Melatonin Improves the Fertilization Capacity of Sex-Sorted Bull Sperm by Inhibiting Apoptosis and Increasing Fertilization Capacitation via MT1
Abstract
:1. Introduction
2. Results
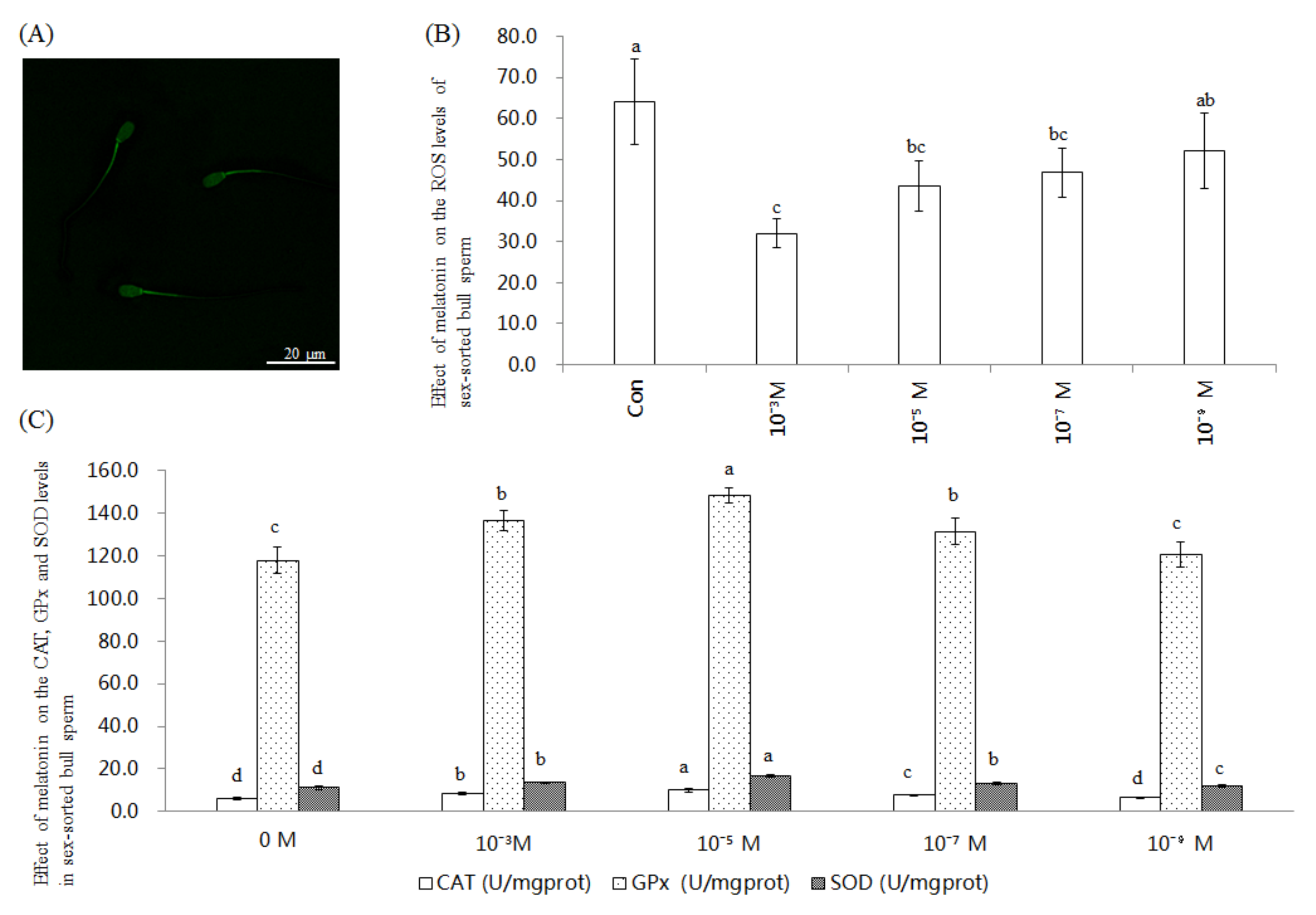
2.1. Effect of Melatonin on ROS Level and CAT, GPx, and SOD Activities in Sex-Sorted Bull Sperm
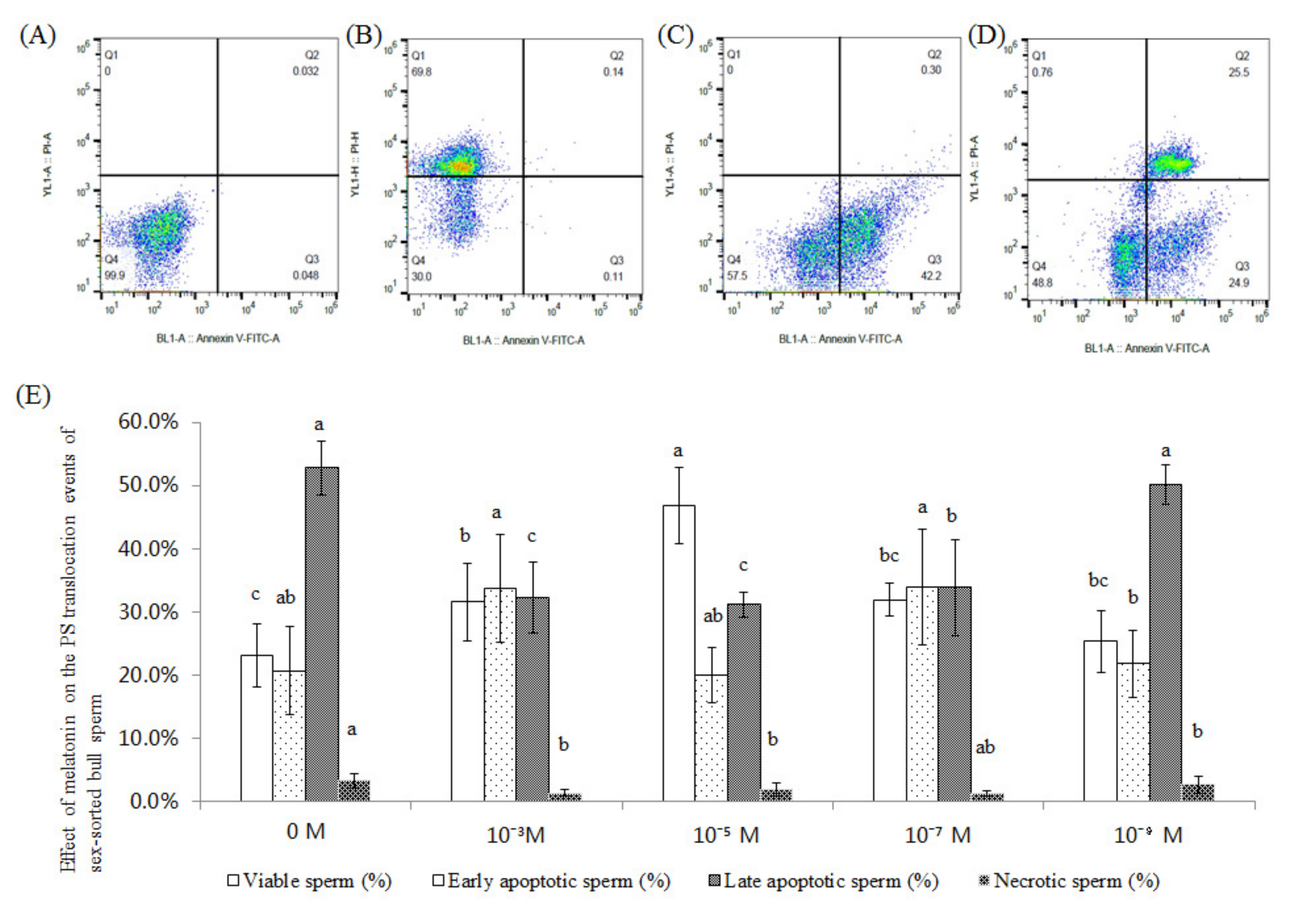
2.2. Effect of Melatonin on the PS Translocation Events of Sex-Sorted Bull Sperm
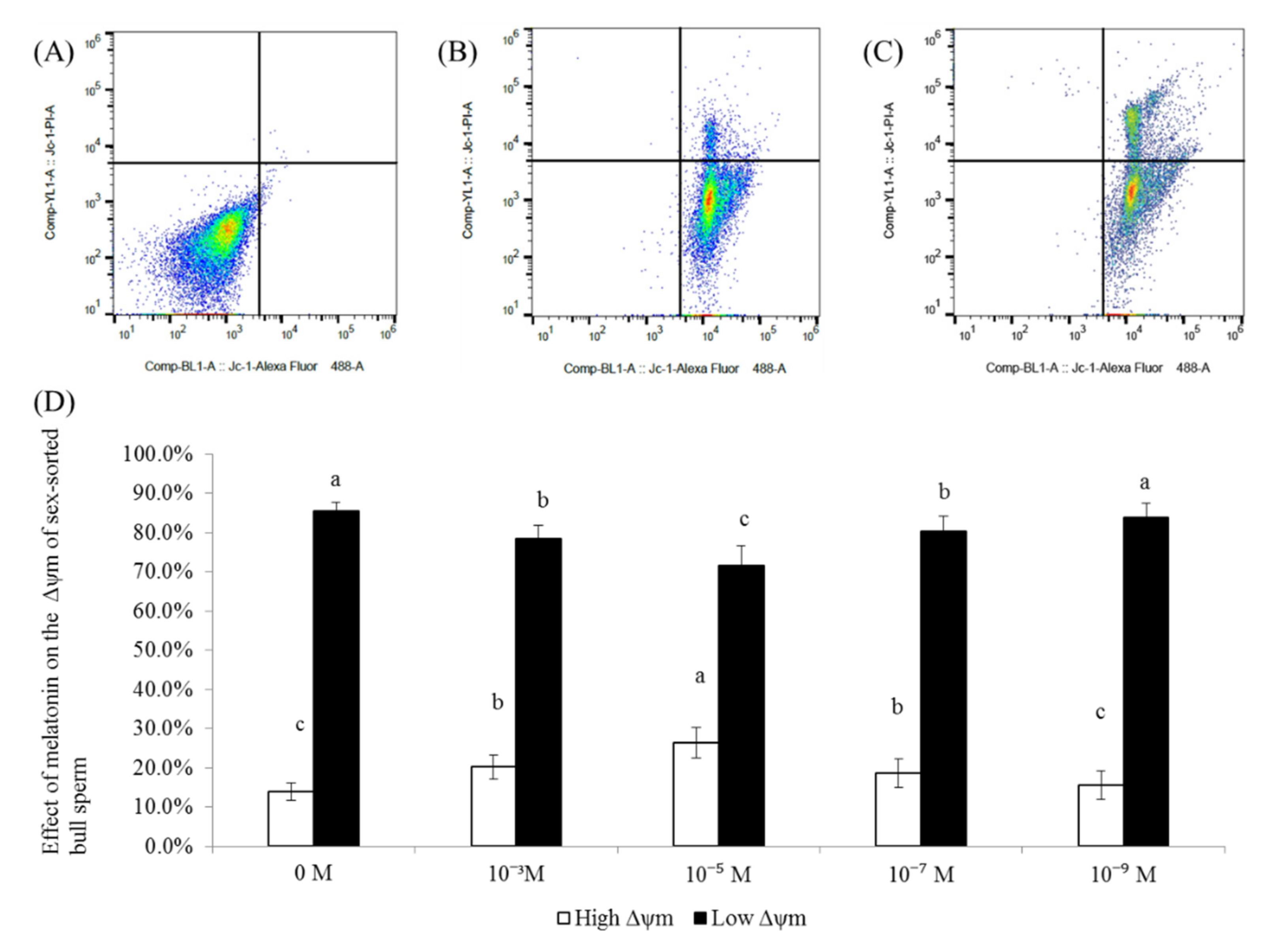
2.3. Effect of Melatonin on the Δψm Levels of Sex-Sorted Bull Sperm
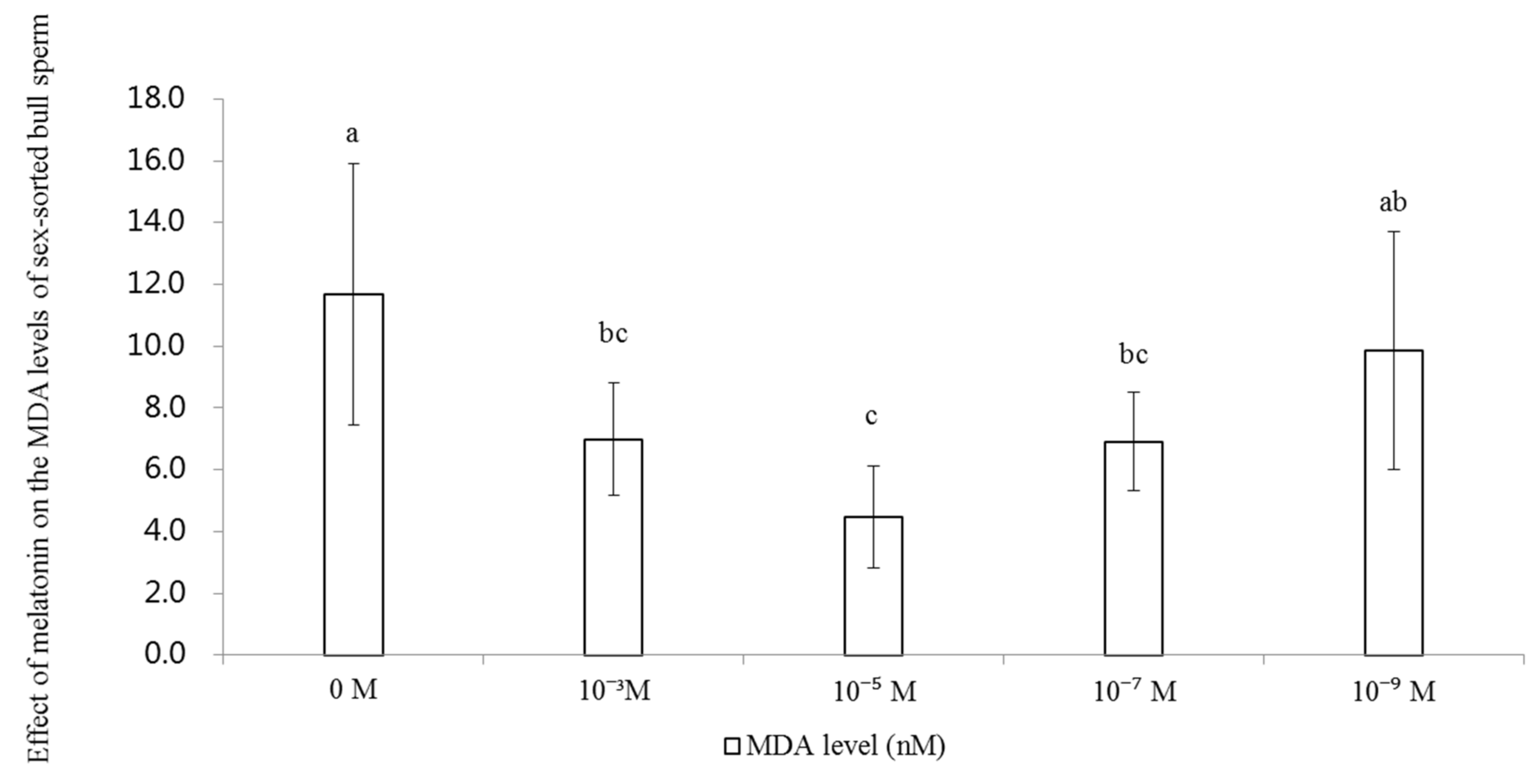
2.4. Effect of Melatonin on the Levels of MDA in Sex-Sorted Bull Sperm
2.5. Effect of Melatonin on the Acrosome Integrity of Sex-Sorted Bull Sperm
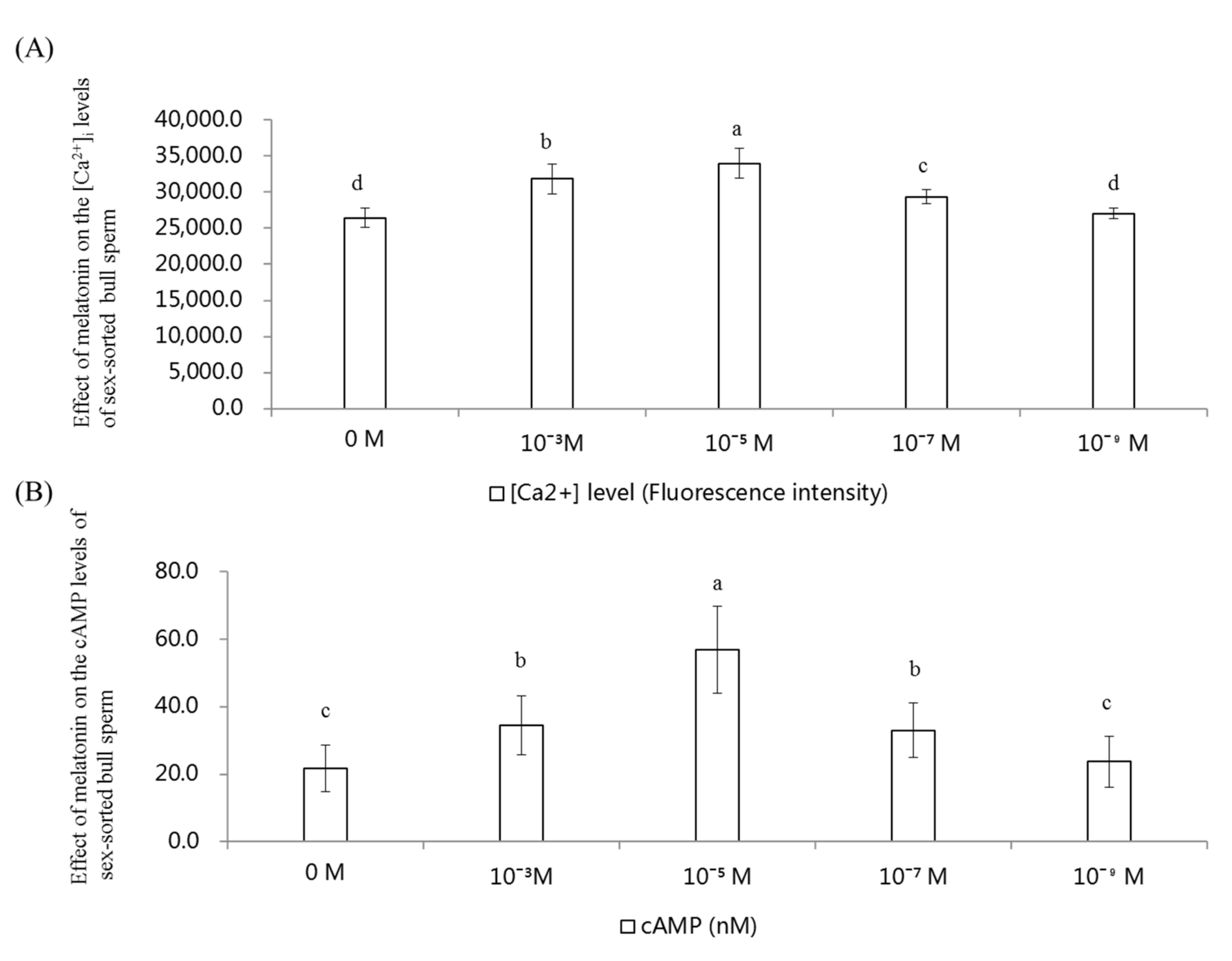
2.6. Effect of Melatonin on the [Ca2+]i and cAMP Levels in Sex-Sorted Bull Sperm
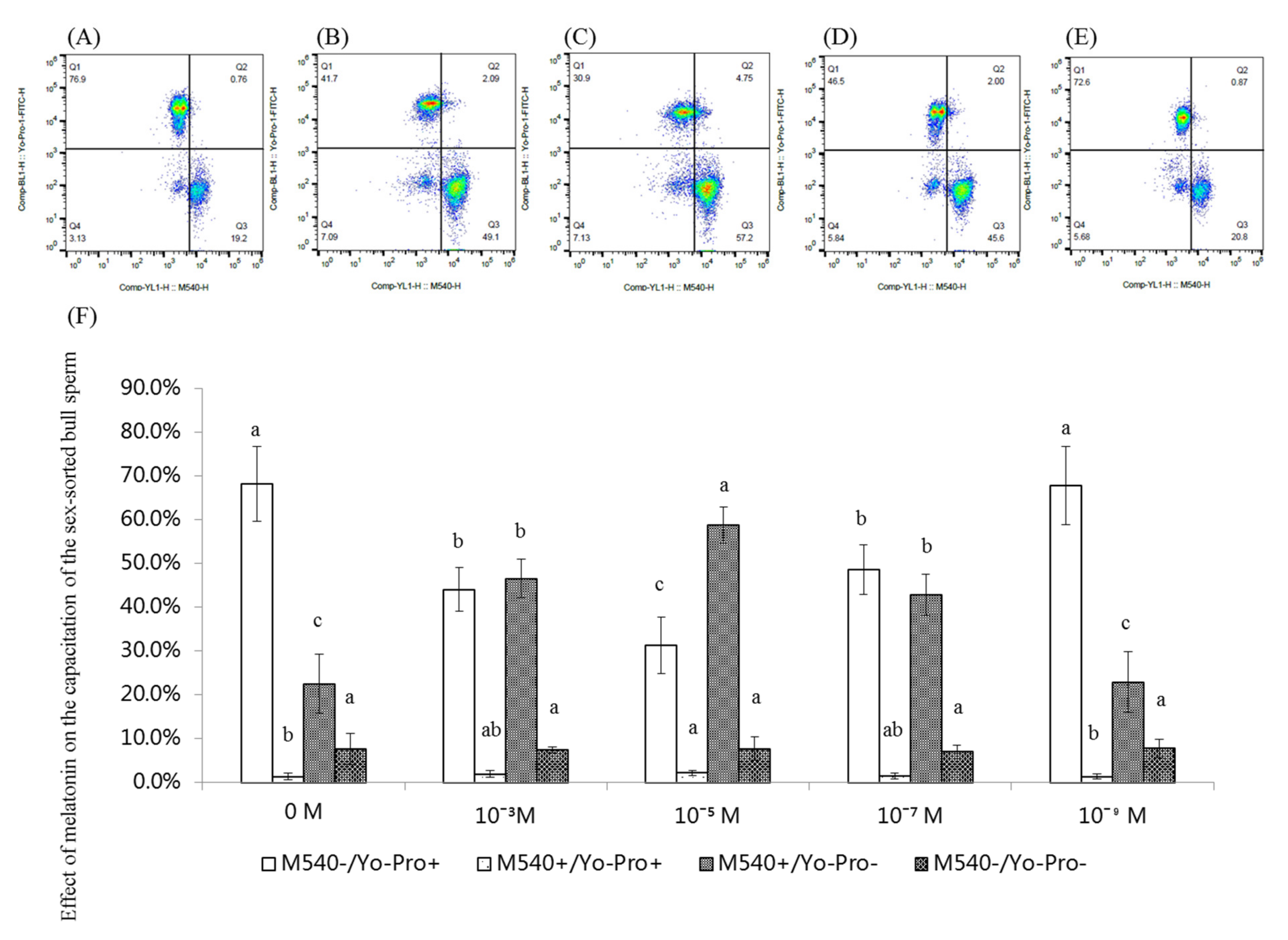
2.7. Effect of Melatonin on the Capacitation of Sex-Sorted Bull Sperm
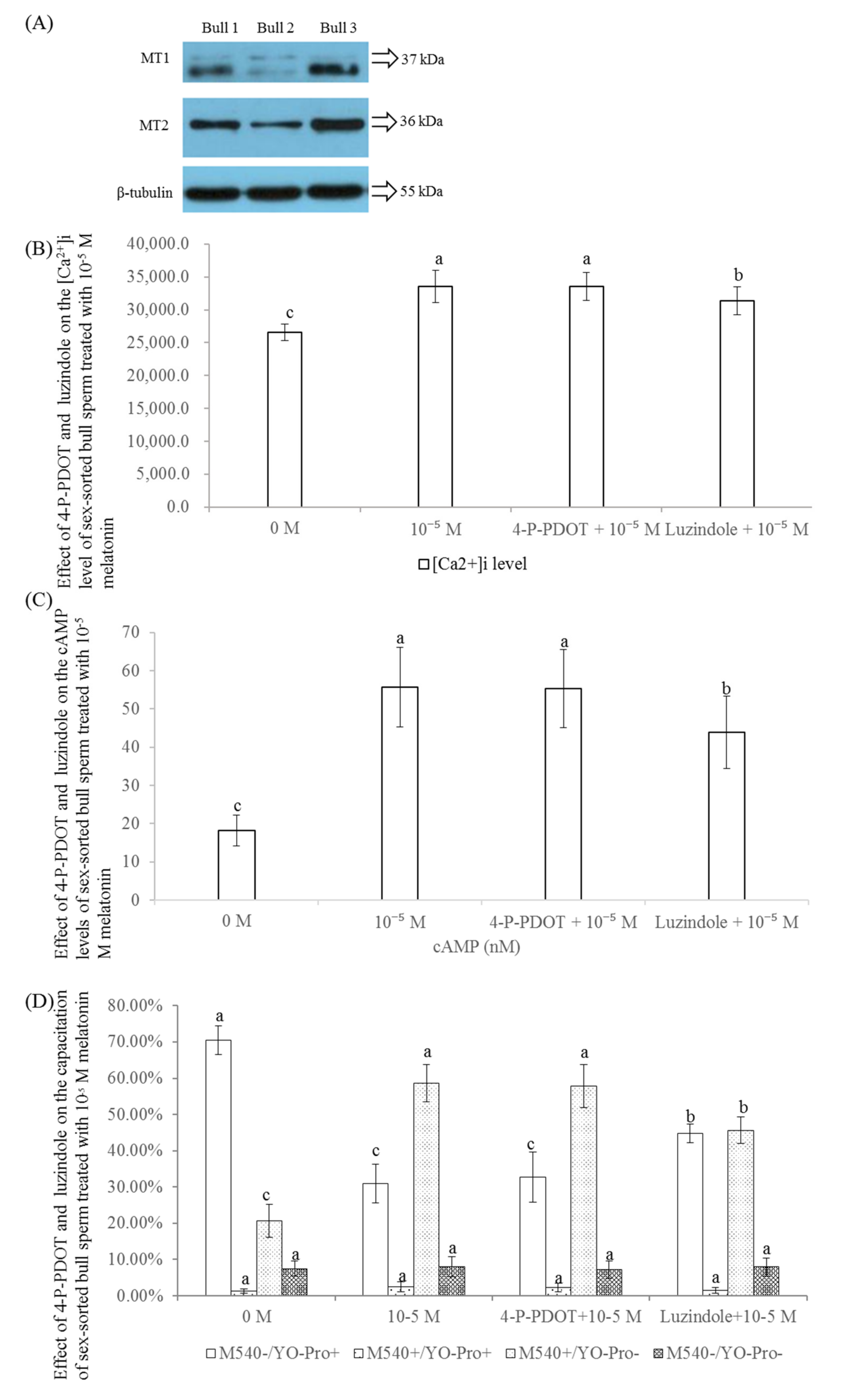
2.8. Effect of 4-P-POD and Luzindole on the Levels of [Ca2+]i, cAMP, and Capacitation of Sex-Sorted Bull Sperm
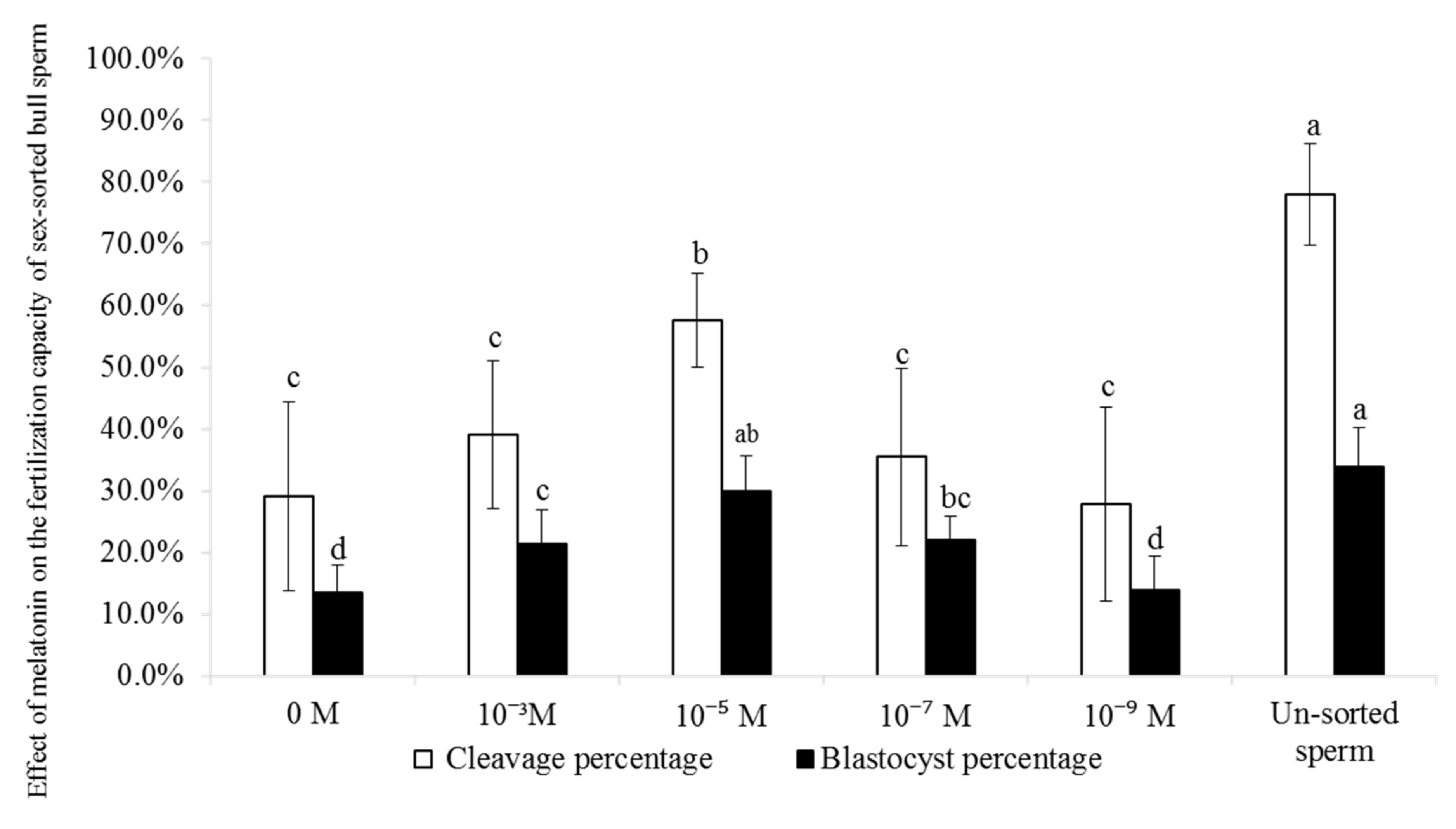
2.9. Effect of Melatonin on IVF Efficiency in Sex-Sorted Bull Sperm
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. The Treatment of Sex-Sorted Semen
4.3. Analysis of the Level of ROS and Endogenous Antioxidant Activity
4.4. Analysis of PS Translocation Events and Level of Δψm
4.5. Analysis of the Level of MDA and Acrosome Integrity of the Sperm
4.6. Analysis of the Level of [Ca2+]i, Intracellular cAMP, and Capacitation of Sperm
4.7. Western Blot Examination of Melatonin Receptors MT1 and MT2
4.8. IVF Assay
4.9. Experimental Design
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Barceló-Fimbres, M.; Campos-Chillón, L.F.; Seidel, G.E., Jr. In vitro fertilization using non-sexed and sexed bovine sperm: Sperm concentration, sorter pressure, and bull effects. Reprod. Domest. Anim. 2011, 46, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Celi, P.; Bathgate, R.; Evans, G.; Maxwell, W.M.; Marti, J.I. Flow-sorted ram spermatozoa are highly susceptible to hydrogen peroxide damage but are protected by seminal plasma and catalase. Reprod. Fertil. Dev. 2010, 22, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Balao da Silva, C.M.; Ortega-Ferrusola, C.; Morrell, J.M.; Rodriguez Martínez, H.; Peña, F.J. Flow Cytometric chromosomal sex sorting of stallion spermatozoa induces oxidative stress on mitochondria and genomic DNA. Reprod. Domest. Anim. 2016, 51, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhao, Y.H.; Hao, H.S.; Wang, H.Y.; Huang, J.M.; Yan, C.L.; Du, W.H.; Pang, Y.W.; Zhang, P.P.; Liu, Y.; et al. Resveratrol significantly improves the fertilisation capacity of bovine sex-sorted semen by inhibiting apoptosis and lipid peroxidation. Sci. Rep. 2018, 8, 7603. [Google Scholar] [CrossRef] [PubMed]
- Plaza Davila, M.; Martin Munoz, P.; Tapia, J.A.; Ortega Ferrusola, C.; Balao da Silva, C.C.; Peña, F.J. Inhibition of mitochondrial complex i leads to decreased motility and membrane integrity related to increased hydrogen peroxide and reduced ATP production, while the inhibition of glycolysis has less impact on sperm motility. PLoS ONE 2015, 10, e0138777. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Ballinger, S.W.; Darley-Usmar, V.M.; Landar, A. Free radicals, mitochondria, and oxidized lipids: The emerging role in signal transduction in vascular cells. Circ. Res. 2006, 99, 924–932. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Peroxiredoxins: Hidden players in the antioxidant defence of human spermatozoa. Basic Clin. Androl. 2014, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Marshburn, P.B.; Giddings, A.; Causby, S.; Matthews, M.L.; Usadi, R.S.; Steuerwald, N.; Hurst, B.S. Influence of ejaculatory abstinence on seminal total antioxidant capacity and sperm membrane lipid peroxidation. Fertil. Steril. 2014, 102, 705–710. [Google Scholar] [CrossRef]
- Partyka, A.; Lukaszewicz, E.; Nizanski, W.; Twardoń, J. Detection of lipid peroxidation in frozen-thawed avian spermatozoa using C (11)-BODIPY (581/591). Theriogenology 2011, 75, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, G.; Venkataraman, P.; Arunkumar, A.; Vignesh, R.C.; Aruldhas, M.M.; Arunakaran, J. Ameliorative effect of vitamins (alpha-tocopherol and ascorbic acid) on PCB (Aroclor 1254) induced oxidative stress in rat epididymal sperm. Reprod. Toxicol. 2007, 23, 239–245. [Google Scholar] [CrossRef]
- Stepniak, J.; Karbownik-Lewinska, M. 17 beta-estradiol prevents experimentally-induced oxidative damage to membrane lipids and nuclear DNA in porcine ovary. Syst. Biol. Reprod. Med. 2016, 62, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Mayo, J.C.; Sainz, R.M.; Leon, J.; Czarnocki, Z. Melatonin as an antioxidant: Biochemical mechanisms and pathophysiological implications in humans. Acta Biochim. Pol. 2003, 50, 1129–1146. [Google Scholar] [PubMed]
- Xia, Y.; Chen, S.; Zeng, S.; Zhao, Y.; Zhu, C.; Deng, B.; Zhu, G.; Yin, Y.; Wang, W.; Hardeland, R.; et al. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 2019, 66, e12547. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, S.; Lu, C.; Yang, W.; Yang, Z.; Hu, W.; Xin, Z.; Yang, Y. Melatonin: Another avenue for treating osteoporosis? J. Pineal Res. 2019, 66, e12548. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolín, I.; Herrera, F.; Martín, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dai, X.; Lu, Y.; Miao, Y.; Zhou, C.; Cui, Z.; Liu, H.; Xiong, B. Melatonin protects oocyte quality from Bisphenol A-induced deterioration in the mouse. J. Pineal Res. 2017, 62, e12396. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Rosales-Corral, S.; Tan, D.X.; Jou, M.J.; Galano, A.; Xu, B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. 2017, 74, 3863–3881. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Ateşşahin, A.; Sahna, E.; Türk, G.; Ceribaşi, A.O.; Yilmaz, S.; Yüce, A.; Bulmuş, O. Chemoprotective effect of melatonin against cisplatin- induced testicular toxicity in rats. J. Pineal Res. 2006, 41, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.M.; Macias-Garcia, B.; Miro-Moran, A.; González-Fernández, L.; Morillo-Rodriguez, A.; Ortega-Ferrusola, C.; Gallardo-Bolaños, J.M.; Stilwell, G.; Tapia, J.A.; Peña, F.J. Melatonin reduces lipid peroxidation and apoptotic-like changes in stallion spermatozoa. J. Pineal Res. 2011, 51, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Casao, A.; Mendoza, N.; Perez-Pe, R.; Grasa, P.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muino-Blanco, T. Melatonin prevents capacitation and apoptotic-like changes of ram spermatozoa and increases fertility rate. J. Pineal Res. 2010, 48, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Succu, S.; Berlinguer, F.; Pasciu, V.; Satta, V.; Leoni, G.G.; Naitana, S. Melatonin protects ram spermatozoa from cryopreservation injuries in a dose-dependent manner. J. Pineal Res. 2011, 50, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, I.; Kohram, H.; Ardabili, F.F. Antioxidative effects of melatonin on kinetics, microscopic and oxidative parameters of cryopreserved bull spermatozoa. Anim. Reprod. Sci. 2013, 139, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Espino, J.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. High endogenous melatonin concentrations enhance sperm quality and short-term in vitro exposure to melatonin improves aspects of sperm motility. J. Pineal Res. 2011, 50, 132–139. [Google Scholar] [CrossRef]
- Deng, S.L.; Sun, T.C.; Yu, K.; Wang, Z.P.; Zhang, B.L.; Zhang, Y.; Wang, X.X.; Lian, Z.X.; Liu, Y.X. Melatonin reduces oxidative damage and upregulates heat shock protein 90 expression in cryopreserved human semen. Free Radic. Biol. Med. 2017, 113, 347–354. [Google Scholar] [CrossRef]
- Zhao, X.M.; Wang, N.; Hao, H.S.; Li, C.Y.; Zhao, Y.H.; Yan, C.L.; Wang, H.Y.; Du, W.H.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2018, 64, e12445. [Google Scholar] [CrossRef]
- Wang, F.; Tian, X.; Zhang, L.; Tan, D.; Reiter, R.J.; Liu, G. Melatonin promotes the in vitro development of pronuclear embryos and increases the efficiency of blastocyst implantation in murine. J. Pineal Res. 2013, 55, 267–274. [Google Scholar] [CrossRef]
- Brazão, V.; Santello, F.H.; Colato, R.P.; Mazotti, T.T.; Tazinafo, L.F.; Toldo, M.P.A.; Do Vale, G.T.; Tirapelli, C.R.; Do Prado, J.C., Jr. Melatonin: Antioxidant and modulatory properties in age-related changes during Trypanosoma cruzi infection. J. Pineal Res. 2017, 63, e12409. [Google Scholar] [CrossRef]
- Yang, Y.; Duan, W.; Jin, Z.; Yi, W.; Yan, J.; Zhang, S.; Wang, N.; Liang, Z.; Li, Y.; Chen, W.; et al. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J. Pineal Res. 2013, 55, 275–286. [Google Scholar] [CrossRef]
- Bejarano, I.; Monllor, F.; Marchena, A.M.; Ortiz, A.; Lozano, G.; Jiménez, M.I.; Gaspar, P.; García, J.F.; Pariente, J.A.; Rodríguez, A.B.; et al. Exogenous melatonin supplementation prevents oxidative stress-evoked DNA damage in human spermatozoa. J. Pineal Res. 2014, 57, 333–339. [Google Scholar] [CrossRef]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef]
- Wang, X.; Sharma, R.K.; Sikka, S.C.; Thomas, A.J., Jr.; Falcone, T.; Agarwal, A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil. Steril. 2003, 80, 531–535. [Google Scholar] [CrossRef]
- Treulen, F.; Uribe, P.; Boguen, R.; Villegas, J.V. Mitochondrial permeability transition increases reactive oxygen species production and induces DNA fragmentation in human spermatozoa. Hum. Reprod. 2015, 30, 767–776. [Google Scholar] [CrossRef] [Green Version]
- Pérez-González, A.; Castañeda-Arriaga, R.; Álvarez-Idaboy, J.R.; Reiter, R.J.; Galano, A. Melatonin and its metabolites as chemical agents capable of directly repairing oxidized DNA. J. Pineal Res. 2019, 66, e12539. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, J.; Lv, D.; Zhu, T.; Wang, F.; Tian, X.; Yao, Y.; Ji, P.; Liu, G. Melatonin regulates the activities of ovary and delays the fertility decline in female animals via MT1/AMPK pathway. J. Pineal Res. 2019, 66, e12550. [Google Scholar] [CrossRef]
- Karimfar, M.H.; Niazvand, F.; Haghani, K.; Ghafourian, S.; Shirazi, R.; Bakhtiyari, S. The protective effects of melatonin against cryopreservation-induced oxidative stress in human sperm. Int. J. Immunopathol. Pharmacol. 2015, 28, 69–76. [Google Scholar] [CrossRef]
- Berlinguer, F.; Pasciu, V.; Succu, S.; Cossu, I.; Caggiu, S.; Addis, D.; Castagna, A.; Fontani, V.; Rinaldi, S.; Passino, E.S. REAC technology as optimizer of stallion spermatozoa liquid storage. Reprod. Biol. Endocrinol. 2017, 15, 11. [Google Scholar] [CrossRef]
- Cheuquemán, C.; Arias, M.E.; Risopatrón, J.; Felmer, R.; Álvarez, J.; Mogas, T.; Sánchez, R. Supplementation of IVF medium with melatonin: Effect on sperm functionality and in vitroproduced bovine embryos. Andrologia 2015, 47, 604–615. [Google Scholar] [CrossRef]
- Martín-Hidalgo, D.; Barón, F.J.; Bragado, M.J.; Carmona, P.; Robina, A.; García-Marín, L.J.; Gil, M.C. The effect of melatonin on the quality of extended boar semen after long-term storage at 17 °C. Theriogenology 2011, 75, 1550–1560. [Google Scholar] [CrossRef]
- Li, R.; Luo, X.; Li, L.; Peng, Q.; Yang, Y.; Zhao, L.; Ma, M.; Hou, Z. The protective effects of melatonin against oxidative stress and inflammation induced by acute cadmium exposure in mice testis. Biol. Trace Elem. Res. 2016, 170, 152–164. [Google Scholar] [CrossRef]
- Esposito, G.; Jaiswal, B.S.; Xie, F.; Krajnc-Franken, M.A.; Robben, T.J.; Strik, A.M.; Kuil, C.; Philipsen, R.L.; Van Duin, M.; Conti, M.; et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc. Natl. Acad. Sci. USA 2004, 101, 2993–2998. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.W.; Gong, Y.; McDonough, C.W.; Langaee, T.Y.; Nasiri Kenari, N.; Beitelshees, A.L.; Gums, J.G.; Chapman, A.B.; Turner, S.T.; Johnson, J.A.; et al. Melatonin pathway and atenolol-related glucose dysregulation: Is there a correlation? Clin. Transl. Sci. 2016, 9, 114–122. [Google Scholar] [CrossRef]
- Chang, M.C. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature 1951, 168, 697–698. [Google Scholar] [CrossRef]
- Lishko, P.V.; Botchkina, I.L.; Kirichok, Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature 2011, 471, 387–391. [Google Scholar] [CrossRef]
- Escoffier, J.; Navarrete, F.; Haddad, D.; Santi, C.M.; Darszon, A.; Visconti, P.E. Flow cytometry analysis reveals that only a subpopulation of mouse sperm undergoes hyperpolarization during capacitation. Biol. Reprod. 2015, 92, 121. [Google Scholar] [CrossRef]
- Harrison, R.A. Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol. Reprod. Dev. 2004, 67, 337–352. [Google Scholar] [CrossRef]
- Arnoult, C.; Lemos, J.R.; Florman, H.M. Voltage-dependent modulation of T-type calcium channels by protein tyrosine phosphorylation. EMBO J. 1997, 16, 1593–1599. [Google Scholar] [CrossRef]
- Casao, A.; Gallego, M.; Abecia, J.A.; Forcada, F.; Pérez-Pé, R.; Muiño-Blanco, T.; Cebrián-Pérez, J.Á. Identification and immunolocalisation of melatonin MT (1) and MT (2) receptors in Rasa Aragonesa ram spermatozoa. Reprod. Fertil. Dev. 2012, 24, 953–961. [Google Scholar] [CrossRef]
- González-Arto, M.; Vicente-Carrillo, A.; Martínez-Pastor, F.; Fernández-Alegre, E.; Roca, J.; Miró, J.; Rigau, T.; Rodríguez-Gil, J.E.; Pérez-Pé, R.; Muiño-Blanco, T.; et al. Melatonin receptors MT1 and MT2 are expressed in spermatozoa from several seasonal and nonseasonal breeder species. Theriogenology 2016, 86, 1958–1968. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; Zhang, L.; He, C.; Ji, P.; Wang, J.; Zhang, Z.; Lv, D.; Abulizi, W.; Wang, X.; et al. Beneficial effects of melatonin on the in vitro maturation of sheep oocytes and its relation to melatonin receptors. Int. J. Mol. Sci. 2017, 18, 834. [Google Scholar] [CrossRef]
- Lacoste, B.; Angeloni, D.; Dominguez-Lopez, S.; Calderoni, S.; Mauro, A.; Fraschini, F.; Descarries, L.; Gobbi, G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015, 58, 397–417. [Google Scholar] [CrossRef]
- Gobbi, G.; Comai, S. Sleep well. Untangling the role of melatonin MT1 and MT2 receptors in sleep. J. Pineal Res. 2019, 66, e12544. [Google Scholar] [CrossRef]
- Zahn, P.K.; Lansmann, T.; Berger, E.; Speckmann, E.J.; Musshoff, U. Gene expression and functional characterization of melatonin receptors in the spinal cord of the rat: Implications for pain modulation. J. Pineal Res. 2003, 35, 24–31. [Google Scholar] [CrossRef]
- Lv, D.; Tan, T.; Zhu, T.; Wang, J.; Zhang, S.; Zhang, L.; Hu, X.; Liu, G.; Xing, Y. Leptin mediates the effects of melatonin on female reproduction in mammals. J. Pineal Res. 2019, 66, e12559. [Google Scholar] [CrossRef]
- Das, A.; Belagodu, A.; Reiter, R.J.; Ray, S.K.; Banik, N.L. Cytoprotective effects of melatonin on C6 astroglial cells exposed to glutamate excitotoxicity and oxidative stress. J. Pineal Res. 2008, 45, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Tian, X.; Zhang, L.; Gao, C.; He, C.; Fu, Y.; Ji, P.; Li, Y.; Li, N.; Liu, G. Beneficial effects of melatonin on in vitro bovine embryonic development are mediated by melatonin receptor 1. J. Pineal Res. 2014, 56, 333–342. [Google Scholar] [CrossRef]
- Fujinoki, M. Melatonin-enhanced hyperactivation of hamster sperm. Reproduction 2008, 136, 533–541. [Google Scholar] [CrossRef] [Green Version]
- Nagata, M.P.B.; Endo, K.; Ogata, K.; Yamanaka, K.; Egashira, J.; Katafuchi, N.; Yamanouchi, T.; Matsuda, H.; Goto, Y.; Sakatani, M.; et al. Live births from artificial insemination of microfluidic-sorted bovine spermatozoa characterized by trajectories correlated with fertility. Proc. Natl. Acad. Sci. USA 2018, 115, E3087–E3096. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.M.; Ren, J.J.; Zhao, S.J.; Cui, L.S.; Hao, H.S.; Wang, H.Y.; Du, W.H.; Qin, T.; Liu, Y.; Wang, D.; et al. Apoptosis-like events and in vitro fertilization capacity of sex-sorted bovine sperm. Reprod. Domest. Anim. 2014, 49, 543–549. [Google Scholar] [CrossRef]
- Brackett, B.G.; Oliphant, G. Capacitation of rabbit spermatozoa in vitro. Biol. Reprod. 1975, 12, 260–274. [Google Scholar] [CrossRef]
- Anzar, M.; He, L.; Buhr, M.M.; Kroetsch, T.G.; Pauls, K.P. Sperm apoptosis in fresh and cryopreserved bull semen detected by flow cytometry and its relationship with fertility. Biol. Reprod. 2002, 66, 354–360. [Google Scholar] [CrossRef]
- Del Olmo, E.; Garcia-Alvarez, O.; Maroto-Morales, A.; Ramón, M.; Iniesta-Cuerda, M.; Martinez-Pastor, F.; Montoro, V.; Soler, A.J.; Garde, J.J.; Fernández-Santos, M.R. Oestrous sheep serum balances ROS levels to supply in vitro capacitation of ram spermatozoa. Reprod. Domest. Anim. 2016, 51, 743–750. [Google Scholar] [CrossRef] [Green Version]
- Karanikola, S.N.; Krücken, J.; Ramünke, S.; De Waal, T.; Höglund, J.; Charlier, J.; Weber, C.; Müller, E.; Kowalczyk, S.J.; Kaba, J.; et al. Development of a multiplex fluorescence immunological assay for the simultaneous detection of antibodies against Cooperia oncophora, Dictyocaulus viviparus and Fasciola hepatica in cattle. Parasit. Vectors 2015, 8, 335. [Google Scholar] [CrossRef]
- Deng, S.L.; Chen, S.R.; Wang, Z.P.; Zhang, Y.; Tang, J.X.; Li, J.; Wang, X.X.; Cheng, J.M.; Jin, C.; Li, X.Y.; et al. Melatonin promotes development of haploid germ cells from early developing spermatogenic cells of Suffolk sheep under in vitro condition. J. Pineal Res. 2016, 60, 435–447. [Google Scholar] [CrossRef]
- Hurtado de Llera, A.; Martin-Hidalgo, D.; Rodriguez-Gil, J.E.; Gil, M.C.; Garcia-Marin, L.J.; Bragado, M.J. AMP-activated kinase, AMPK, is involved in the maintenance of plasma membrane organization in boar spermatozoa. Biochim. Biophys. Acta. 2013, 1828, 2143–2151. [Google Scholar] [CrossRef] [Green Version]
- Steckler, D.; Stout, T.A.; Durandt, C.; Nöthling, J.O. Validation of merocyanine 540 staining as a technique for assessing capacitation-related membrane destabilization of fresh dog sperm. Theriogenology 2015, 83, 1451–1460. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.T.; Koo, O.J.; Kwon, D.K.; Park, H.J.; Jang, G.; Kang, S.K.; Lee, B.C. Effects of melatonin on in vitro maturation of porcine oocyte and expression of melatonin receptor RNA in cumulus and granulosa cells. J. Pineal Res. 2009, 46, 22–28. [Google Scholar] [CrossRef]
- Rosenkrans, C.F.; First, N.L. Effect of free amino acids and vitamins on cleavage and developmental rate of bovine zygotes in vitro. J. Anim. Sci. 1994, 72, 434–437. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-Y.; Hao, H.-S.; Zhao, Y.-H.; Zhang, P.-P.; Wang, H.-Y.; Pang, Y.-W.; Du, W.-H.; Zhao, S.-J.; Liu, Y.; Huang, J.-M.; et al. Melatonin Improves the Fertilization Capacity of Sex-Sorted Bull Sperm by Inhibiting Apoptosis and Increasing Fertilization Capacitation via MT1. Int. J. Mol. Sci. 2019, 20, 3921. https://doi.org/10.3390/ijms20163921
Li C-Y, Hao H-S, Zhao Y-H, Zhang P-P, Wang H-Y, Pang Y-W, Du W-H, Zhao S-J, Liu Y, Huang J-M, et al. Melatonin Improves the Fertilization Capacity of Sex-Sorted Bull Sperm by Inhibiting Apoptosis and Increasing Fertilization Capacitation via MT1. International Journal of Molecular Sciences. 2019; 20(16):3921. https://doi.org/10.3390/ijms20163921
Chicago/Turabian StyleLi, Chong-Yang, Hai-Sheng Hao, Ya-Han Zhao, Pei-Pei Zhang, Hao-Yu Wang, Yun-Wei Pang, Wei-Hua Du, Shan-Jiang Zhao, Yan Liu, Jin-Ming Huang, and et al. 2019. "Melatonin Improves the Fertilization Capacity of Sex-Sorted Bull Sperm by Inhibiting Apoptosis and Increasing Fertilization Capacitation via MT1" International Journal of Molecular Sciences 20, no. 16: 3921. https://doi.org/10.3390/ijms20163921





