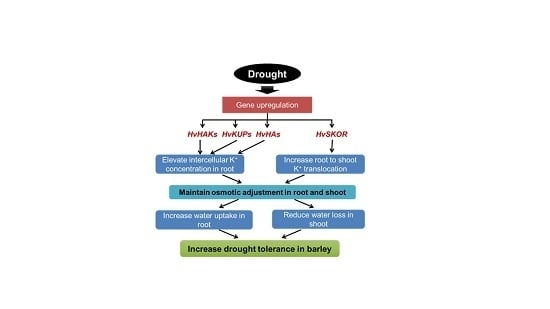
The Ability to Regulate Transmembrane Potassium Transport in Root Is Critical for Drought Tolerance in Barley
Abstract
1. Introduction
2. Results
2.1. Barley Genotypes Differ Significantly in Their Sensitivity to Drought
2.2. Genotypic Difference in Drought Sensitivity is Highly Dependent on the Ability of Water Retention by Osmotic Adjustment
2.3. Drought Tolerance is Highly Correlated with the Ability of K+ Uptake and Accumulation in Barley
2.4. Drought Tolerant Barley Genotypes are Capable of Maintaining H+-Pumping Activity
2.5. Drought Stress Induces Changes in Relative Expression of Plasma Membrane K+ Transporting and H+ Pumping-Related Genes
3. Discussion
3.1. K+ Uptake Confers Osmotic Adjustment and Contributes to Genotypic Difference in Drought Tolerance in Barley
3.2. Regulation of the K Transporters and Channels is Critical for Root K+ Uptake and Root to Shoot K+ Translocation under Drought Stress in Barley
3.3. Plasma Membrane H+-ATPase Activity and/or Expression Plays an Important Role in Regulating the Activity of K Transporters and Channels under Drought Stress
4. Materials and Methods
4.1. Plant Materials, Growth Conditions and Treatments
4.2. Biomass and Ratio of Wilting Leaves
4.3. SPAD, Chlorophyll Fluorescence and Stomatal Conductance
4.4. Relative Water Content (RWC)
4.5. Sap Osmolality
4.6. K+ Content
4.7. Ion flux Measurements
4.8. qRT-PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AHA | Arabidopsis plasma membrane H+-ATPase |
| AKT1 | Arabidopsis K+ transporter 1 |
| BSM | Basic salt medium |
| CBL1 | Calcineurin B-like protein 1 |
| CIPK23 | CBL-interacting protein kinase 23 |
| DW | Dry weight |
| FW | Fresh weight |
| gs | Stomatal conductance |
| HAK | High-affinity K+ transporter |
| ILK1 | Integrin-linked kinase1 |
| KIR | K inward rectifier |
| KUP | K+ uptake transporter |
| LIX | Liquid ion exchanger |
| MIFE | Microelectrode ion flux estimation |
| PEG | Polyethylene glycol |
| PM | Plasma membrane |
| PSII | Photosystem II |
| qRT-PCR | Quantitative reverse transcriptase PCR |
| RWC | Relative water content |
| SKOR | Stelar K+ outward rectifier |
| TW | Turgid weight |
References
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. In Sustainable Agriculture; Springer: Dordrecht, The Netherlands, 2009; pp. 153–188. [Google Scholar]
- Intergovernmental Panel on Climate Change. Climate Change 2014 Synthesis Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2014. [Google Scholar]
- United Nations, Department of Economic and Social Affairs. Population Division. World Population Prospects: The 2017 Revision, Key Findings and Advance Table; United Nations: New York, NY, USA, 2017. [Google Scholar]
- Wani, S.H.; Sah, S.K. Biotechnology and abiotic stress tolerance in rice. J. Rice Res. 2014, 2, e105. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.A. Global synthesis of drought effects on cereal, legume, tuber and root crops production: A review. Agric. Water Manag. 2017, 179, 18–33. [Google Scholar] [CrossRef]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; Devoil, P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- Morgan, J.M.; Hare, R.A.; Fletcher, R.J. Genetic variation in osmoregulation in bread and durum wheats and its relationship to grain yield in a range of field environments. Aust. J. Agric. Res. 1986, 37, 449–457. [Google Scholar] [CrossRef]
- Morgan, J.M. The use of coleoptile responses to water stress to differentiate wheat genotypes for osmoregulation, growth and yield. Ann. Bot. 1988, 62, 193–198. [Google Scholar] [CrossRef]
- Rascio, A.; Cedola, M.C.; Toponi, M.; Flagella, Z.; Wittmer, G. Leaf morphology and water status changes in Triticum durum under water stress. Physiol. Plant. 1990, 78, 462–467. [Google Scholar] [CrossRef]
- Costa, J.M.; Corey, A.; Hayes, P.M.; Jobet, C.; Kleinhofs, A.; Kopisch-Obusch, A.; Kramer, S.F.; Kudrna, D.; Li, M.; Riera-Lizarazu, O.; et al. Molecular mapping of the Oregon Wolfe Barleys: a phenotypically polymorphic doubled-haploid population. Appl. Genet. 2001, 103, 415–424. [Google Scholar] [CrossRef]
- Mascher, M.; Gundlach, H.; Himmelbach, A.; Beier, S.; Twardziok, S.O.; Wicker, T.; Bayer, M. A chromosome conformation capture ordered sequence of the barley genome. Nature 2017, 544, 427–433. [Google Scholar] [CrossRef]
- Nevo, E.; Chen, G. Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ. 2010, 33, 670–685. [Google Scholar] [CrossRef]
- Araus, J.L.; Slafer, G.A.; Reynolds, M.P.; Royo, C. Plant breeding and drought in C3 cereals: what should we breed for? Ann. Bot. 2002, 89, 925–940. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.P.; Forster, B.P.; Robinson, D.; Handley, L.L.; Gordon, D.C.; Russell, J.R.; Powell, W. Wild barley: a source of genes for crop improvement in the 21st century? J. Exp. Bot. 2000, 51, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Wright, G.C.; Siddique, K.H.H. Adaptation of grain legumes (pulses) to water-limited environments. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2001; pp. 193–231. [Google Scholar]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought - from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Xing, W.; Rajashekar, C.B. Alleviation of water stress in beans by exogenous glycine betaine. Plant Sci. 1999, 148, 185–192. [Google Scholar] [CrossRef]
- Agboma, P.C.; Sinclair, T.R.; Jokinen, K.; Peltonen-Sainio, P.; Pehu, E. An evaluation of the effect of exogenous glycinebetaine on the growth and yield of soybean: timing of application, watering regimes and cultivars. Field Crop. Res. 1997, 54, 51–64. [Google Scholar] [CrossRef]
- Agboma, P.C.; Jones, M.G.K.; Peltonen-Sainio, P.; Rita, H.; Pehu, E. Exogenous glycinebetaine enhances grain yield of maize, sorghum and wheat grown under two supplementary watering regimes. J. Agron. Crop Sci. 1997, 178, 29–37. [Google Scholar] [CrossRef]
- Garg, A.K.; Kim, J.K.; Owens, T.G.; Ranwala, A.P.; Do Choi, Y.; Kochian, L.V.; Wu, R.J. Trehalose accumulation in rice plants confers high tolerance levels to different abiotic stresses. Proc. Natl. Acad. Sci. USA 2002, 99, 15898–15903. [Google Scholar] [CrossRef] [PubMed]
- Serraj, R.; Sinclair, T.R. Osmolyte accumulation: can it really help increase crop yield under drought conditions? Plant Cell Environ. 2002, 25, 333–341. [Google Scholar] [CrossRef]
- Shabala, S.; Demidchik, V.; Shabala, L.; Cuin, T.A.; Smith, S.J.; Miller, A.J.; Davies, J.M.; Newman, I.A. Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasma membrane K+-permeable channels. Plant Physiol. 2006, 141, 1653–1665. [Google Scholar] [CrossRef]
- Leigh, R.A.; Wyn Jones, R.G. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984, 97, 1–13. [Google Scholar] [CrossRef]
- Asif, M.; Yilmaz, O.; Ozturk, L. Potassium deficiency impedes elevated carbon dioxide-induced biomass enhancement in well-watered or drought-stressed bread wheat. J. Plant Nutr. Soil Sci. 2017, 180, 474–481. [Google Scholar] [CrossRef]
- Oosterhuis, D.M.; Loka, D.A.; Kawakami, E.M.; Pettigrew, W.T. The physiology of potassium in crop production. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; pp. 203–233. [Google Scholar]
- Sardans, J.; Peñuelas, J. Potassium: a neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef]
- Zain, N.A.M.; Ismail, M.R. Effects of potassium rates and types on growth, leaf gas exchange and biochemical changes in rice (Oryza sativa) planted under cyclic water stress. Agric. Water Manag. 2016, 164, 83–90. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.A.; Abd-Alla, M.H. The role of potassium fertilizer in nodulation and nitrogen fixation of faba bean (Vicia faba L.) plants under drought stress. Biol. Fertil. Soils 1995, 20, 147–150. [Google Scholar] [CrossRef]
- Zahoor, R.; Dong, H.; Abid, M.; Zhao, W.; Wang, Y.; Zhou, Z. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017, 137, 73–83. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Hajirezaei, M.R.; Seiler, C.; Sreenivasulu, N.; von Wirén, N. A potential role of flag leaf potassium in conferring tolerance to drought-induced leaf senescence in barley. Front. Plant Sci. 2016, 7, 206. [Google Scholar] [CrossRef]
- Osakabe, Y.; Arinaga, N.; Umezawa, T.; Katsura, S.; Nagamachi, K.; Tanaka, H.; Ohiraki, H.; Yamada, K.; Seo, S.; Abo, M.; et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell 2013, 25, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, W.; Zeng, F.; Chen, Z.; Zhang, G.; Wu, F. K+ Uptake, H+-ATPase pumping activity and Ca2+ efflux mechanism are involved in drought tolerance of barley. Environ. Exp. Bot. 2016, 129, 57–66. [Google Scholar] [CrossRef]
- Ashraf, M.H.P.J.C.; Harris, P.J.C. Photosynthesis under stressful environments: an overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Kim, E.J.; Kwak, J.M.; Uozumi, N.; Schroeder, J.I. AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell 1998, 10, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ragel, P.; Ródenas, R.; García-Martín, E.; Andrés, Z.; Villalta, I.; Nieves-Cordones, M.; Rivero, R.M.; Martinez, V.; Pardo, J.M.; Quintero, F.J.; et al. The CBL-interacting protein kinase CIPK23 regulates HAK5-mediated high-affinity K+ uptake in Arabidopsis roots. Plant Physiol. 2015, 169, 2863–2873. [Google Scholar] [PubMed]
- Santa-María, G.E.; Rubio, F.; Dubcovsky, J.; Rodríguez-Navarro, A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell 1997, 9, 2281–2289. [Google Scholar] [PubMed]
- Gaymard, F.; Pilot, G.; Lacombe, B.; Bouchez, D.; Bruneau, D.; Boucherez, J.; Michaux-ferriere, N.; Thibaud, J.; Sentenac, H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell 1998, 94, 647–655. [Google Scholar] [CrossRef]
- Hirsch, R.E.; Lewis, B.D.; Spalding, E.P.; Sussman, M.R. A role for the AKT1 potassium channel in plant nutrition. Science 1998, 280, 918–921. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agr. Res. 2011, 6, 2026–2032. [Google Scholar]
- Shabala, S.N.; Lew, R.R. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol. 2002, 129, 290–299. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, M.; Newman, I.A.; Mendham, N.J.; Zhang, G.; Shabala, S. Potassium and sodium relations in salinised barley tissues as a basis of differential salt tolerance. Funct. Plant Biol. 2007, 34, 150–162. [Google Scholar] [CrossRef]
- Römheld, V.; Kirkby, E.A. Research on potassium in agriculture: needs and prospects. Plant Soil 2010, 335, 155–180. [Google Scholar] [CrossRef]
- Chen, Z.; Newman, I.; Zhou, M.; Mendham, N.; Zhang, G.; Shabala, S. Screening plants for salt tolerance by measuring K+ flux: a case study for barley. Plant Cell Environ. 2005, 28, 1230–1246. [Google Scholar] [CrossRef]
- Chen, Z.; Pottosin, I.I.; Cuin, T.A.; Fuglsang, A.T.; Tester, M.; Jha, D.; Zepeda-Jazo, I.; Zhou, M.; Palmgren, M.G.; Newman, I.A.; et al. Root plasma membrane transporters controlling K+/Na+ homeostasis in salt-stressed barley. Plant Physiol. 2007, 145, 1714–1725. [Google Scholar] [CrossRef] [PubMed]
- Cuin, T.A.; Betts, S.A.; Chalmandrier, R.; Shabala, S. A root’s ability to retain K+ correlates with salt tolerance in wheat. J. Exp. Bot. 2008, 59, 2697–2706. [Google Scholar] [CrossRef] [PubMed]
- Cuin, T.A.; Zhou, M.; Parsons, D.; Shabala, S. Genetic behaviour of physiological traits conferring cytosolic K+/Na+ homeostasis in wheat. Plant Biol. 2012, 14, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.B.; Zeng, F.; Shabala, L.; Böhm, J.; Zhang, G.; Zhou, M.; Shabala, S. The ability to regulate voltage-gated K+-permeable channels in the mature root epidermis is essential for waterlogging tolerance in barley. J. Exp. Bot. 2017, 69, 667–680. [Google Scholar] [CrossRef] [PubMed]
- Anschütz, U.; Becker, D.; Shabala, S. Going beyond nutrition: regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef] [PubMed]
- Nieves-Cordones, M.; Caballero, F.; Martínez, V.; Rubio, F. Disruption of the Arabidopsis thaliana inward-rectifier K+ channel AKT1 improves plant responses to water stress. Plant Cell Physiol. 2011, 53, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, H.; Chen, L.; Wang, Y.; Liu, L.; He, L.; Wu, W. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 2006, 125, 1347–1360. [Google Scholar] [CrossRef]
- Li, J.; Long, Y.; Qi, G.; Xu, Z.; Wu, W.; Wang, Y. The Os-AKT1 channel is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell 2014, 26, 3387–3402. [Google Scholar] [CrossRef]
- Li, W.; Xu, G.; Alli, A.; Yu, L. Plant HAK/KUP/KT K+ transporters: function and regulation. Semin. Cell Dev. Biol. 2018, 74, 133–141. [Google Scholar] [CrossRef]
- Brauer, E.K.; Ahsan, N.; Dale, R.; Kato, N.; Coluccio, A.E.; Piñeros, M.A.; Kochian, L.V.; Thelen, J.J.; Popescu, S.C. The Raf-like kinase ILK1 and the high affinity K+ transporter HAK5 are required for innate immunity and abiotic stress response. Plant Physiol. 2016, 171, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, C.; Gao, Z.; Zhang, Y.; Jiang, H.; Zhu, L.; Ren, D.; Yu, L.; Xu, G.; Qian, Q. OsHAK1, a high-affinity potassium transporter, positively regulates responses to drought stress in rice. Front. Plant Sci. 2017, 8, 1885. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.H.; De Boer, A.H. Properties of two outward-rectifying channels in root xylem parenchyma cells suggest a role in K+ homeostasis and long-distance signaling. Plant Physiol. 1997, 115, 1707–1719. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Straltsova, D.; Medvedev, S.S.; Pozhvanov, G.A.; Sokolik, A.; Yurin, V. Stress-induced electrolyte leakage: the role of K+-permeable channels and involvement in programmed cell death and metabolic adjustment. J. Exp. Bot. 2014, 65, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mata, C.; Wang, J.; Gajdanowicz, P.; Gonzalez, W.; Hills, A.; Donald, N.; Riedelsberger, J.; Amtmann, A.; Dreyer, I.; Blatt, M.R. A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. J. Biol. Chem. 2010, 285, 29286–29294. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ma, Q.; Kumar, T.; Duan, H.; Zhang, J.; Yuan, H.; Wang, Q.; Khan, S.A.; Wang, P.; Wang, S. ZxSKOR is important for salinity and drought tolerance of Zygophyllum xanthoxylum by maintaining K+ homeostasis. Plant Growth Regul. 2016, 80, 195–205. [Google Scholar] [CrossRef]
- Shabala, S.; Bose, J.; Fuglsang, A.T.; Pottosin, I. On a quest for stress tolerance genes: membrane transporters in sensing and adapting to hostile soils. J. Exp. Bot. 2015, 67, 1015–1031. [Google Scholar] [CrossRef]
- Elmore, J.M.; Coaker, G. The role of the plasma membrane H+-ATPase in plant - microbe interactions. Mol. Plant. 2011, 4, 416–427. [Google Scholar] [CrossRef]
- Gaxiola, R.A.; Palmgren, M.G.; Schumacher, K. Plant proton pumps. FEBS Lett. 2007, 581, 2204–2214. [Google Scholar] [CrossRef]
- Liu, J.; Elmore, J.M.; Fuglsang, A.T.; Palmgren, M.G.; Staskawicz, B.J.; Coaker, G. RIN4 functions with plasma membrane H+-ATPases to regulate stomatal apertures during pathogen attack. PLoS Biol. 2009, 7, e1000139. [Google Scholar] [CrossRef]
- Haruta, M.; Burch, H.L.; Nelson, R.B.; Barrett-Wilt, G.; Kline, K.G.; Mohsin, S.B.; Young, J.C.; Otegui, M.S.; Sussman, M.R. Molecular characterization of mutant Arabidopsis plants with reduced plasma membrane proton pump activity. J. Biol. Chem. 2010, 285, 17918–17929. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, B.; Zhang, W.; Liu, Y. Effect of osmotic stress on the activity of H+-ATPase and the levels of covalently and noncovalently conjugated polyamines in plasma membrane preparation from wheat seedling roots. Plant Sci. 2005, 168, 1599–1607. [Google Scholar] [CrossRef]
- Fuglsang, A.T.; Paez-Valencia, J.; Gaxiola, R.A. Plant proton pumps: regulatory circuits involving H+-ATPase and H+-PPase. In Transporters and Pumps in Plant Signaling; Springer: Berlin, Germany, 2011; pp. 39–64. [Google Scholar]
- Palmgren, M.G.; Nissen, P. P-type ATPases. Annu. Rev. Biophys. 2011, 40, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Kerkeb, L.; Venema, K.; Donaire, J.P.; Rodríguez-Rosales, M.P. Enhanced H+/ATP coupling ratio of H+-ATPase and increased 14-3-3 protein content in plasma membrane of tomato cells upon osmotic shock. Physiol. Plant. 2002, 116, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yamaji, N.; Yamane, M.; Kashino-Fujii, M.; Sato, K.; Ma, J. The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol. 2016, 172, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- TOMOS, A.D.; LEIGH, R.A.; SHAW, C.A.; JONES, R.G.W. A comparison of methods for measuring turgor pressures and osmotic pressures of cells of red beet storage tissue. J. Exp. Bot. 1984, 35, 1675–1683. [Google Scholar] [CrossRef]
- Zeng, F.; Shabala, L.; Zhou, M.; Zhang, G.; Shabala, S. Barley responses to combined waterlogging and salinity stress: separating effects of oxygen deprivation and elemental toxicity. Front. Plant Sci. 2013, 4, 313. [Google Scholar] [CrossRef]
- Zeng, F.; Konnerup, D.; Shabala, L.; Zhou, M.; Colmer, T.D.; Zhang, G.; Shabala, S. Linking oxygen availability with membrane potential maintenance and K+ retention of barley roots: implications for waterlogging stress tolerance. Plant Cell Environ. 2014, 37, 2325–2338. [Google Scholar] [CrossRef] [PubMed]
- Newman, I.A. Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ. 2001, 24, 1–14. [Google Scholar] [CrossRef]
- Shabala, S. Non-invasive microelectrode ion flux measurements in plant stress physiology. In Plant Electrophysiology; Volkov, A.G., Ed.; Springer: Berlin, Germany, 2006; pp. 35–71. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jarošová, J.; Kundu, J.K. Validation of reference genes as internal control for studying viral infections in cereals by quantitative real-time RT-PCR. BMC Plant Biol. 2010, 10, 146. [Google Scholar] [CrossRef] [PubMed]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, K.; Gao, H.; Wu, X.; Zhang, S.; Han, Z.; Chen, X.; Zhang, G.; Zeng, F. The Ability to Regulate Transmembrane Potassium Transport in Root Is Critical for Drought Tolerance in Barley. Int. J. Mol. Sci. 2019, 20, 4111. https://doi.org/10.3390/ijms20174111
Cai K, Gao H, Wu X, Zhang S, Han Z, Chen X, Zhang G, Zeng F. The Ability to Regulate Transmembrane Potassium Transport in Root Is Critical for Drought Tolerance in Barley. International Journal of Molecular Sciences. 2019; 20(17):4111. https://doi.org/10.3390/ijms20174111
Chicago/Turabian StyleCai, Kangfeng, Huaizhou Gao, Xiaojian Wu, Shuo Zhang, Zhigang Han, Xiaohui Chen, Guoping Zhang, and Fanrong Zeng. 2019. "The Ability to Regulate Transmembrane Potassium Transport in Root Is Critical for Drought Tolerance in Barley" International Journal of Molecular Sciences 20, no. 17: 4111. https://doi.org/10.3390/ijms20174111
APA StyleCai, K., Gao, H., Wu, X., Zhang, S., Han, Z., Chen, X., Zhang, G., & Zeng, F. (2019). The Ability to Regulate Transmembrane Potassium Transport in Root Is Critical for Drought Tolerance in Barley. International Journal of Molecular Sciences, 20(17), 4111. https://doi.org/10.3390/ijms20174111