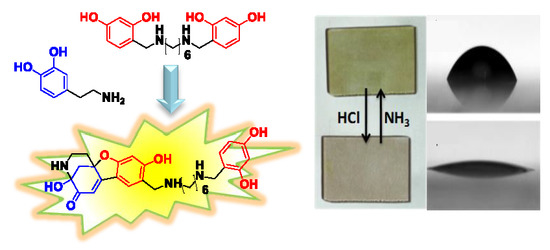
Reaction-Based, Fluorescent Film Deposition from Dopamine and a Diamine-Tethered, Bis–Resorcinol Coupler
Abstract
1. Introduction
2. Results
3. Discussion
- 1)
- The demonstration of the uncommon emission properties of the dopamine–resorcinol coupling product in the solid state as thin film;
- 2)
- Verification of the role of HMDA as an almost universal coupler, inducing film deposition under a variety of conditions;
- 3)
- The added value of including, in the film-forming adducts, strongly basic secondary amine sites susceptible of reversible protonation, to serve as on–off switches for surface property modification.
4. Materials and Methods
4.1. Synthesis and Structural Characterization of Bis–Res
4.2. General Procedure for the Oxidative Coupling of Compound 1 with Dopamine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HMDA | Hexamethylenediamine |
| Res | Resorcinol |
| WCA | Water contact angle |
References
- Li, D.; Zhang, Y.; Fan, Z.; Yu, J. AIE luminogen-functionalised mesoporous nanomaterials for efficient detection of volatile gases. Chem. Commun. 2015, 51, 13830–13833. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Shang, C.; Wang, Z.; Qi, Y.; Miao, R.; Liu, K.; Liu, T.; Fang, Y. Non-contact identification and differentiation of illicit drugs using fluorescent films. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, X.; Ma, H.; Zhang, Z.; Zhang, M.; Hao, S. A simple fluorescent film probe for the detection of fluoride anion in organic solution. Dyes Pigment. 2018, 153, 200–205. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, X.; Obolda, A.; Abdurahman, A.; Li, F.; Zhang, M. A rapid-response fluorescent film probe to DNT based on novel AIE materials. Sens. Actuators B Chem. 2019, 281, 971–976. [Google Scholar] [CrossRef]
- He, M.; Peng, H.; Wang, G.; Chang, X.; Miao, R.; Wang, W.; Fang, Y. Fabrication of a new fluorescent film and its superior sensing performance to N-methamphetamine in vapor phase. Sens. Actuators B Chem. 2016, 227, 255–262. [Google Scholar] [CrossRef]
- Philip, S.A. Organic solid-state fluorescence: Strategies for generating switchable and tunable fluorescent materials. Chempluschem 2012, 77, 518–531. [Google Scholar]
- Jorge, P.A.S.; Caldas, P.; Rosa, C.C.; Oliva, A.G.; Santos, J.L. Optical fiber probes for fluorescence based oxygen sensing. Sens. Actuators B Chem. 2004, 103, 290–299. [Google Scholar] [CrossRef]
- Liu, K.; Liu, T.; Chen, X.; Sun, X.; Fang, Y. Fluorescent films based on molecular-gel networks and their sensing performances. ACS Appl. Mater. Interfaces 2013, 5, 9830–9836. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chang, X.; He, M.; Shang, C.; Wang, G.; Yin, S.; Peng, H. Functionality-oriented derivatization of naphthalene diimide: A molecular gel strategy-based fluorescent film for aniline vapor detection. ACS Appl. Mater. Interfaces 2016, 8, 18584–18592. [Google Scholar] [CrossRef]
- Deng, Z.; Liu, C.; Jin, Y.; Pu, J.; Wang, B.; Chen, J. High quantum yield blue- and orange-emitting carbon dots: One-step microwave synthesis and applications as fluorescent film, fingerprint and cellular imaging. Analyst 2019. [Google Scholar] [CrossRef]
- Bandi, R.; Devulapalli, N.P.; Dadigala, R.; Gangapuram, B.R.; Guttena, V. Facile conversion of toxic cigarette butts to N,S-codoped carbon dots and their application in fluorescent film, security ink, bioimaging, sensing and logic gate operation. ACS Omega 2018, 3, 13454–13466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wang, Z.; Tang, Y.; Zheng, Y.; Wang, Q. Optical detection of anthrax biomarkers in an aqueous medium: The combination of carbon quantum dots and europium ions within alginate hydrogels. J. Mater. Sci. 2019, 54, 2526–2534. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, K.; Wang, G.; Shang, C.; Peng, H.; Liu, T.; Fang, Y. Detection of gaseous amines with a fluorescent film based on a perylene bisimide-functionalized copolymer. New J. Chem. 2018, 42, 12737–12744. [Google Scholar] [CrossRef]
- Anbuselvan, C. Synthesis and spectral studies of Schiff base receptor for fluorescence detection of Hg(II). Asian J. Chem. 2019, 31, 527–532. [Google Scholar] [CrossRef]
- Kaewtong, C.; Kampaengsri, S.; Singhana, B.; Pulpoka, B. Highly selective detection of Au3+ using rhodamine-based modified polyacrylic acid (PAA)-coated ITO. Dyes Pigment. 2017, 141, 277–285. [Google Scholar] [CrossRef]
- Wu, M.-J.; Hu, H.-H.; Siao, C.-Z.; Liao, Y.-M.; Chen, J.-H.; Li, M.-Y.; Lin, T.-Y.; Chen, Y.-F. All organic label-like copper(II) ions fluorescent film sensors with high sensitivity and stretchability. ACS Sens. 2018, 3, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhang, P.; Zhou, H.; Xu, J.; Li, Y.; Lu, M.; Lei, L.; Zhang, Q.; Zhang, Y.; Chen, S. Poly-β-hydroxybutyrate sensitizing effect on the photophysical properties of environment friendly fluorescent films containing europium complex. J. Lumin. 2016, 178, 172–177. [Google Scholar] [CrossRef]
- Crescenzi, O.; Napolitano, A.; Prota, G.; Peter, M.G. Oxidative coupling of DOPA with resorcinol and phloroglucinol: Isolation of adducts with an unusual tetrahydromethanobenzofuro[2,3-d]azocine skeleton. Tetrahedron 1991, 47, 6243–6250. [Google Scholar] [CrossRef]
- Acuña, A.U.; Álvarez-Pérez, M.; Liras, M.; Coto, P.B.; Amat-Guerri, F. Synthesis and photophysics of novel biocompatible fluorescent oxocines and azocines in aqueous solution. Phys. Chem. Chem. Phys. 2013, 15, 16704–16712. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Li, X.; Guo, X.; Zhang, B.; Jia, X.; Dai, B. A simple, fast and low-cost turn-on fluorescence method for dopamine detection using in situ reaction. Anal. Chim. Acta 2016, 944, 51–56. [Google Scholar] [CrossRef]
- Zhao, J.; Bao, X.; Wang, S.; Lu, S.; Sun, J.; Yang, X. In situ fluorogenic and chromogenic reactions for the sensitive dual-readout assay of tyrosinase activity. Anal. Chem. 2017, 89, 10529–10530. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, S.; Lu, S.; Bao, X.; Sun, J.; Yang, X. An enzyme cascade-triggered fluorogenic and chromogenic reaction applied in enzyme activity assay and immunoassay. Anal. Chem. 2018, 90, 7754–7760. [Google Scholar] [CrossRef] [PubMed]
- Iacomino, M.; Alfieri, M.L.; Crescenzi, O.; d’Ischia, M.; Napolitano, A. Unimolecular variant of the fluorescence turn-on oxidative coupling of catecholamines with resorcinols. ACS Omega 2019, 4, 1541–1548. [Google Scholar] [CrossRef]
- Iacomino, M.; Paez, J.I.; Avolio, R.; Carpentieri, A.; Panzella, L.; Falco, G.; Pizzo, E.; Errico, M.E.; Napolitano, A.; del Campo, A. Multifunctional thin films and coatings from caffeic acid and a cross-linking diamine. Langmuir 2017, 33, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; d’Ischia, M. The chemistry of polydopamine film formation: The amine-quinone interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; Ball, V.; d’Ischia, M. Hexamethylenediamine-mediated polydopamine film deposition: Inhibition by resorcinol as a strategy for mapping quinone targeting mechanisms. Front. Chem. 2019, 7, 407. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Lyu, Q.; Zhang, J.; Neoh, K.G.; Chai Li Lin, C. A one step method for the functional and property modification of DOPA based nanocoatings. Nanoscale 2017, 9, 12409–12415. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Yang, Z.; Zhou, S.; Luo, R.; Maitz, M.F.; Zhao, Y.; Wang, J.; Xiong, K.; Huang, N. A simple one-step modification of various materials for introducing effective multi-functional groups. Colloids Surf. B Biointerfaces 2014, 113, 125–133. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Chen, Y.; Zhao, S.; Chen, M.; Li, X.; Maitz, M.F.; Wang, J.; Huang, N. Application of phenol/amine copolymerized film modified magnesium alloys: Anticorrosion and surface biofunctionalization. ACS Appl. Mater. Interfaces 2015, 7, 24510–24522. [Google Scholar] [CrossRef]
- Kord Forooshani, P.; Lee, B.P. Recent approaches in designing bioadhesive materials inspired by mussel adhesive protein. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef] [PubMed]
- Memoli, S.; Napolitano, A.; d’Ischia, M.; Misuraca, G.; Palumbo, A.; Prota, G. Diffusible melanin-related metabolites are potent inhibitors of lipid peroxidation. Biochim. Biophys. Acta 1997, 1346, 61–68. [Google Scholar] [CrossRef]
- Heacock, R.A.; Marion, L. The infrared spectra of secondary amines and their salts. Can. J. Chem. 1956, 34, 1782–1795. [Google Scholar] [CrossRef]
- Putz, A.-M.; Putz, M.V. Spectral inverse quantum (Spectral-IQ) method for modeling mesoporous systems: Application on silica films by FTIR. Int. J. Mol. Sci. 2012, 13, 15925–15941. [Google Scholar] [CrossRef] [PubMed]







| Coating | λmax/ex (nm) | λem (nm) | Thickness (nm) | Roughness (nm) | Water Contact Angle (deg) |
|---|---|---|---|---|---|
| Bis-Res/DA | 420 | 464 | 55 ± 2.7 | 15 | 52 ± 3.6 |
| Bis-Res/DA-HCl | 400 | no | 48 ± 0.2 | 16 | Not calculable |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfieri, M.L.; Iacomino, M.; Napolitano, A.; d’Ischia, M. Reaction-Based, Fluorescent Film Deposition from Dopamine and a Diamine-Tethered, Bis–Resorcinol Coupler. Int. J. Mol. Sci. 2019, 20, 4532. https://doi.org/10.3390/ijms20184532
Alfieri ML, Iacomino M, Napolitano A, d’Ischia M. Reaction-Based, Fluorescent Film Deposition from Dopamine and a Diamine-Tethered, Bis–Resorcinol Coupler. International Journal of Molecular Sciences. 2019; 20(18):4532. https://doi.org/10.3390/ijms20184532
Chicago/Turabian StyleAlfieri, Maria Laura, Mariagrazia Iacomino, Alessandra Napolitano, and Marco d’Ischia. 2019. "Reaction-Based, Fluorescent Film Deposition from Dopamine and a Diamine-Tethered, Bis–Resorcinol Coupler" International Journal of Molecular Sciences 20, no. 18: 4532. https://doi.org/10.3390/ijms20184532
APA StyleAlfieri, M. L., Iacomino, M., Napolitano, A., & d’Ischia, M. (2019). Reaction-Based, Fluorescent Film Deposition from Dopamine and a Diamine-Tethered, Bis–Resorcinol Coupler. International Journal of Molecular Sciences, 20(18), 4532. https://doi.org/10.3390/ijms20184532