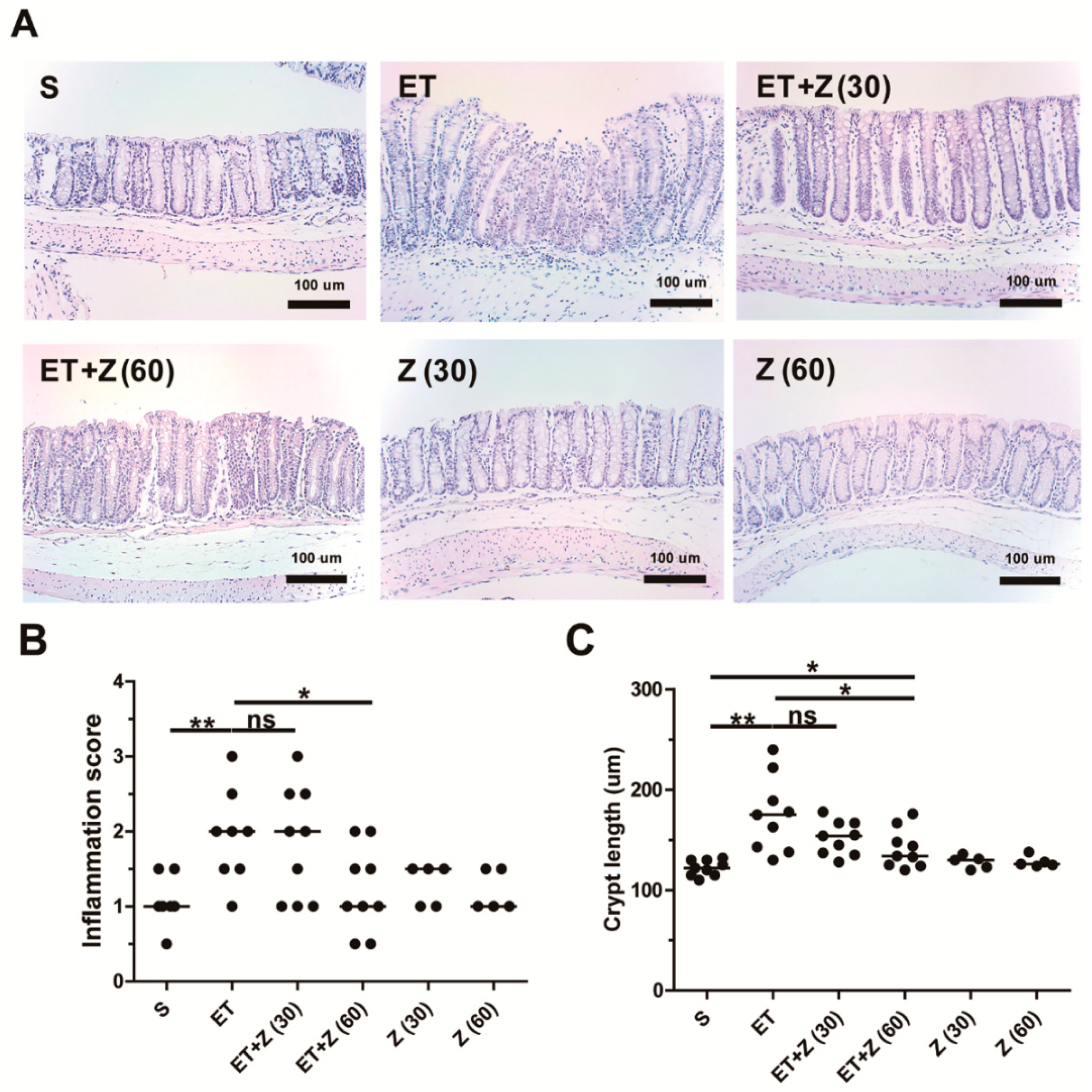
2.2. Zerumbone Decreased ETBF-Induced Histologic Damage in Mouse Colon
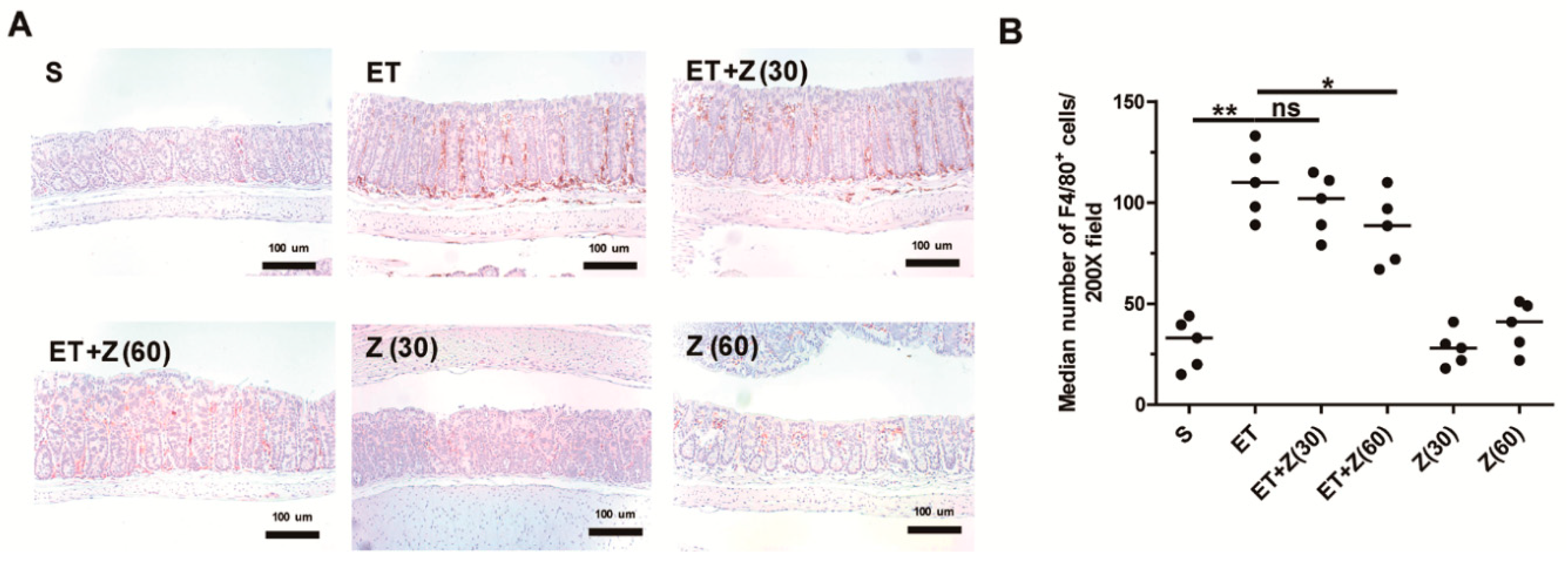
2.3. Zerumbone Decreased Infiltration of Macrophage in Distal Colon of ETBF-Infected Mice
2.4. Zerumbone Reduced Expression of Pro-Inflammatory Cytokines and NF-κB Pathway Related Proteins in Colon of ETBF-Infected Mice
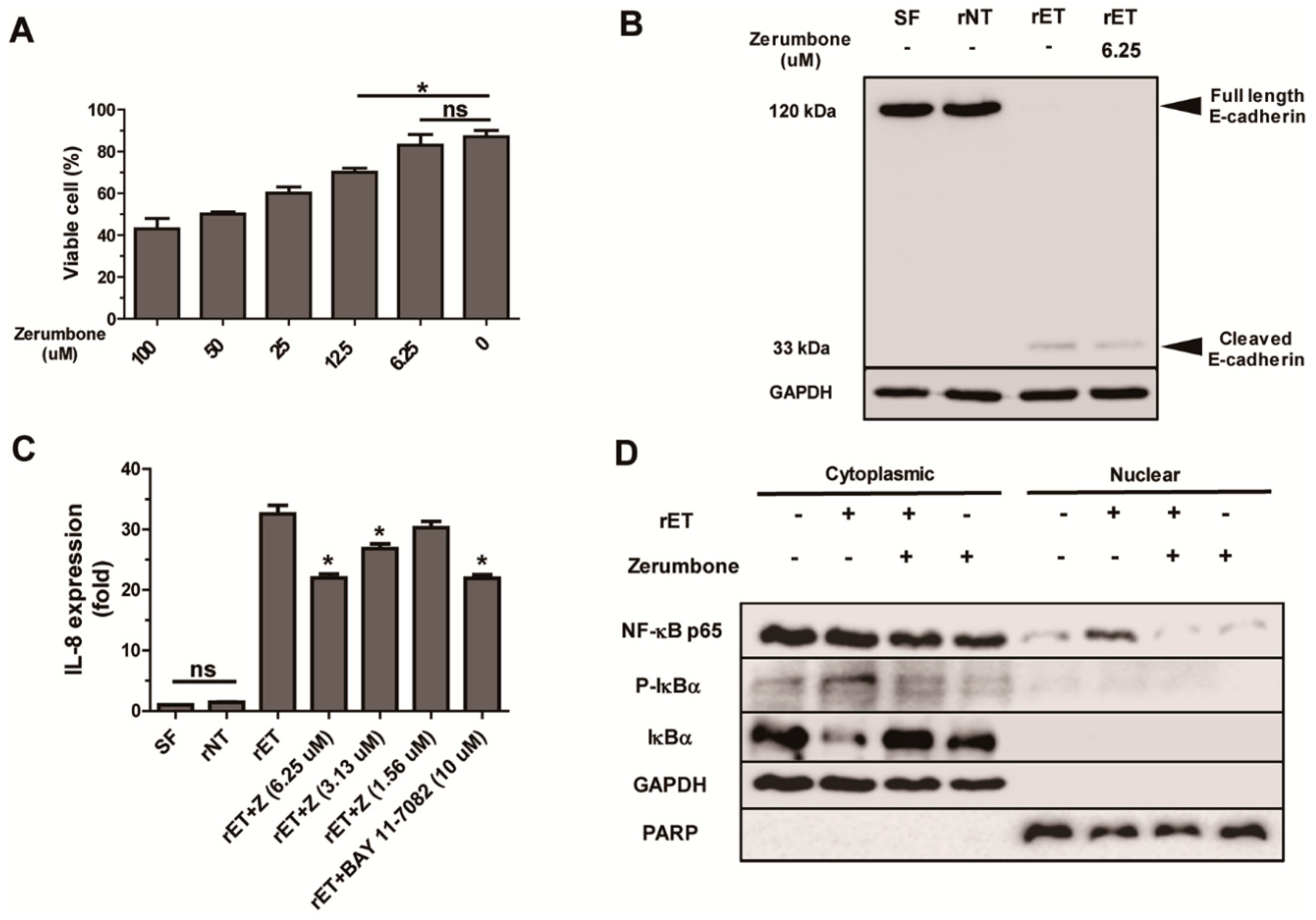
2.5. Zerumbone Inhibited NF-κB Signaling and IL-8 Expression, but not Cleavage of E-Cadherin, by BFT in HT29/C1 Cells
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cell Culture
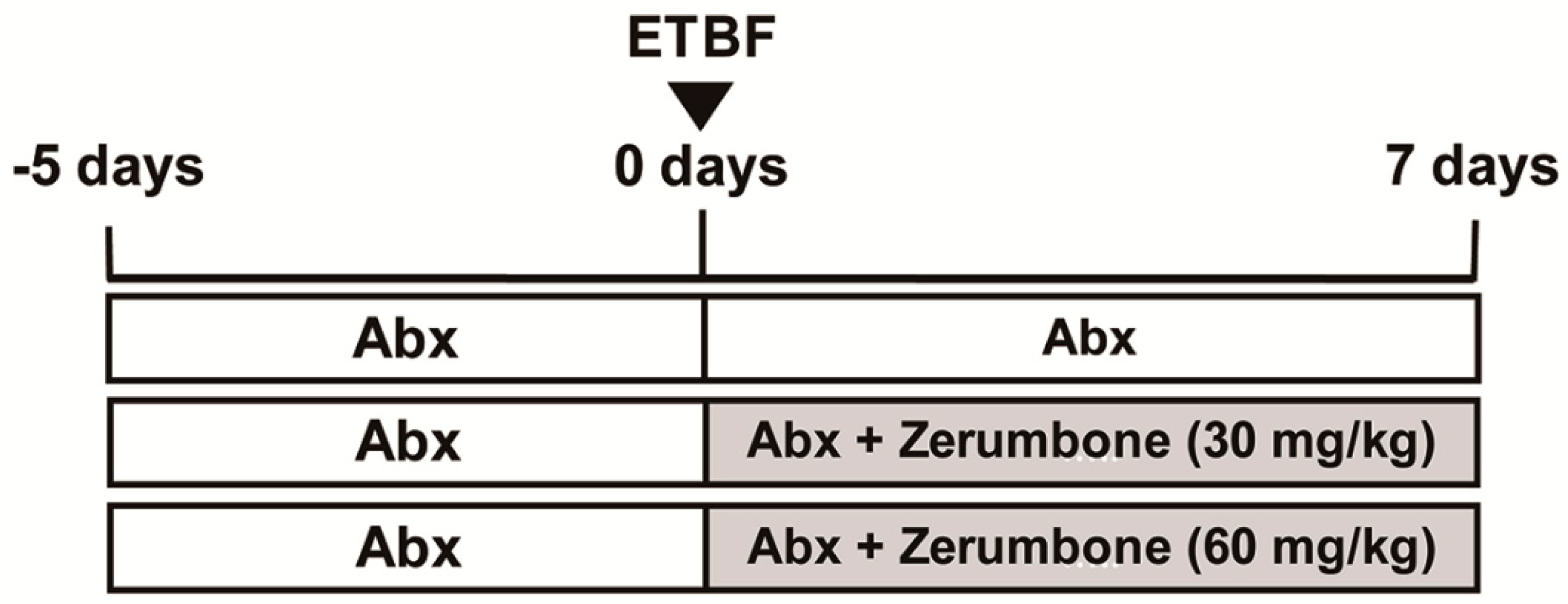
4.3. ETBF Infection in Mice and Zerumbone Treatment
4.4. Western Blot Analysis
4.5. Quantitative RT-PCR (qRT-PCR)
4.6. Trypan Blue Exclusion Assay
4.7. ELISA
4.8. Nitric Oxide Assay
4.9. Histologic Assessment of Colonic Inflammation
4.10. Immunohistochemistry
4.11. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Correa, P.; Piazuelo, M.B. Helicobacter pylori Infection and Gastric Adenocarcinoma. US Gastroenterol. Hepatol. Rev. 2011, 7, 59–64. [Google Scholar] [PubMed]
- El-Serag, H.B. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology 2012, 142, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Triantafillidis, J.K.; Nasioulas, G.; Kosmidis, P.A. Colorectal Cancer and Inflammatory Bowel Disease: Epidemiology, Risk factors, Mechanisms of Carcinogenesis and Prevention Strategies. Anticancer. Res. 2009, 29, 2727–2737. [Google Scholar] [PubMed]
- Zhao, S.; Lieberman, T.D.; Poyet, M.; Kauffman, K.M.; Gibbons, S.M.; Groussin, M.; Xavier, R.J.; Alm, E.J. Adaptive Evolution within Gut Microbiomes of Healthy People. Cell. Host. Microbe. 2019, 25, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, C.; Kujawski, M.; Chu, H.; Li, L.; Mazmanian, S.K.; Cantin, E.M. Bacteroides fragilis Polysaccharide A Induces IL-10 Secreting B and T Cells That Prevent Viral Encephalitis. Nat. Commun. 2019, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.L.; Firehammer, B.D.; Shoop, D.S.; Border, M.M. Bacteroides fragilis: A Possible Cause of Acute Diarrheal Disease in Newborn Lambs. Infect. Immun. 1984, 44, 241–244. [Google Scholar] [PubMed]
- Sears, C.L. The toxins of Bacteroides fragilis. Toxicon 2001, 39, 1737–1746. [Google Scholar] [CrossRef]
- Sears, C.L.; Islam, S.; Saha, A.; Arjumand, M.; Alam, N.H.; Faruque, A.S.; Salam, M.A.; Shin, J.; Hecht, D.; Weintraub, A.; et al. Association of Enterotoxigenic Bacteroides fragilis Infection with Inflammatory Diarrhea. Clin. Infect. Dis. 2008, 47, 797–803. [Google Scholar] [CrossRef]
- Basset, C.; Holton, J.; Bazeos, A.; Vaira, D.; Bloom, S. Are Helicobacter Species and Enterotoxigenic Bacteroides fragilis Involved in Inflammatory Bowel Disease? Dig. Dis. Sci. 2004, 49, 1425–1432. [Google Scholar] [CrossRef]
- Boleij, A.; Hechenbleikner, E.M.; Goodwin, A.C.; Badani, R.; Stein, E.M.; Lazarev, M.G.; Ellis, B.; Carroll, K.C.; Albesiano, E.; Wick, E.C.; et al. The Bacteroides fragilis Toxin Gene Is Prevalent in the Colon Mucosa of Colorectal Cancer Patients. Clin. Infect. Dis. 2015, 60, 208–215. [Google Scholar] [CrossRef]
- Dejea, C.M.; Fathi, P.; Craig, J.M.; Boleij, A.; Taddese, R.; Geis, A.L.; Wu, X.; DeStefano Shields, C.E.; Hechenbleikner, E.M.; Huso, D.L.; et al. Patients with Familial Adenomatous Polyposis Harbor Colonic Biofilms Containing Tumorigenic Bacteria. Science 2018, 359, 592–597. [Google Scholar] [CrossRef]
- Rhee, K.J.; Wu, S.; Wu, X.; Huso, D.L.; Karim, B.; Franco, A.A.; Rabizadeh, S.; Golub, J.E.; Mathews, L.E.; Shin, J.; et al. Induction of Persistent Colitis by a Human Commensal, Enterotoxigenic Bacteroides fragilis, in Wild-Type C57BL/6 Mice. Infect. Immun. 2009, 77, 1708–1718. [Google Scholar] [CrossRef]
- Rabizadeh, S.; Rhee, K.J.; Wu, S.; Huso, D.; Gan, C.M.; Golub, J.E.; Wu, X.; Zhang, M.; Sears, C.L. Enterotoxigenic Bacteroides fragilis: A Potential Instigator of Colitis. Inflamm. Bowel. Dis. 2007, 13, 1475–1483. [Google Scholar] [CrossRef]
- Wu, S.; Rhee, K.J.; Albesiano, E.; Rabizadeh, S.; Wu, X.; Yen, H.R.; Huso, D.L.; Brancati, F.L.; Wick, E.; McAllister, F.; et al. A Human Colonic Commensal Promotes Colon Tumorigenesis via Activation of T Helper Type 17 T Cell Responses. Nat. Med. 2009, 15, 1016–1022. [Google Scholar] [CrossRef]
- Wareham, D.W.; Wilks, M.; Ahmed, D.; Brazier, J.S.; Millar, M. Anaerobic Sepsis Due to Multidrug-Resistant Bacteroides fragilis: Microbiological Cure and Clinical Response with Linezolid Therapy. Clin. Infect. Dis. 2005, 40, e67–e68. [Google Scholar] [CrossRef]
- Choi, V.M.; Herrou, J.; Hecht, A.L.; Teoh, W.P.; Turner, J.R.; Crosson, S.; Bubeck Wardenburg, J. Activation of Bacteroides fragilis Toxin by a Novel Bacterial Protease Contributes to Anaerobic Sepsis in Mice. Nat. Med. 2016, 22, 563–567. [Google Scholar] [CrossRef]
- Wu, S.; Lim, K.C.; Huang, J.; Saidi, R.F.; Sears, C.L. Bacteroides fragilis Enterotoxin Cleaves the Zonula Adherens Protein, E-cadherin. Proc. Natl. Acad. Sci. USA 1998, 95, 14979–14984. [Google Scholar] [CrossRef]
- Sanfilippo, L.; Li, C.K.; Seth, R.; Balwin, T.J.; Menozzi, M.G.; Mahida, Y.R. Bacteroides fragilis Enterotoxin Induces the Expression of IL-8 and Transforming Growth Factor-Beta (TGF-β) by Human Colonic Epithelial Cells. Clin. Exp. Immunol. 2000, 119, 456–463. [Google Scholar] [CrossRef]
- Kim, J.M.; Cho, S.J.; Oh, Y.K.; Jung, H.Y.; Kim, Y.J.; Kim, N. Nuclear Factor-Kappa B Activation Pathway in Intestinal Epithelial Cells Is a Major Regulator of Chemokine Gene Expression and Neutrophil Migration Induced by Bacteroides fragilis Enterotoxin. Clin. Exp. Immunol. 2002, 130, 59–66. [Google Scholar] [CrossRef]
- Wu, S.; Powell, J.; Mathioudakis, N.; Kane, S.; Fernandez, E.; Sears, C.L. Bacteroides fragilis Enterotoxin Induces Intestinal Epithelial Cell Secretion of Interleukin-8 through Mitogen-Activated Protein Kinases and a Tyrosine Kinase-Regulated Nuclear Factor-kB pathway. Infect. Immun. 2004, 72, 5832–5839. [Google Scholar] [CrossRef]
- Housseau, F.; Wu, S.; Wick, E.C.; Fan, H.; Wu, X.; Llosa, N.J.; Smith, K.N.; Tam, A.; Ganguly, S.; Wanyiri, J.W.; et al. Redundant Innate and Adaptive Sources of IL17 Production Drive Colon Tumorigenesis. Cancer. Res. 2016, 76, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Hogenauer, C.; Hammer, H.F.; Krejs, G.J.; Reisinger, E.C. Mechanisms and Management of Antibiotic-Associated Diarrhea. Clin. Infect. Dis. 1998, 27, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frohlich, E.E.; Farzi, A.; Mayerhofer, R.; Reichmann, F.; Jacan, A.; Wagner, B.; Zinser, E.; Bordag, N.; Magnes, C.; Frohlich, E.; et al. Cognitive Impairment by Antibiotic-induced Gut Dysbiosis: Analysis of Gut Microbiota-Brain Communication. Brain. Behav. Immun. 2016, 56, 140–155. [Google Scholar] [CrossRef] [PubMed]
- DeStefano Shields, C.E.; van Meerbeke, S.W.; Housseau, F.; Wang, H.; Huso, D.L.; Casero, R.A., Jr.; O’Hagan, H.M.; Sears, C.L. Reduction of Murine Colon Tumorigenesis Driven by Enterotoxigenic Bacteroides fragilis Using Cefoxitin Treatment. J. Infect. Dis. 2016, 214, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Van Hoek, A.H.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J. Acquired Antibiotic Resistance Genes: An Overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalghatgi, S.; Spina, C.S.; Costello, J.C.; Liesa, M.; Morones-Ramirez, J.R.; Slomovic, S.; Molina, A.; Shirihai, O.S.; Collins, J.J. Bactericidal Antibiotics Induce Mitochondrial Dysfunction and Oxidative Damage in Mammalian Cells. Sci. Transl. Med. 2013, 5, 192ra185. [Google Scholar] [CrossRef]
- Jernberg, C.; Lofmark, S.; Edlund, C.; Jansson, J.K. Long-Term Impacts of Antibiotic Exposure on the Human Intestinal Microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the Gut Microbiota in Disease. Microb. Ecol. Health. Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Kitayama, T.; Yamamoto, K.; Utsumi, R.; Takatani, M.; Hill, R.K.; Kawai, Y.; Sawada, S.; Okamoto, T. Chemistry of Zerumbone. 2. Regulation of Ring Bond Cleavage and Unique Antibacterial Activities of Zerumbone Derivatives. Biosci. Biotechnol. Biochem. 2001, 65, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Sung, B.; Jhurani, S.; Ahn, K.S.; Mastuo, Y.; Yi, T.; Guha, S.; Liu, M.; Aggarwal, B.B. Zerumbone Down-regulates Chemokine Receptor CXCR4 Expression Leading to Inhibition of CXCL12-Induced Invasion of Breast and Pancreatic Tumor Cells. Cancer Res. 2008, 68, 8938–8944. [Google Scholar] [CrossRef] [PubMed]
- Yodkeeree, S.; Sung, B.; Limtrakul, P.; Aggarwal, B.B. Zerumbone Enhances TRAIL-Induced Apoptosis through the Induction of Death Receptors in Human Colon Cancer Cells: Evidence for an Essential Role of Reactive Oxygen Species. Cancer Res. 2009, 69, 6581–6589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santosh Kumar, S.C.; Srinivas, P.; Negi, P.S.; Bettadaiah, B.K. Antibacterial and Antimutagenic Activities of Novel Zerumbone Analogues. Food Chem. 2013, 141, 1097–1103. [Google Scholar] [CrossRef]
- Sulaiman, M.R.; Perimal, E.K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Tasrip, N.A.; Mokhtar, F.; Zakaria, Z.A.; Lajis, N.H.; Israf, D.A. Anti-Inflammatory Effect of Zerumbone on Acute and Chronic Inflammation Models in Mice. Fitoterapia 2010, 81, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Fatima, A.; Abdul, A.B.H.; Abdullah, R.; Karjiban, R.A.; Lee, V.S. Docking Studies Reveal Zerumbone Targets b-catenin of the Wnt-b-catenin Pathway in Breast Cancer. J. Serb. Chem. Soc. 2018, 83, 575–591. [Google Scholar] [CrossRef]
- Sidahmed, H.M.; Hashim, N.M.; Abdulla, M.A.; Ali, H.M.; Mohan, S.; Abdelwahab, S.I.; Taha, M.M.; Fai, L.M.; Vadivelu, J. Antisecretory, Gastroprotective, Antioxidant and Anti-Helicobcter Pylori Activity of Zerumbone from Zingiber Zerumbet (L.) Smith. PLoS. ONE 2015, 10, e0121060. [Google Scholar] [CrossRef]
- Kim, H.R.; Rhee, K.J.; Eom, Y.B. Anti-Biofilm and Antimicrobial Effects of Zerumbone against Bacteroides fragilis. Anaerobe 2019, 57, 99–106. [Google Scholar] [CrossRef]
- Schenk, M.; Bouchon, A.; Seibold, F.; Mueller, C. TREM-1–expressing Intestinal Macrophages Crucially Amplify Chronic Inflammation in Experimental Colitis and Inflammatory Bowel Diseases. J. Clin. Invest. 2007, 117, 3097–3310. [Google Scholar] [CrossRef]
- Magnusson, M.K.; Brynjolfsson, S.F.; Dige, A.; Uronen-Hansson, H.; Borjesson, L.G.; Bengtsson, J.L.; Gudjonsson, S.; Ohman, L.; Agnholt, J.; Sjovall, H.; et al. Macrophage and Dendritic Cell Subsets in IBD: ALDH+ Cells Are Reduced in Colon Tissue of Patients with Ulcerative Colitis Regardless of Inflammation. Mucosal. Immunol. 2016, 9, 171–182. [Google Scholar] [CrossRef]
- Thiele Orberg, E.; Fan, H.; Tam, A.J.; Dejea, C.M.; Destefano Shields, C.E.; Wu, S.; Chung, L.; Finard, B.B.; Wu, X.; Fathi, P.; et al. The Myeloid Immune Signature of Enterotoxigenic Bacteroides fragilis-induced Murine Colon Tumorigenesis. Mucosal. Immunol. 2017, 10, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Orberg, E.T.; Geis, A.L.; Chan, J.L.; Fu, K.; DeStefano Shields, C.E.; Dejea, C.M.; Fathi, P.; Chen, J.; Finard, B.B.; et al. Bacteroides fragilis Toxin Coordinates a Pro-carcinogenic Inflammatory Cascade via Targeting of Colonic Epithelial Cells. Cell Host Microbe. 2018, 23, 421. [Google Scholar] [CrossRef] [PubMed]
- Boncompain, G.; Schneider, B.; Delevoye, C.; Kellermann, O.; Dautry-Varsat, A.; Subtil, A. Production of Reactive Oxygen Species Is Turned on and Rapidly Shut Down in Epithelial Cells Infected with Chlamydia trachomatis. Infect. Immun. 2010, 78, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Urbano, R.; Karlinsey, J.E.; Libby, S.J.; Doulias, P.T.; Ischiropoulos, H.; Warheit-Niemi, H.I.; Liggitt, D.H.; Horswill, A.R.; Fang, F.C. Host Nitric Oxide Disrupts Microbial Cell-to-Cell Communication to Inhibit Staphylococcal Virulence. Cell Host Microbe. 2018, 23, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The Nuclear Factor NF-kB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Pahl, H.L. Activators and Target Genes of Rel/NF-kB Transcription Factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Karin, M. Dangerous Liaisons: STAT3 and NF-kB Collaboration and Crosstalk in Cancer. Cytokine Growth Factor Rev. 2010, 21, 11–19. [Google Scholar] [CrossRef]
- Ruan, Q.G.; Kameswaran, V.; Zheng, S.J.; Wang, J.M.; Liou, H.C.; Beg, A.; Chen, Y.H. The Th17 Immune Response is Controlled by the Rel-RORg-RORgT Transcriptional Axis. J. Immunol. 2010, 184, 2321–2333. [Google Scholar]
- Kuhl, A.A.; Kakirman, H.; Janotta, M.; Dreher, S.; Cremer, P.; Pawlowski, N.N.; Loddenkemper, C.; Heimesaat, M.M.; Grollich, K.; Zeitz, M.; et al. Aggravation of Different Types of Experimental Colitis by Depletion or Adhesion Blockade of Neutrophils. Gastroenterology 2007, 133, 1882–1892. [Google Scholar] [CrossRef]
- Jones, G.R.; Bain, C.C.; Fenton, T.M.; Kelly, A.; Brown, S.L.; Ivens, A.C.; Travis, M.A.; Cook, P.C.; MacDonald, A.S. Dynamics of Colon Monocyte and Macrophage Activation During Colitis. Front. Immunol. 2018, 9, 2764. [Google Scholar] [CrossRef] [PubMed]
- Prame Kumar, K.; Nicholls, A.J.; Wong, C.H.Y. Partners in Crime: Neutrophils and Monocytes/Macrophages in Inflammation and Disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.; Lee, Y.; Kim, M.; Seo, Y.H.; Kim, W.H.; Yong, D.; Jeong, S.H.; Lee, K.; Chong, Y. Antimicrobial Susceptibility of Clinical Isolates of Bacteroides fragilis Group Organisms Recovered from 2009 to 2012 in a Korean Hospital. Ann. Lab. Med. 2015, 35, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Merchan, C.; Parajuli, S.; Siegfried, J.; Scipione, M.R.; Dubrovskaya, Y.; Rahimian, J. Multidrug-Resistant Bacteroides fragilis Bacteremia in a US Resident: An Emerging Challenge. Case Rep. Infect. Dis. 2016, 2016, 3607125. [Google Scholar] [PubMed]
- Niestepski, S.; Harnisz, M.; Korzeniewska, E.; Aguilera-Arreola, M.G.; Contreras-Rodriguez, A.; Filipkowska, Z.; Osinska, A. The Emergence of Antimicrobial Resistance in Environmental Strains of the Bacteroides fragilis Group. Environ. Int. 2019, 124, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Boyanova, L.; Kolarov, R.; Mitov, I. Recent Evolution of Antibiotic Resistance in the Anaerobes as Compared to Previous Decades. Anaerobe 2015, 31, 4–10. [Google Scholar] [CrossRef]
- Panda, S.; El khader, I.; Casellas, F.; Lopez Vivancos, J.; Garcia Cors, M.; Santiago, A.; Cuenca, S.; Guarner, F.; Manichanh, C. Short-term Effect of Antibiotics on Human Gut Microbiota. PLoS. ONE 2014, 9, e95476. [Google Scholar] [CrossRef]
- Fatima, A.; Abdul, A.B.; Abdullah, R.; Karjiban, R.A.; Lee, V.S. Binding Mode Analysis of Zerumbone to Key Signal Proteins in the Tumor Necrosis Factor Pathway. Int. J. Mol. Sci. 2015, 16, 2747–2766. [Google Scholar] [CrossRef]
- Tomkovich, S.; Dejea, C.M.; Winglee, K.; Drewes, J.L.; Chung, L.; Housseau, F.; Pope, J.L.; Gauthier, J.; Sun, X.; Muhlbauer, M.; et al. Human Colon Mucosal Biofilms from Healthy or Colon Cancer Hosts Are Carcinogenic. J. Clin. Invest. 2019, 130, 1699–1712. [Google Scholar] [CrossRef]
- Viljoen, K.S.; Dakshinamurthy, A.; Goldberg, P.; Blackburn, J.M. Quantitative Profiling of Colorectal Cancer-Associated Bacteria Reveals Associations between Fusobacterium spp., Enterotoxigenic Bacteroides fragilis (ETBF) and Clinicopathological Features of Colorectal Cancer. PLoS. ONE 2015, 10, e0119462. [Google Scholar] [CrossRef]
- He, Z.; Huang, Z.; Zhou, W.; Tang, Z.; Ma, R.; Liang, J. Anti-biofilm Activities from Resveratrol against Fusobacterium nucleatum. Front. Microbiol. 2016, 7, 1065. [Google Scholar] [CrossRef] [PubMed]
- Buret, A.G.; Motta, J.P.; Allain, T.; Ferraz, J.; Wallace, J.L. Pathobiont Release from Dysbiotic Gut Microbiota Biofilms in Intestinal Inflammatory Diseases: A Role for Iron? J. Biomed. Sci. 2019, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Eom, Y.B. Zerumbone inhibits Candida albicans Biofilm Formation and Hyphal Growth. Can. J. Microbiol. 2019. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Jo, M.; Hong, J.E.; Park, C.O.; Lee, C.G.; Yun, M.; Rhee, K.-J. Zerumbone Suppresses Enterotoxigenic Bacteroides fragilis Infection-Induced Colonic Inflammation through Inhibition of NF-κΒ. Int. J. Mol. Sci. 2019, 20, 4560. https://doi.org/10.3390/ijms20184560
Hwang S, Jo M, Hong JE, Park CO, Lee CG, Yun M, Rhee K-J. Zerumbone Suppresses Enterotoxigenic Bacteroides fragilis Infection-Induced Colonic Inflammation through Inhibition of NF-κΒ. International Journal of Molecular Sciences. 2019; 20(18):4560. https://doi.org/10.3390/ijms20184560
Chicago/Turabian StyleHwang, Soonjae, Minjeong Jo, Ju Eun Hong, Chan Oh Park, Chang Gun Lee, Miyong Yun, and Ki-Jong Rhee. 2019. "Zerumbone Suppresses Enterotoxigenic Bacteroides fragilis Infection-Induced Colonic Inflammation through Inhibition of NF-κΒ" International Journal of Molecular Sciences 20, no. 18: 4560. https://doi.org/10.3390/ijms20184560




