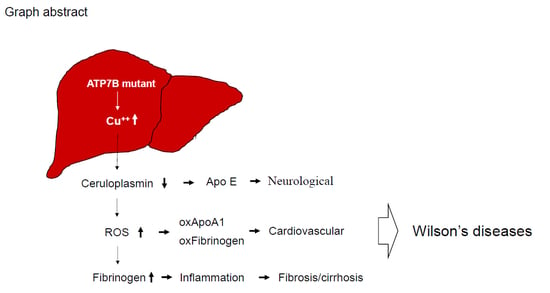
Characterization of Fibrinogen as a Key Modulator in Patients with Wilson’s Diseases with Functional Proteomic Tools
Abstract
1. Introduction
2. Results
2.1. Enzymatic Analysis of Ceruloplasmin Activity and Evaluation of Protein Carbonylation
2.2. Identification of WD-Associated Proteins by Proteomics Analysis
2.3. Biological Network Analysis
2.4. Fibrinogen Level Was Detected by 2DE-Immunoblots
2.5. Evaluating Fibrinogen Expression in Liver Tissue by Immunohistochemistry
2.6. Estimation of Fibrinogen Expression in Healthy Controls, Patients with WD and Liver Fibrosis and Blood-Stasis Subjects
2.7. Characterize the Interplay between Ceruloplasmin and Fibrinogen with Cell Model
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Subjects
5.2. In Situ Oxidase Activity of Ceruloplasmin
5.3. Two-Dimensional Polyacrylamide Gel Electrophoresis (2-DE)
5.4. Albumin Removal
5.5. Tryptic In-Gel Digestion of Proteins & MALDI-TOF Mass Spectrometry
5.6. Biological Network Analysis Using Metacore™
5.7. D-Oxyblot and 2D-Immunoblots
5.8. Immunohistochemistry
5.9. Analysis of Plasma Fibrinogen in Clinical Samples
5.10. Immunofluorescence
5.11. siRNA Administration
5.12. Western Blot Analysis
5.13. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| WD | Wilson’s disease |
| DNPH | 2,4-Dinitrophenylhydrazine |
| MALDI-TOF-MS | Matrix-Assisted Laser Desorption/ Ionization Time of Flight Mass Spectrometry |
| SOD | Superoxide dismutase |
| 2-DE | Two-dimensional electrophoresis |
| CP | Ceruloplasmin |
| PMF | Peptide mass fingerprinting |
| ROS | Reactive oxygen species |
| IPG | Immobilized pH gradient |
| IHC | Immunohistochemistry |
| NBT | p-Nitrobluetetrazolium chloride |
| BCIP | 5-Bromo-4-chloro-3-indolyl phosphate |
| DTE | Dithioerythritol |
| SDS-PAGE | Sodium dodecyl sulfate-polyacrylamide gel electrophoresis. |
References
- Dzieżyc, K.; Litwin, T.; Chabik, G.; Gramza, K.; Członkowska, A. Families with Wilson’s disease in subsequent generations: Clinical and genetic analysis. Mov. Disord. 2014, 29, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Medici, V.; Weiss, K.H. Genetic and environmental modifiers of Wilson disease. Handb. Clin. Neurol. 2017, 142, 35–41. [Google Scholar] [PubMed]
- Kluska, A.; Kulecka, M.; Litwin, T.; Dziezyc, K.; Balabas, A.; Piatkowska, M.; Paziewska, A.; Dabrowska, M.; Mikula, M.; Kaminska, D.; et al. Whole-exome sequencing identifies novel pathogenic variants across the ATP7B gene and some modifiers of Wilson’s disease phenotype. Liver Int. 2019, 39, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Chakraborty, K.; Shukla, A. Cellular copper homeostasis: Current concepts on its interplay with glutathione homeostasis and its implication in physiology and human diseases. Metallomics 2017, 9, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef]
- Roberts, E.A. Update on the Diagnosis and Management of Wilson Disease. Curr. Gastroenterol. Rep. 2018, 20, 56. [Google Scholar] [CrossRef]
- Wu, F.; Wang, J.; Pu, C.; Qiao, L.; Jiang, C. Wilson’s disease: A comprehensive review of the molecular mechanisms. Int. J. Mol. Sci. 2015, 16, 6419–6431. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The many “faces” of copper in medicine and treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef]
- Lorincz, M.T. Recognition and treatment of neurologic Wilson’s disease. Semin. Neurol. 2012, 32, 538–543. [Google Scholar] [CrossRef]
- Macintyre, G.; Gutfreund, K.S.; Martin, W.R.; Camicioli, R.; Cox, D.W. Value of an enzymatic assay for the determination of serum ceruloplasmin. J. Lab. Clin. Med. 2004, 144, 294–301. [Google Scholar] [CrossRef]
- Danesh, J.; Lewington, S.; Thompson, S.G.; Lowe, G.D.; Collins, R.; Kostis, J.B.; Wilson, A.C.; Folsom, A.R.; Wu, K.; Benderly, M.; et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: An individual participant meta-analysis. JAMA 2005, 294, 1799–1809. [Google Scholar] [PubMed]
- Stec, J.J.; Silbershatz, H.; Tofler, G.H.; Matheney, T.H.; Sutherland, P.; Lipinska, I.; Massaro, J.M.; Wilson, P.F.; Muller, J.E.; D’Agostino, R.B., Sr. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham Offspring Population. Circulation 2000, 102, 1634–1638. [Google Scholar] [CrossRef] [PubMed]
- Ferenci, P. Regional distribution of mutations of the ATP7B gene in patients with Wilson disease: Impact on genetic testing. Hum. Genet. 2006, 120, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Greco, V.; Piras, C.; Pieroni, L.; Urbani, A. Direct Assessment of Plasma/Serum Sample Quality for Proteomics Biomarker Investigation. Methods Mol. Biol. 2017, 1619, 3–21. [Google Scholar] [PubMed]
- Gallart-Palau, X.; Serra, A.; Sze, S.K. Uncovering Neurodegenerative Protein Modifications via Proteomic Profiling. Int. Rev. Neurobiol. 2015, 121, 87–116. [Google Scholar] [PubMed]
- Cauza, E.; Maier-Dobersberger, T.; Polli, C.; Kaserer, K.; Kramer, L.; Ferenci, P. Screening for Wilson’s disease in patients with liver diseases by serum ceruloplasmin. J. Hepatol. 1997, 27, 358–362. [Google Scholar] [CrossRef]
- Xu, R.; Jiang, Y.F.; Zhang, Y.H.; Yang, X. The optimal threshold of serum ceruloplasmin in the diagnosis of Wilson’s disease: A large hospital-based study. PLoS ONE 2018, 13, e0190887. [Google Scholar] [CrossRef] [PubMed]
- Vassiliev, V.B.; Kachurin, A.M.; Beltramini, M.; Rocco, G.P.; Salvato, B.; Gaitskhoki, V.S. Copper depletion/repletion of human ceruloplasmin is followed by the changes in its spectral features and functional properties. J. Inorg. Biochem. 1997, 65, 167–174. [Google Scholar] [CrossRef]
- Merle, U.; Eisenbach, C.; Weiss, K.H.; Tuma, S.; Stremmel, W. Serum ceruloplasmin oxidase activity is a sensitive and highly specific diagnostic marker for Wilson’s disease. J. Hepatol. 2009, 51, 925–930. [Google Scholar] [CrossRef]
- Hahn, S.H. Population screening for Wilson’s disease. Ann. NY Acad. Sci. 2014, 1315, 64–69. [Google Scholar] [CrossRef]
- Geyer, P.E.; Kulak, N.A.; Pichler, G.; Holdt, L.M.; Teupser, D.; Mann, M. Plasma Proteome Profiling to Assess Human Health and Disease. Cell Syst. 2016, 2, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, R.I.; Weisel, J.W. What Is the Biological and Clinical Relevance of Fibrin? Semin Thromb. Hemost. 2016, 42, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liu, Z.; Qiu, Y.; Zhang, J.; Wu, H.; Liang, R.; Chen, G.; Qin, G.; Li, Y.; Zou, D. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2018, 44, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Wolberg, A.S. Fibrinogen and fibrin: An illustrated review. Res. Pract. Thromb. Haemost. 2019, 3, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Weber, L.; Gotthardt, D.; Seessle, J.; Stremmel, W.; Pfeiffenberger, J.; Weiss, K.H. Coagulation Parameters in Wilson Disease. J. Gastrointest. Liver Dis. 2015, 24, 183–188. [Google Scholar]
- Davalos, D.; Akassoglou, K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopathol. 2012, 34, 43–62. [Google Scholar] [CrossRef]
- Zischka, H.; Lichtmannegger, J. Pathological mitochondrial copper overload in livers of Wilson’s disease patients and related animal models. Ann. NY Acad. Sci. 2014, 1315, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Clayton, P.T. Inherited disorders of transition metal metabolism: An update. J. Inherit. Metab. Dis. 2017, 40, 519–529. [Google Scholar] [CrossRef]
- Yu, Y.; Guerrero, C.R.; Liu, S.; Amato, N.J.; Sharma, Y.; Gupta, S.; Wang, Y. Comprehensive Assessment of Oxidatively Induced Modifications of DNA in a Rat Model of Human Wilson’s Disease. Mol. Cell Proteom. 2016, 15, 810–817. [Google Scholar] [CrossRef]
- Rosenfeld, M.A.; Vasilyeva, A.D.; Yurina, L.V.; Bychkova, A.V. Oxidation of proteins: Is it a programmed process? Free Radic. Res. 2018, 52, 14–38. [Google Scholar] [CrossRef]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass. Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Baraibar, M.A.; Friguet, B. Oxidative proteome modifications target specific cellular pathways during oxidative stress, cellular senescence and aging. Exp. Gerontol. 2013, 48, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Scheiber, I.F.; Brůha, R.; Dušek, P. Pathogenesis of Wilson disease. Handb. Clin. Neurol. 2017, 142, 43–55. [Google Scholar] [PubMed]
- Scheiber, I.F.; Mercer, J.F.; Dringen, R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014, 116, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Boukhenouna, S.; Wilson, M.A.; Bahmed, K.; Kosmider, B. Reactive Oxygen Species in Chronic Obstructive Pulmonary Disease. Oxid. Med. Cell Longev. 2018, 2018, 5730395. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Levin, L.A. Ceruloplasmin detection by SDS-PAGE, immunoblotting, and in situ oxidase activity. Methods Mol. Biol. 2002, 186, 265–271. [Google Scholar]
- Wang, P.W.; Chang, W.N.; Lu, C.H.; Chao, D.; Schrag, C.; Pan, T.L. New insights into the pathological mechanisms of cerebrotendinous xanthomatosis in the Taiwanese using genomic and proteomic tools. Proteomics 2006, 6, 1029–1037. [Google Scholar] [CrossRef]
- Pan, T.L.; Wang, P.W.; Huang, C.C.; Yeh, C.T.; Hu, T.H.; Yu, J.S. Network analysis and proteomic identification of vimentin as a key regulator associated with invasion and metastasis in human hepatocellular carcinoma cells. J. Proteomics. 2012, 75, 4676–4692. [Google Scholar] [CrossRef]
- Wang, P.W.; Wu, T.H.; Pan, T.L.; Chen, M.H.; Goto, S.; Chen, C.L. Integrated Proteome and Cytokine Profiles Reveal Ceruloplasmin Eliciting Liver Allograft Tolerance via Antioxidant Cascades. Front. Immunol. 2018, 9, 2216. [Google Scholar] [CrossRef]
- Pan, T.L.; Goto, S.; Lin, Y.C.; Lord, R.; Chiang, K.C.; Lai, C.Y.; Chen, Y.S.; Eng, H.L.; Cheng, Y.F.; Tatsuma, T.; et al. The fas and fas ligand pathways in liver allograft tolerance. Clin. Exp. Immunol. 1999, 118, 180–187. [Google Scholar] [CrossRef]
- Pan, T.L.; Wang, P.W.; Leu, Y.L.; Wu, T.H.; Wu, T.S. Inhibitory effects of Scutellaria baicalensis extract on hepatic stellate cells through inducing G2/M cell cycle arrest and activating ERK-dependent apoptosis via Bax and caspase pathway. J. Ethnopharmacol. 2012, 139, 829–837. [Google Scholar] [CrossRef]










| Spot No. | Protein Name | Accession number | Score (Coverage) | Mw (kDa)/pI | Function |
|---|---|---|---|---|---|
| 1 | Apo A1 | P02647 | 215 (61%) | 30.76/5.56 | Participates in the reverse transport of cholesterol from tissues to the liver for excretion by promoting cholesterol efflux from tissues and by acting as a cofactor for the lecithin cholesterol acyltransferase (LCAT). |
| 2 | Apo J (clusterin) | P10909 | 38 (19%) | 49.34/6.27 | Prevents stress-induced aggregation of blood plasma proteins. |
| 3 | α2-HS-glycoprotein | P02765 | 72 (28%) | 40.10/5.43 | Promotes endocytosis, possesses opsonic properties and influences the mineral phase of bone. Shows affinity for calcium and barium ions. |
| 4 | α1-B-glycoprotein | P04219 | 130 (43%) | 54.81/5.65 | . |
| 5 | Haptoglobin α chain | P00738 | 80 (51%) | 13.74/6.10 | Haptoglobin also acts as an antioxidant, has antibacterial activity, and plays a role in modulating many aspects of the acute phase response. |
| 6 | Fibrinogen-γ-A chain | P02679 | 158 (58%) | 50.82/5.70 | Together with fibrinogen-α (FGA) and fibrinogen-β (FGB), polymerizes to form an insoluble fibrin matrix. |
| 7 | Fibrinogen-γ-B chain | P02679 | 156 (56%) | 52.11/5.37 | Together with fibrinogen-α (FGA) and fibrinogen-β (FGB), polymerizes to form an insoluble fibrin matrix. |
| 8 | antithrombin | P01008 | 182 (58%) | 53.04/6.32 | Most important serine protease inhibitor in plasma that regulates the blood coagulation cascade. AT-III inhibits thrombin, matriptase-3/TMPRSS7, as well as factors IXa, Xa and XIa. |
| 9 | Fibrinogen- | P02675 | 225 (62%) | 56.58/8.54 | Cleaved by the protease thrombin to yield monomers which, together with fibrinogen-α (FGA) and fibrinogen-γ (FGG), polymerize to form an insoluble fibrin matrix. Fibrin deposition is also associated with infection, where it protects against IFNG-mediated hemorrhage. |
| 10 | Serotransferrin | P02787 | 93 (37%) | 74.28/6.63 | Transferrins are iron binding transport proteins which can bind two Fe3+ ions in association with the binding of an anion, usually bicarbonate. |
| 11 | α1-antiproteinase | P01009 | 148 (47%) | 46.88/5.37 | Inhibitor of serine proteases. Its primary target is elastase, but it also has a moderate affinity for plasmin and thrombin. |
| 12 | Immunoglobulin α2 heavy chain | P01876 | 133 (37%) | 54.82/6.02 | Constant region of immunoglobulin heavy chains. Immunoglobulins, also known as antibodies, are membrane-bound or secreted glycoproteins produced by B lymphocytes. |
| 13 | Haptoglobin β chain | P00738 | 126 (46%) | 38.87/6.26 | Haptoglobin captures, and combines with free plasma hemoglobin to allow hepatic recycling of heme iron and to prevent kidney damage. |
| 14 | α2-microglobulin | P01023 | 172 (25%) | 164.6/6.00 | Is able to inhibit all four classes of proteinases by a unique ‘trapping’ mechanism. |
| 15 | Complement C3 | P01024 | 107 (24%) | 188.57/6.02 | C3 plays a central role in the activation of the complement system. |
| 16 | Fibrinogen-α | P02671 | 127 (38%) | 94.97/5.70 | Cleaved by the protease thrombin to yield monomers which, together with fibrinogen-β (FGB) and fibrinogen-γ (FGG), polymerize to form an insoluble fibrin matrix. |
| 17 | ApoE | P02649 | 82 (43%) | 36.15/5.65 | APOE is an apolipoprotein, a protein associating with lipid particles, that mainly functions in lipoprotein-mediated lipid transport between organs via the plasma and interstitial fluids |
| 18 | Plasminogen | P00747 | 72 (23%) | 90.57/7.04 | Plasmin dissolves the fibrin of blood clots and acts as a proteolytic factor in a variety of other processes including embryonic development, tissue remodeling, tumor invasion, and inflammation. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.-W.; Lin, T.-Y.; Hung, Y.-C.; Chang, W.-N.; Yang, P.-M.; Chen, M.-H.; Yeh, C.-T.; Pan, T.-L. Characterization of Fibrinogen as a Key Modulator in Patients with Wilson’s Diseases with Functional Proteomic Tools. Int. J. Mol. Sci. 2019, 20, 4528. https://doi.org/10.3390/ijms20184528
Wang P-W, Lin T-Y, Hung Y-C, Chang W-N, Yang P-M, Chen M-H, Yeh C-T, Pan T-L. Characterization of Fibrinogen as a Key Modulator in Patients with Wilson’s Diseases with Functional Proteomic Tools. International Journal of Molecular Sciences. 2019; 20(18):4528. https://doi.org/10.3390/ijms20184528
Chicago/Turabian StyleWang, Pei-Wen, Tung-Yi Lin, Yu-Chiang Hung, Wen-Neng Chang, Pei-Ming Yang, Mu-Hong Chen, Chau-Ting Yeh, and Tai-Long Pan. 2019. "Characterization of Fibrinogen as a Key Modulator in Patients with Wilson’s Diseases with Functional Proteomic Tools" International Journal of Molecular Sciences 20, no. 18: 4528. https://doi.org/10.3390/ijms20184528
APA StyleWang, P.-W., Lin, T.-Y., Hung, Y.-C., Chang, W.-N., Yang, P.-M., Chen, M.-H., Yeh, C.-T., & Pan, T.-L. (2019). Characterization of Fibrinogen as a Key Modulator in Patients with Wilson’s Diseases with Functional Proteomic Tools. International Journal of Molecular Sciences, 20(18), 4528. https://doi.org/10.3390/ijms20184528