Embryonic Ontogeny of 5-Hydroxyindoles and 5-Methoxyindoles Synthesis Pathways in the Goose Pineal Organ
Abstract
1. Introduction
2. Results
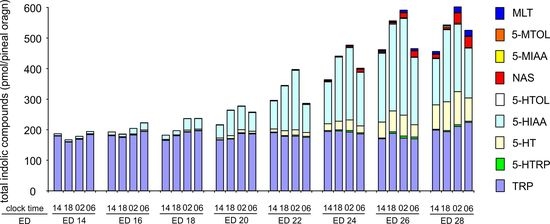
2.1. Content of Tryptophan, 5-Hydroxy- and 5-Methoxyindoles in the Pineal Organ during Embryonic Development
2.2. Diurnal Profiles of 5-Hydroxy- and 5-Methoxyindoles in the Embryonic Pineal Organs on ED 24, ED 26 and ED 28
2.3. Changes in Proportions Between Indoles during Embryonic Development
3. Discussion
4. Materials and Methods
4.1. Egg Incubation
4.2. Tissue Collection
4.3. Analytical Procedure
4.3.1. Chemicals
4.3.2. Sample Preparation
4.3.3. HPLC Assay
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TRP | tryptophan |
| 5-HTRP | 5-hydroxytryptophan |
| 5-HT | serotonin |
| NAS | N-acetylserotonin |
| 5-HIAA | 5-hydroxyindole acetic acid |
| 5-HTOL | 5-hydroxytryptophol |
| 5-MIAA | 5-methoxyindole acetic acid |
| 5-MTOL | 5-methoxytryptophol |
| 5-MTAM | 5-methoxytryptamine |
References
- Zeman, M.; Gwinner, E.; Somogyiová, E. Development of melatonin rhythm in the pineal gland and eyes of chick embryo. Experientia 1992, 48, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Gwinner, E.; Herichová, I.; Lamosová, D.; Kost’ál, L. Perinatal development of circadian melatonin production in domestic chicks. J. Pineal Res. 1999, 26, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Herichová, I.; Zeman, M.; Macková, M.; Griac, P. Rhythms of the pineal N-acetyltransferase mRNA and melatonin concentrations during embryonic and post-embryonic development in chicken. Neurosci. Lett. 2001, 298, 123–126. [Google Scholar] [CrossRef]
- Zeman, M.; Pavlik, P.; Lamosová, D.; Herichová, I.; Gwinner, E. Entrainment of rhythmic melatonin production by light and temperature in the chick embryo. Avian Poult. Biol. Rev. 2004, 15, 197–204. [Google Scholar] [CrossRef]
- Herichová, I.; Monosíková, J.; Zeman, M. Ontogeny of melatonin, Per2 and E4bp4 light responsiveness in the chicken embryonic pineal gland. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.; Herichová, I. Circadian melatonin production develops faster in birds than in mammals. Gen. Comp. Endocrinol. 2011, 172, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Drozdova, A.; Okuliarova, M.; Zeman, M. The effect of different wavelengths of light during incubation on the development of rhythmic pineal melatonin biosynthesis in chick embryos. Animal 2019, 7, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takayama, H.; Nakamura, Y.; Reiter, R.J.; Sugino, N. Fetal/placental regulation of maternal melatonin in rats. J. Pineal Res. 2008, 44, 335–340. [Google Scholar] [CrossRef]
- Voiculescu, S.E.; Zygouropoulos, N.; Zahiu, C.D.; Zagrean, A.M. Role of melatonin in embryo fetal development. J. Med. Life. 2014, 7, 488–492. [Google Scholar]
- Akasaka, K.; Nasu, T.; Katayama, T.; Murakami, N. Development of regulation of melatonin release in pineal cells in chick embryo. Brain Res. 1995, 692, 283–286. [Google Scholar] [CrossRef]
- Lamosová, D.; Zeman, M.; Macková, M.; Gwinner, E. Development of rhythmic melatonin synthesis in cultured pineal glands and pineal cells isolated from chick embryo. Experientia 1995, 51, 970–975. [Google Scholar]
- Macková, M.; Lamosová, D.; Zeman, M. Regulation of rhythmic melatonin production in pineal cells of chick embryo by cyclic AMP. Cell. Mol. Life Sci. 1998, 54, 461–466. [Google Scholar] [CrossRef]
- Faluhelyi, N.; Reglodi, D.; Csernus, V. Development of the circadian melatonin rhythm and its responsiveness to PACAP in the embryonic chicken pineal gland. Ann. N. Y. Acad. Sci. 2005, 1040, 305–309. [Google Scholar] [CrossRef]
- Csernus, V.J.; Nagy, A.D.; Faluhelyi, N. Development of the rhythmic melatonin secretion in the embryonic chicken pineal gland. Gen. Comp. Endocrinol. 2007, 152, 148–153. [Google Scholar] [CrossRef]
- Faluhelyi, N.; Csernus, V. The effects of environmental illumination on the in vitro melatonin secretion from the embryonic and adult chicken pineal gland. Gen. Comp. Endocrinol. 2007, 152, 154–158. [Google Scholar] [CrossRef]
- Faluhelyi, N.; Matkovits, A.; Párniczky, A.; Csernus, V. The in vitro and in ovo effects of environmental illumination and temperature on the melatonin secretion from the embryonic chicken pineal gland. Ann. N. Y. Acad. Sci. 2009, 1163, 383–385. [Google Scholar] [CrossRef]
- Kommedal, S.; Csernus, V.; Nagy, A.D. The embryonic pineal gland of the chicken as a model for experimental jet lag. Gen. Comp. Endocrinol. 2013, 188, 226–231. [Google Scholar] [CrossRef]
- Faluhelyi, N.; Reglodi, D.; Lengvári, I.; Csernus, V. Development of the circadian melatonin rhythm and the effect of PACAP on melatonin release in the embryonic chicken pineal gland. An in vitro study. Regul. Pept. 2004, 123, 23–28. [Google Scholar] [CrossRef]
- Faluhelyi, N.; Reglodi, D.; Csernus, V. The effects of PACAP and VIP on the in vitro melatonin secretion from the embryonic chicken pineal gland. Ann. N. Y. Acad. Sci. 2006, 1070, 271–275. [Google Scholar] [CrossRef]
- Wainwright, S.D. Course of the increase in hydroxyindole-O-methyltransferase activity in the pineal gland of the chick embryo and young chick. J. Neurochem. 1974, 22, 193–196. [Google Scholar]
- Binkley, S.; Geller, E.B. Pineal Enzymes in Chickens: Development of Daily Rhythmicity. Gen. Comp. Endocrinol. 1975, 27, 424–429. [Google Scholar] [CrossRef]
- Fraser, I.H.; Wainwright, S.D. The influence of lighting conditions upon the level and course of increase in specific activity of serotonin N-acetyltransferase in the developing chick pineal gland. Can. J. Biochem. 1976, 54, 103–109. [Google Scholar] [CrossRef]
- Zeman, M.; Illnerová, H. Ontogeny of N-acetyltransferase activity rhythm in pineal gland of chick embryo. Comp. Biochem. Physiol. A Physiol. 1990, 97, 175–178. [Google Scholar] [CrossRef]
- Bernard, M.; Voisin, P.; Guerlotté, J.; Collin, J.P. Molecular and cellular aspects of hydroxyindole-O-methyltransferase expression in the developing chick pineal gland. Brain Res. Dev. Brain Res. 1991, 59, 75–81. [Google Scholar] [CrossRef]
- Grechez-Cassiau, A.; Grève, P.; Guerlotté, J.; Collin, J.P.; Voisin, P. Hydroxyindole-O-methyltransferase gene expression in the pineal gland of chicken embryo: Development of messenger RNA levels and regulation by serum. Brain Res. Dev. Brain Res. 1995, 88, 204–211. [Google Scholar] [CrossRef]
- Obłap, R.; Olszańska, B. Presence and developmental regulation of serotonin N-acetyltransferase transcripts in oocytes and early quail embryos (Coturnix coturnix japonica). Mol. Reprod. Dev. 2003, 65, 132–140. [Google Scholar] [CrossRef]
- Obłap, R.; Olszańska, B. Transition from embryonic to adult transcription pattern of serotonin N-acetyltransferase gene in avian pineal gland. Mol. Reprod. Dev. 2004, 67, 145–153. [Google Scholar] [CrossRef]
- Olszańska, B.; Majewski, P.; Lewczuk, B.; Stepińska, U. Melatonin and its synthesizing enzymes (arylalkylamine N-acetyltransferase-like and hydroxyindole-O-methyltransferase) in avian eggs and early embryos. J. Pineal Res. 2007, 42, 310–318. [Google Scholar]
- Möller, W.; Möller, G. Structural and functional differentiation of the embryonic chick pineal organ in vivo and in vitro. A scanning electron-microscopic and radioimmunoassay study. Cell Tissue Res. 1990, 260, 337–348. [Google Scholar] [CrossRef]
- Haldar, C.; Araki, M. Morphometric analysis of photoreceptive, neuronal and endocrinal cell differentiation of avian pineal cells: An in vitro immunohistochemical study on the developmental transition from neuronal to photo-endocrinal property. Zool. Sci. 2002, 19, 781–787. [Google Scholar] [CrossRef]
- Gwinner, E.; Zeman, M.; Klaassen, M. Synchronization by low-amplitude light-dark cycles of 24-hour pineal and plasma melatonin rhythms of hatchling European starlings (Sturnus vulgaris). J. Pineal Res. 1997, 23, 176–181. [Google Scholar] [CrossRef]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Nowicki, M. Post-hatching development of the turkey pineal organ: Histological and immunohistochemical studies. Neuro Endocrinol. Lett. 2005, 26, 383–392. [Google Scholar]
- Prusik, M.; Lewczuk, B.; Nowicki, M.; Przybylska-Gornowicz, B. Histology and ultrastructure of the pineal gland of the domestic goose. Histol. Histopathol. 2006, 21, 1075–1090. [Google Scholar]
- Prusik, M.; Lewczuk, B. Regulation of melatonin secretion in the avian pineal gland. Med. Weter 2008, 64, 617–736. (In Polish) [Google Scholar]
- Prusik, M.; Lewczuk, B. Structure of the avian pineal gland. Med. Weter 2008, 64, 734–848. (In Polish) [Google Scholar]
- Piesiewicz, A.; Podobas, E.; Kędzierska, U.; Joachimiak, E.; Markowska, M.; Majewski, P.; Skwarlo-Sonta, K. Season-related differences in the biosynthetic activity of the neonatal chicken pineal gland. Open Ornithol. J. 2010, 3, 134–140. [Google Scholar] [CrossRef]
- Lewczuk, B.; Ziółkowska, N.; Prusik, M.; Przybylska-Gornowicz, B. Diurnal profiles of melatonin synthesis-related indoles, catecholamines and their metabolites in the duck pineal organ. Int. J. Mol. Sci. 2014, 15, 12604–12630. [Google Scholar] [CrossRef]
- Prusik, M.; Lewczuk, B.; Ziółkowska, N.; Przybylska-Gornowicz, B. Regulation of melatonin secretion in the pineal organ of the domestic duck—An in vitro study. Pol. J. Vet. Sci. 2015, 18, 635–644. [Google Scholar] [CrossRef]
- Adamska, I.; Lewczuk, B.; Markowska, M.; Majewski, P.M. Daily profiles of melatonin synthesis-related indoles in the pineal glands of young chickens (Gallus gallus domesticus L.). J. Photochem. Photobiol. B. Biol. 2016, 164, 335–343. [Google Scholar] [CrossRef]
- Ziółkowska, N.; Lewczuk, B.; Prusik, M. Diurnal and circadian variations in indole contents in the goose pineal gland. Chronobiol. Int. 2018, 25, 1–16. [Google Scholar] [CrossRef]
- Li, S.; Bai, S.; Qin, X.; Zhang, J.; Irwin, D.M.; Zhang, S.; Wang, Z. Comparison of whole embryonic development in the duck (Anas platyrhynchos) and goose (Anser cygnoides) with the chicken (Gallus gallus). Poult. Sci. 2019, 98, 3278–3291. [Google Scholar] [CrossRef]
- Florez, J.C.; Takahashi, J.S. Regulation of tryptophan hydroxylase by cyclic AMP, calcium, norepinephrine, and light in cultured chick pineal cells. J. Neurochem. 1996, 67, 242–250. [Google Scholar] [CrossRef]
- Florez, J.C.; Takahashi, J.S. Quantitative two-dimensional gel electrophoretic analysis of clock-controlled proteins in cultured chick pineal cells: Circadian regulation of tryptophan hydroxylase. J. Biol. Rhythms 1996, 11, 241–257. [Google Scholar] [CrossRef]
- Green, C.B.; Besharse, J.C.; Zatz, M. Tryptophan hydroxylase mRNA levels are regulated by the circadian clock, temperature, and cAMP in chick pineal cells. Brain Res. 1996, 738, 1–7. [Google Scholar] [CrossRef]
- Florez, J.C.; Seidenman, K.J.; Barrett, R.K.; Sangoram, A.M.; Takahashi, J.S. Molecular cloning of chick pineal tryptophan hydroxylase and circadian oscillation of its mRNA levels. Brain Res. Mol. Brain Res. 1996, 42, 25–30. [Google Scholar] [CrossRef]
- Liu, T.; Borjigin, J. N-acetyltransferase is not the rate-limiting enzyme of melatonin synthesis at night. J. Pineal Res. 2005, 39, 91–96. [Google Scholar] [CrossRef]
- Chattoraj, A.; Liu, T.; Zhang, L.S.; Huang, Z.; Borjigin, J. Melatonin formation in mammals: In vivo perspectives. Rev. Endocr. Metab. Disord. 2009, 10, 237–243. [Google Scholar] [CrossRef]
- Okabayashi, N.; Yasuo, S.; Watanabe, M.; Namikawa, T.; Ebihara, S.; Yoshimura, T. Ontogeny of circadian clock gene expression in the pineal and the suprachiasmatic nucleus of chick embryo. Brain Res. 2003, 990, 231–234. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Rosiak, J.; Vivien-Roels, B.; Skene, D.J.; Pevet, P.; Nowak, J.Z. Daily variation in the concentration of 5-methoxytryptophol and melatonin in the duck pineal gland and plasma. J. Pineal Res. 2002, 32, 214–218. [Google Scholar] [CrossRef]
- Simonneaux, V.; Ribelayga, C. Generation of the melatonin endocrine message in mammals: A review of the complex regulations of melatonin synthesis by norepinephrine, peptides and other pineal transmitters. Pharmacol. Rev. 2003, 55, 325–395. [Google Scholar] [CrossRef]






| Days of Incubation | Temperature (°C) | Relative Humidity (%) | Ventilation (%) |
|---|---|---|---|
| 1–16 | 37.8 | 55 | 40 |
| 17–26 | 37.4 | 60 | 60 |
| 27 | 37.0 | 60 | 60 |
| 28 | 37.0 | 65 | 60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanuszewska, M.; Prusik, M.; Lewczuk, B. Embryonic Ontogeny of 5-Hydroxyindoles and 5-Methoxyindoles Synthesis Pathways in the Goose Pineal Organ. Int. J. Mol. Sci. 2019, 20, 3948. https://doi.org/10.3390/ijms20163948
Hanuszewska M, Prusik M, Lewczuk B. Embryonic Ontogeny of 5-Hydroxyindoles and 5-Methoxyindoles Synthesis Pathways in the Goose Pineal Organ. International Journal of Molecular Sciences. 2019; 20(16):3948. https://doi.org/10.3390/ijms20163948
Chicago/Turabian StyleHanuszewska, Maria, Magdalena Prusik, and Bogdan Lewczuk. 2019. "Embryonic Ontogeny of 5-Hydroxyindoles and 5-Methoxyindoles Synthesis Pathways in the Goose Pineal Organ" International Journal of Molecular Sciences 20, no. 16: 3948. https://doi.org/10.3390/ijms20163948
APA StyleHanuszewska, M., Prusik, M., & Lewczuk, B. (2019). Embryonic Ontogeny of 5-Hydroxyindoles and 5-Methoxyindoles Synthesis Pathways in the Goose Pineal Organ. International Journal of Molecular Sciences, 20(16), 3948. https://doi.org/10.3390/ijms20163948