The Role of MicroRNAs in Early Chondrogenesis of Human Induced Pluripotent Stem Cells (hiPSCs)
Abstract
:1. Introduction
2. Results
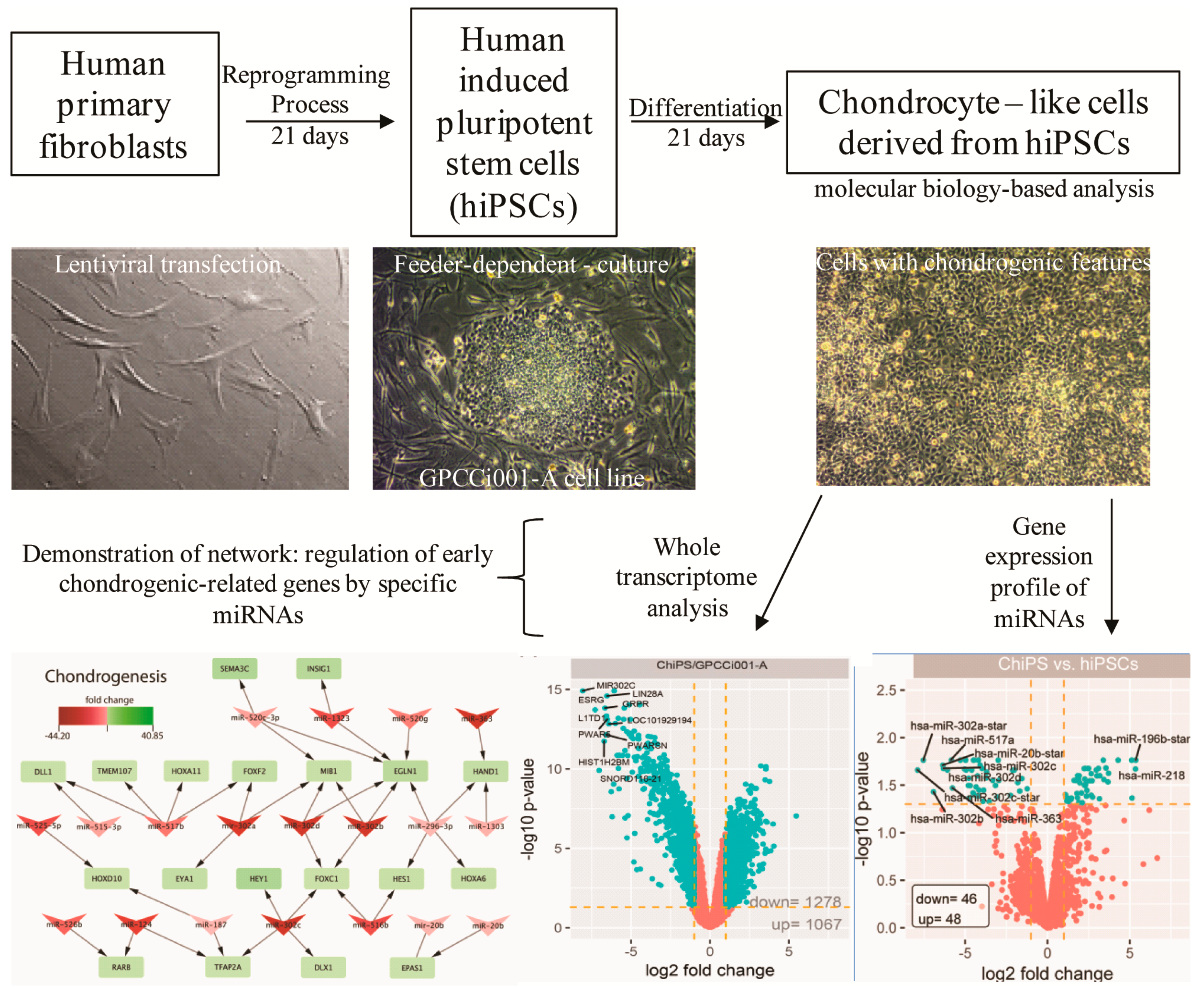
2.1. Chondrogenic Differentiation of hiPSCs: We Obtained Chondrogenic Progenitors from hiPSCs
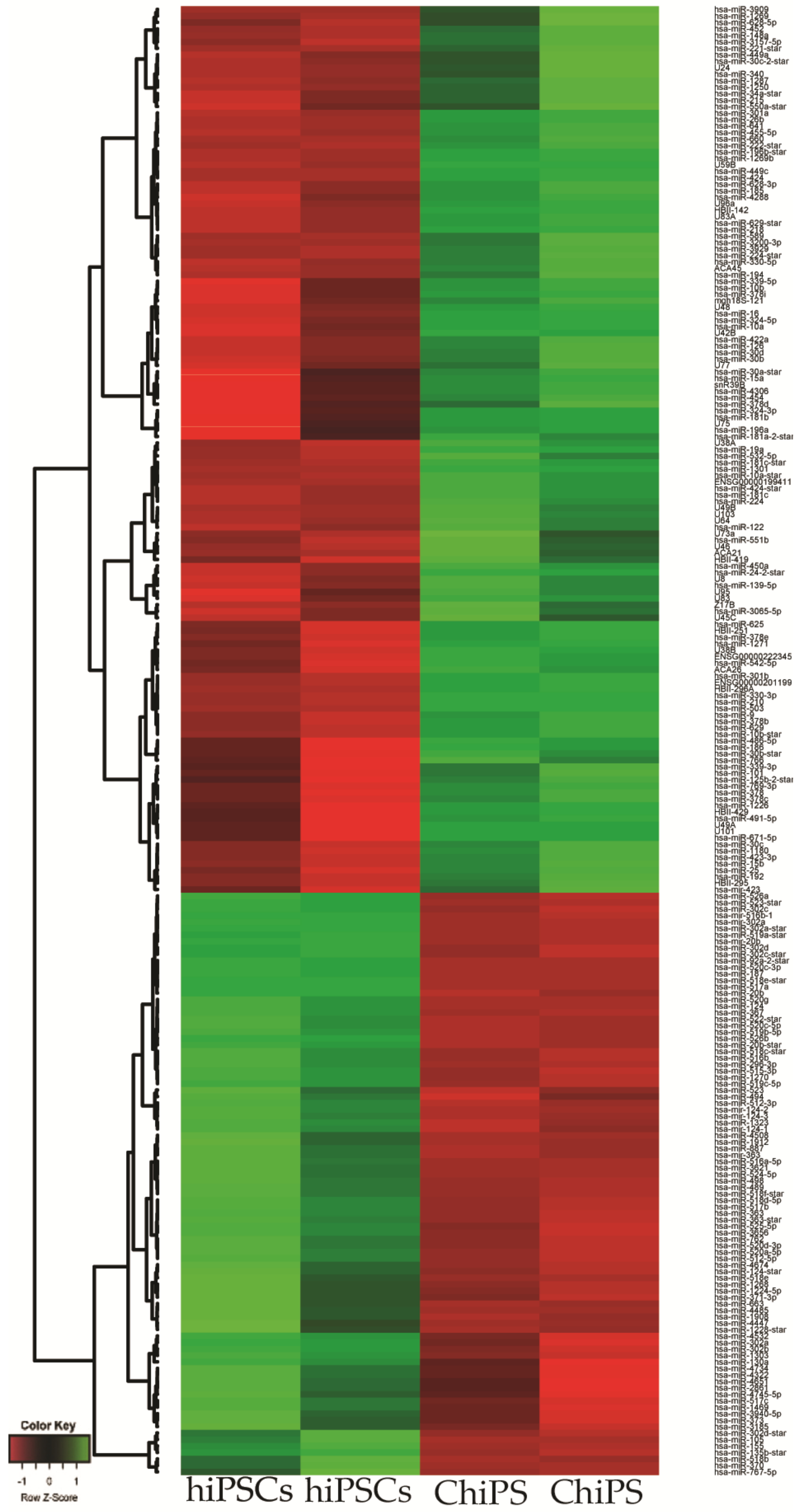
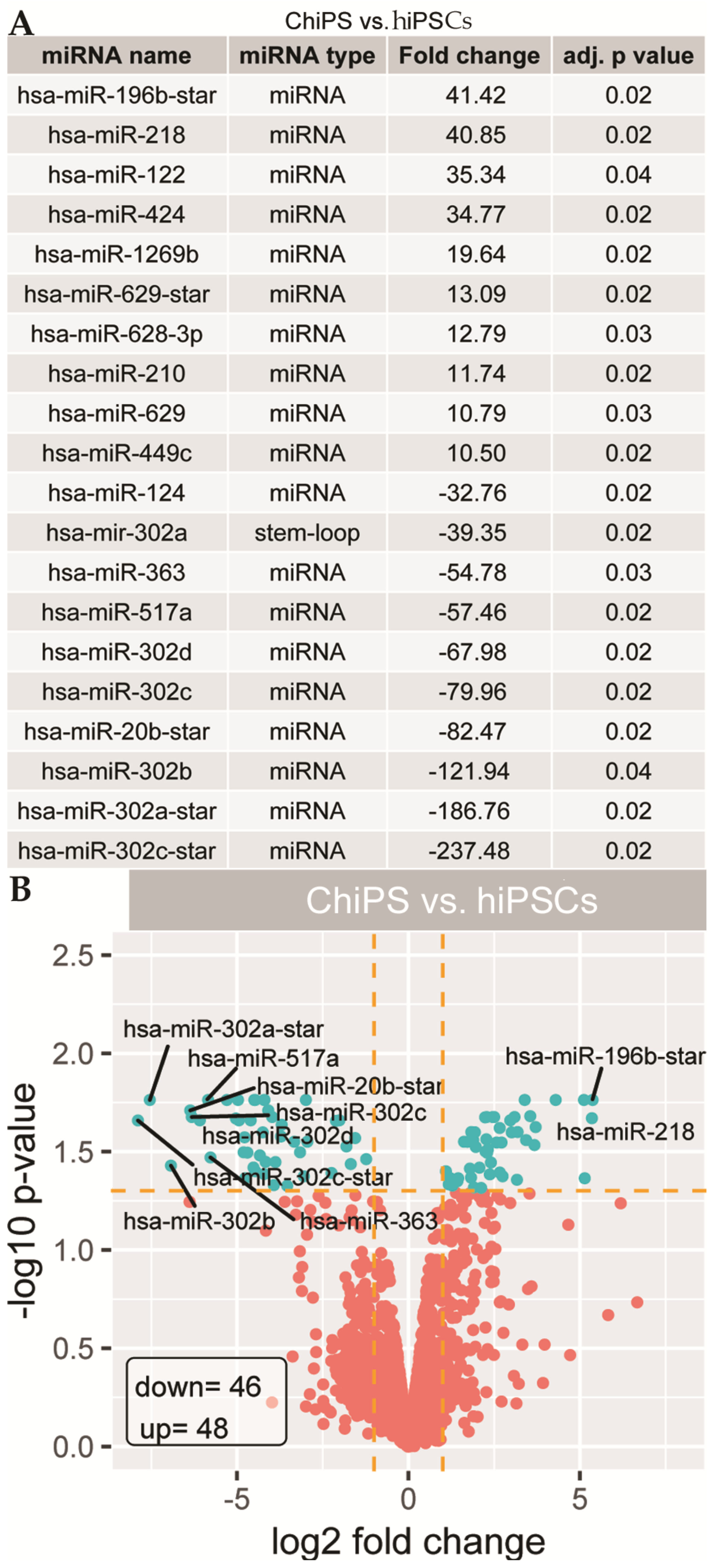
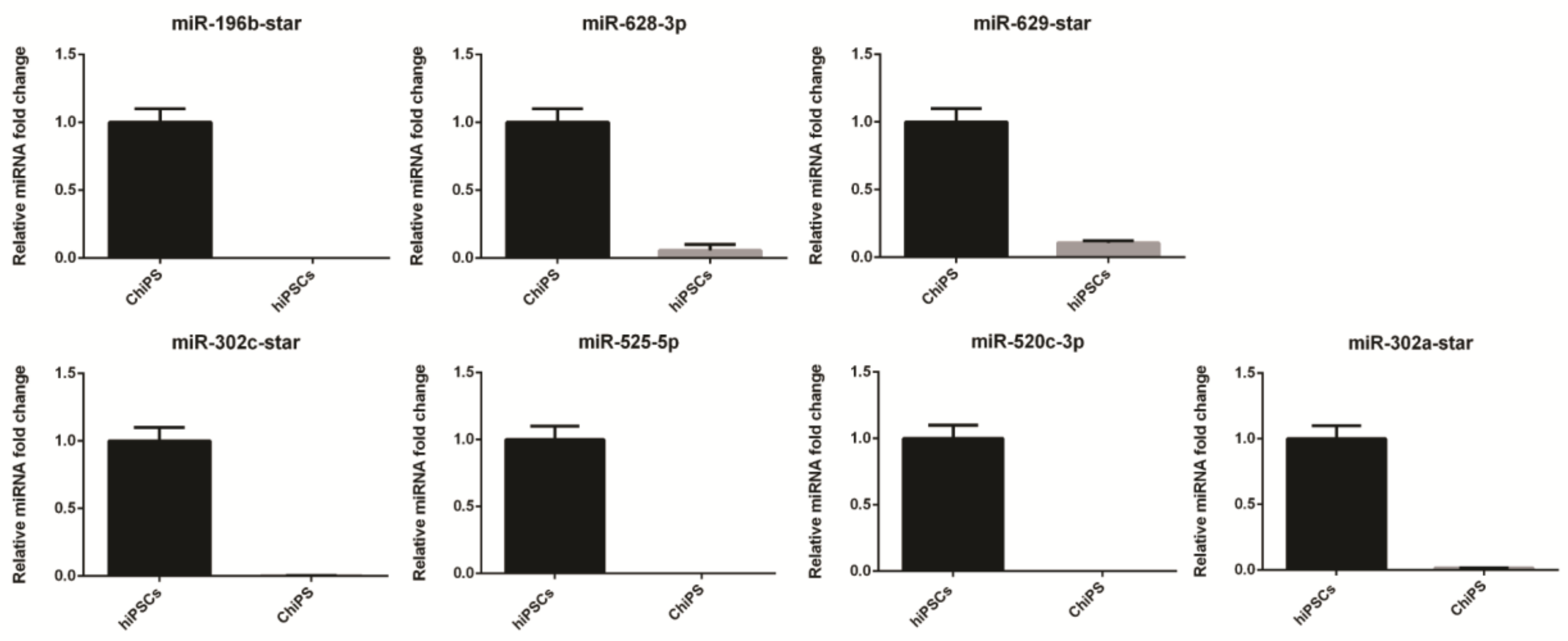
2.2. Analysis of Microarray Experiments of miRNA Expression Profiling: ChiPS and hiPSCs Are Characterized by Distinct miRNA Expression Profiles
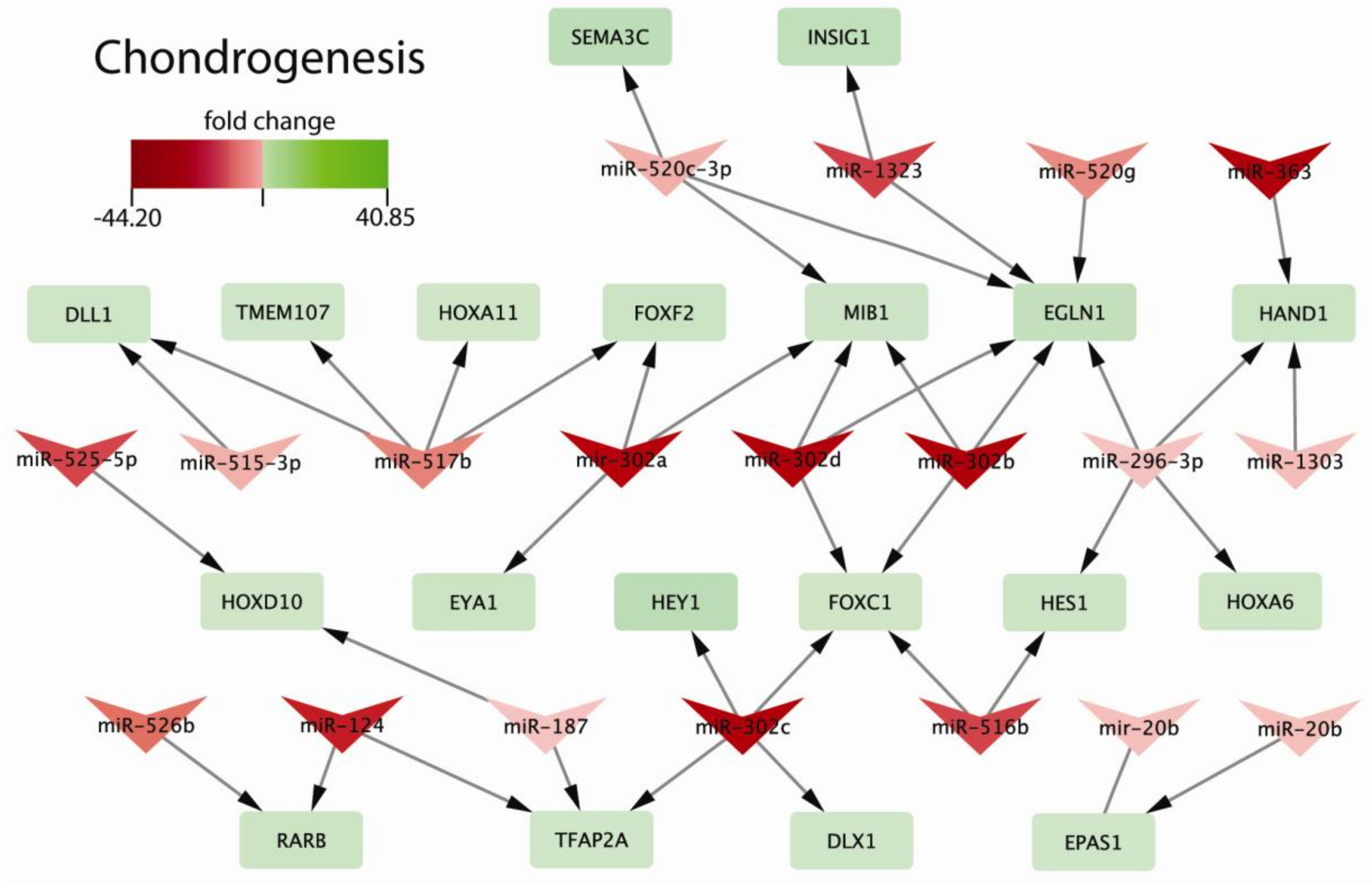
2.3. Biological Processes Regulated by miRNAs during Chondrogenic Differentiation: miRNAs Strongly Regulate Signaling Pathways Engaged in Early Chondrogenesis
3. Discussion
4. Materials and Methods
4.1. Differentiation of hiPSCs into Chondrogenic Progenitors
4.2. Immunofluorescence Analysis
4.3. Total RNA and miRNA-Enriched Fraction Purification
4.4. Gene Expression Profile of miRNAs
4.5. miRNA-Target Gene Prediction
4.6. Validation of miRNAs Expression
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACAN | Aggrecan |
| BMP-4 | Bone morphogenetic protein 4 |
| bMSCs | Bone mesenchymal stem cells |
| COL2A1 | Collagen type II alpha 1 chain |
| COL10A1 | Collagen type X alpha 1 chain |
| COL11A1 | Collagen type XI alpha 1 chain |
| COMP | Cartilage oligomeric matrix protein |
| DAVID | Database for Annotation, Visualization, and Integrated Discovery |
| EBs | Embryoid bodies |
| FGF-2 | Basic fibroblast growth factor |
| FGFR1 | Fibroblast growth factor receptor 1 |
| FOX | Forkhead box (Fox gene family) |
| GAG | glycosaminoglycan |
| GEO | Gene Expression Omnibus |
| GFs | Growth factors |
| GO | Gene ontology |
| GPCCi001-A | Human induced pluripotent stem cell line created in Greater Poland Cancer Centre |
| hADSCs | Human adipose-derived stem cells |
| hiPSCs | Human induced pluripotent stem cells |
| HPGD | 15-hydroxyprostaglandin dehydrogenase |
| HOX | Homeobox genes |
| IGF-1 | Insulin-like growth factor 1 |
| ITS | Insulin–transferrin–selenium |
| KEGG | Kyoto Encyclopedia Genes and Genomes |
| MMP-13 | Matrix metallopeptidase 13 |
| MSCs | Mesenchymal stem cells |
| PDGF-BB | Platelet-derived growth factor |
| RARB | Retinoic acid receptor, beta polypeptide |
| RMA | Robust Multiarray Average Normalization Algorithm |
| SCs | Stem cells |
| SEMA3C | Semaphorin 3C |
| SONFH | steroid-induced osteonecrosis of the femoral head |
| SOX | SRY (sex determining region Y)-box genes |
| TGF-β3 | Transforming growth factor beta 3 |
| VEGF | Vascular endothelial growth factor |
| VEGFR2 | Vascular endothelial growth factor receptor 2 |
| Wnt | Wingless-type MMTV integration site family |
References
- Pei, D.; Xu, J.; Zhuang, Q.; Tse, H.F.; Esteban, M.A. Induced pluripotent stem cell technology in regenerative medicine and biology. Adv. Biochem. Eng. Biotechnol. 2010, 123, 127–141. [Google Scholar]
- Kitada, M.; Wakao, S.; Dezawa, M. Muse cells and induced pluripotent stem cell: Implication of the elite model. Cell. Mol. Life Sci. 2012, 69, 3739–3750. [Google Scholar] [CrossRef]
- Yamashita, A.; Tamamura, Y.; Morioka, M.; Karagiannis, P.; Shima, N.; Tsumaki, N. Considerations in hiPSC-derived cartilage for articular cartilage repair. Inflamm. Regen. 2018, 38, 17. [Google Scholar] [CrossRef] [Green Version]
- Nejadnik, H.; Diecke, S.; Lenkov, O.D.; Chapelin, F.; Donig, J.; Tong, X.; Derugin, N.; Chan, R.C.; Gaur, A.; Yang, F.; et al. Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem Cell Rev. 2015, 11, 242–253. [Google Scholar] [CrossRef]
- Dimitrion, P.; Zhi, Y.; Clayton, D.; Apodaca, G.L.; Wilcox, M.R.; Johnson, J.W.; Nimgaonkar, V.; D’Aiuto, L. Low-Density Neuronal Cultures from Human Induced Pluripotent Stem Cells. Mol. Neuropsychiatry 2017, 3, 28–36. [Google Scholar] [CrossRef]
- Suchorska, W.M.; Augustyniak, E.; Richter, M.; Trzeciak, T. Comparison of Four Protocols to Generate Chondrocyte-Like Cells from Human Induced Pluripotent Stem Cells (hiPSCs). Stem Cell Rev. 2017, 13, 299–308. [Google Scholar] [CrossRef]
- Stelcer, E.; Kulcenty, K.; Rucinski, M.; Jopek, K.; Richter, M.; Trzeciak, T.; Suchorska, W.M. Chondrogenic differentiation in vitro of hiPSCs activates pathways engaged in limb development. Stem Cell Res. 2018, 30, 53–60. [Google Scholar] [CrossRef]
- Lee, J.; Taylor, S.E.; Smeriglio, P.; Lai, J.; Maloney, W.J.; Yang, F.; Bhutani, N. Early induction of a prechondrogenic population allows efficient generation of stable chondrocytes from human induced pluripotent stem cells. FASEB J. 2015, 29, 3399–3410. [Google Scholar] [CrossRef] [Green Version]
- Stelcer, E.; Kulcenty, K.; Rucinski, M.; Jopek, K.; Richter, M.; Trzeciak, T.; Suchorska, W.M. Forced differentiation in vitro leads to stress-induced activation of DNA damage response in hiPSC-derived chondrocyte-like cells. PLoS ONE 2018, 13, e0198079. [Google Scholar] [CrossRef]
- Li, N.; Long, B.; Han, W.; Yuan, S.; Wang, K. MicroRNAs: Important regulators of stem cells. Stem Cell Res. Ther. 2017, 8, 110. [Google Scholar] [CrossRef]
- Wang, T.; Shi, S.B.; Sha, H.Y. MicroRNAs in regulation of pluripotency and somatic cell reprogramming: Small molecule with big impact. RNA Biol. 2013, 10, 1255–1261. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Cavalcanti, M.; Trubiani, O.; Diomede, F. MicroRNA 210 Mediates VEGF Upregulation in Human Periodontal Ligament Stem Cells Cultured on 3DHydroxyapatite Ceramic Scaffold. Int. J. Mol. Sci. 2018, 19, 3916. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Gugliandolo, A.; Orsini, T.; Fontana, A.; Ventrella, A.; Mazzon, E.; Bramanti, P.; Diomede, F.; Trubiani, O. Engineered Extracellular Vesicles from Human Periodontal-Ligament Stem Cells Increase VEGF/VEGFR2 Expression During Bone Regeneration. Front. Physiol. 2019, 10, 512. [Google Scholar] [CrossRef]
- Oliveto, S.; Mancino, M.; Manfrini, N.; Biffo, S. Role of microRNAs in translation regulation and cancer. World J. Biol. Chem. 2017, 8, 45–56. [Google Scholar] [CrossRef]
- Lach, M.S.; Wroblewska, J.P.; Augustyniak, E.; Kulcenty, K.; Suchorska, W.M. A feeder- and xeno-free human induced pluripotent stem cell line obtained from primary human dermal fibroblasts with epigenetic repression of reprogramming factors expression: GPCCi001-A. Stem Cell Res. 2017, 20, 34–37. [Google Scholar] [CrossRef]
- Stelcer, E.; Kulcenty, K.; Rucinski, M.; Jopek, K.; Trzeciak, T.; Richter, M.; Wroblewska, J.; Suchorska, W. Expression of Pluripotency Genes in Chondrocyte-Like Cells Differentiated from Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2018, 19, 550. [Google Scholar] [CrossRef]
- Barter, M.J.; Tselepi, M.; Gomez, R.; Woods, S.; Hui, W.; Smith, G.R.; Shanley, D.P.; Clark, I.M.; Young, D.A. Genome-Wide MicroRNA and Gene Analysis of Mesenchymal Stem Cell Chondrogenesis Identifies an Essential Role and Multiple Targets for miR-140-5p. Stem Cells 2015, 33, 3266–3280. [Google Scholar] [CrossRef] [Green Version]
- Mahboudi, H.; Soleimani, M.; Enderami, S.E.; Kehtari, M.; Ardeshirylajimi, A.; Eftekhary, M.; Kazemi, B. Enhanced chondrogenesis differentiation of human induced pluripotent stem cells by MicroRNA-140 and transforming growth factor beta 3 (TGFbeta3). Biologicals 2018, 52, 30–36. [Google Scholar] [CrossRef]
- Chen, S.; Fu, P.; Cong, R.; Wu, H.; Pei, M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015, 2, 76–95. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Chang, W.H.; Fong, B.; Gao, F.; Liu, C.; Al Alam, D.; Bellusci, S.; Lu, W. Regulation of induced pluripotent stem (iPS) cell induction by Wnt/beta-catenin signaling. J. Biol. Chem. 2014, 289, 9221–9232. [Google Scholar] [CrossRef]
- Fernandez, A.; Huggins, I.J.; Perna, L.; Brafman, D.; Lu, D.; Yao, S.; Gaasterland, T.; Carson, D.A.; Willert, K. The WNT receptor FZD7 is required for maintenance of the pluripotent state in human embryonic stem cells. Proc. Natl. Acad Sci. USA 2014, 111, 1409–1414. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Xu, Z.; Shao, J.; Fu, P.; Wu, H. MicroRNA-218 promotes early chondrogenesis of mesenchymal stem cells and inhibits later chondrocyte maturation. BMC Biotechnol. 2019, 19, 6. [Google Scholar] [CrossRef]
- Yang, B.; Guo, H.; Zhang, Y.; Chen, L.; Ying, D.; Dong, S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS ONE 2011, 6, e21679. [Google Scholar] [CrossRef]
- Gao, F.; Peng, C.; Zheng, C.; Zhang, S.; Wu, M. miRNA-101 promotes chondrogenic differentiation in rat bone marrow mesenchymal stem cells. Exp. Ther. Med. 2019, 17, 175–180. [Google Scholar] [CrossRef]
- Guerit, D.; Brondello, J.M.; Chuchana, P.; Philipot, D.; Toupet, K.; Bony, C.; Jorgensen, C.; Noël, D. FOXO3A regulation by miRNA-29a Controls chondrogenic differentiation of mesenchymal stem cells and cartilage formation. Stem Cells Dev. 2014, 23, 1195–1205. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Human FOX gene family (Review). Int. J. Oncol. 2004, 25, 1495–1500. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, Y.; Zhang, Z.; Zhang, H.; Duan, X.; Liu, J.; Li, X.; Liao, W. Expression of microRNAs during chondrogenesis of human adipose-derived stem cells. Osteoarthr. Cartil. 2012, 20, 1638–1646. [Google Scholar] [CrossRef] [Green Version]
- Yekta, S.; Shih, I.H.; Bartel, D.P. MicroRNA-directed cleavage of HOXB8 mRNA. Science 2004, 304, 594–596. [Google Scholar] [CrossRef]
- McAlinden, A.; Varghese, N.; Wirthlin, L.; Chang, L.W. Differentially expressed microRNAs in chondrocytes from distinct regions of developing human cartilage. PLoS ONE 2013, 8, e75012. [Google Scholar] [CrossRef]
- Wang, A.; Ren, M.; Song, Y.; Wang, X.; Wang, Q.; Yang, Q.; Liu, H.; Du, Z.; Zhang, G.; Wang, J. MicroRNA Expression Profiling of Bone Marrow Mesenchymal Stem Cells in Steroid-Induced Osteonecrosis of the Femoral Head Associated with Osteogenesis. Med. Sci. Monit. 2018, 24, 1813–1825. [Google Scholar] [CrossRef] [Green Version]
- Mandourah, A.Y.; Ranganath, L.; Barraclough, R.; Vinjamuri, S.; Hof, R.V.; Hamill, S.; Czanner, G.; Dera, A.A.; Wang, D.; Barraclough, D. L Circulating microRNAs as potential diagnostic biomarkers for osteoporosis. Sci. Rep. 2018, 8, 8421. [Google Scholar] [CrossRef]
- Chamorro-Jorganes, A.; Araldi, E.; Suarez, Y. MicroRNAs as pharmacological targets in endothelial cell function and dysfunction. Pharmacol. Res. 2013, 75, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.; Zhang, Z.; Hu, S.; Zhang, Z.; Chang, Z.; Huang, Z.; Liao, W.; Kang, Y. Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res. Ther. 2018, 9, 247. [Google Scholar] [CrossRef]
- Gautier, L.; Cope, L.; Bolstad, B.M.; Irizarry, R.A. Affy—Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004, 20, 307–315. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Cava, C.; Colaprico, A.; Bertoli, G.; Graudenzi, A.; Silva, T.C.; Olsen, C.; Noushmehr, H.; Bontempi, G.; Mauri, G.; Castiglioni, I. SpidermiR: An R/Bioconductor Package for Integrative Analysis with miRNA Data. Int. J. Mol. Sci. 2017, 18, 274. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T., Jr.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Jopek, K.; Tyczewska, M.; Celichowski, P.; Malendowicz, L.K.; Rucinski, M. Transcriptome Profile in Unilateral Adrenalectomy-Induced Compensatory Adrenal Growth in the Rat. Int. J. Mol. Sci. 2018, 19, 1111. [Google Scholar] [CrossRef]
- Fresno, C.; Fernandez, E.A. RDAVIDWebService: A versatile R interface to DAVID. Bioinformatics 2013, 29, 2810–2811. [Google Scholar] [CrossRef]
- Benjamini, Y.; Cohen, R. Weighted false discovery rate controlling procedures for clinical trials. Biostatistics 2017, 18, 91–104. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelcer, E.; Kulcenty, K.; Rucinski, M.; Jopek, K.; Richter, M.; Trzeciak, T.; Suchorska, W.M. The Role of MicroRNAs in Early Chondrogenesis of Human Induced Pluripotent Stem Cells (hiPSCs). Int. J. Mol. Sci. 2019, 20, 4371. https://doi.org/10.3390/ijms20184371
Stelcer E, Kulcenty K, Rucinski M, Jopek K, Richter M, Trzeciak T, Suchorska WM. The Role of MicroRNAs in Early Chondrogenesis of Human Induced Pluripotent Stem Cells (hiPSCs). International Journal of Molecular Sciences. 2019; 20(18):4371. https://doi.org/10.3390/ijms20184371
Chicago/Turabian StyleStelcer, Ewelina, Katarzyna Kulcenty, Marcin Rucinski, Karol Jopek, Magdalena Richter, Tomasz Trzeciak, and Wiktoria Maria Suchorska. 2019. "The Role of MicroRNAs in Early Chondrogenesis of Human Induced Pluripotent Stem Cells (hiPSCs)" International Journal of Molecular Sciences 20, no. 18: 4371. https://doi.org/10.3390/ijms20184371
APA StyleStelcer, E., Kulcenty, K., Rucinski, M., Jopek, K., Richter, M., Trzeciak, T., & Suchorska, W. M. (2019). The Role of MicroRNAs in Early Chondrogenesis of Human Induced Pluripotent Stem Cells (hiPSCs). International Journal of Molecular Sciences, 20(18), 4371. https://doi.org/10.3390/ijms20184371





