New Aspects Towards a Molecular Understanding of the Allicin Immunostimulatory Mechanism via Colec12, MARCO, and SCARB1 Receptors
Abstract
:1. Introduction
2. Results
2.1. Blood Oxidative Stress Is Related to Immunoglobulin Secretion in a Dose-Dependent Manner after Allicin Treatment
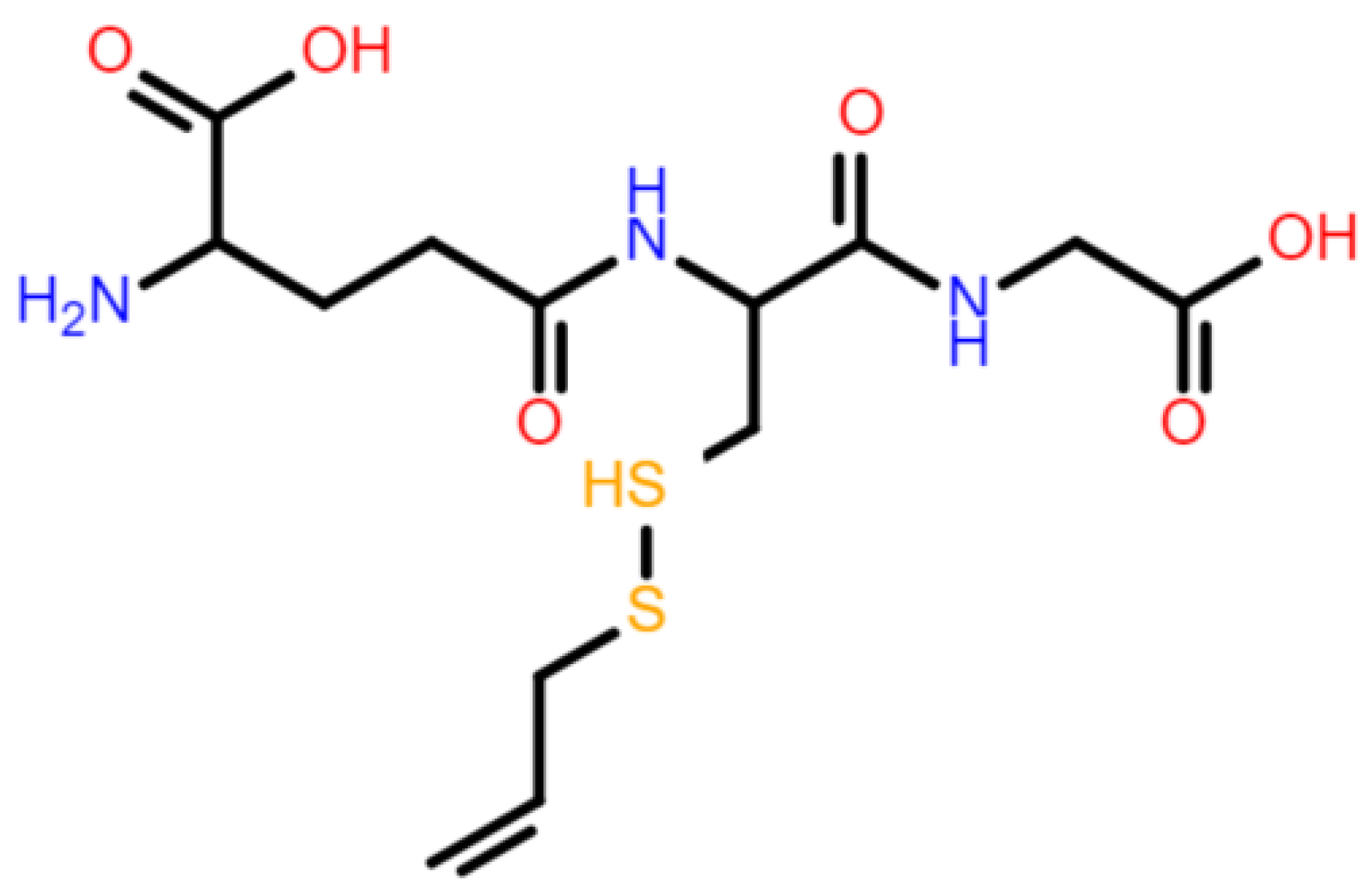
2.2. Serum GSH Concentration Was Decreased after Allicin Administration by Forming S-Allylmercaptoglutathione (GSSA)
2.3. CD19+ Lymphocytes Were Stimulated by GSSA (GSH–Allicin) Exposure
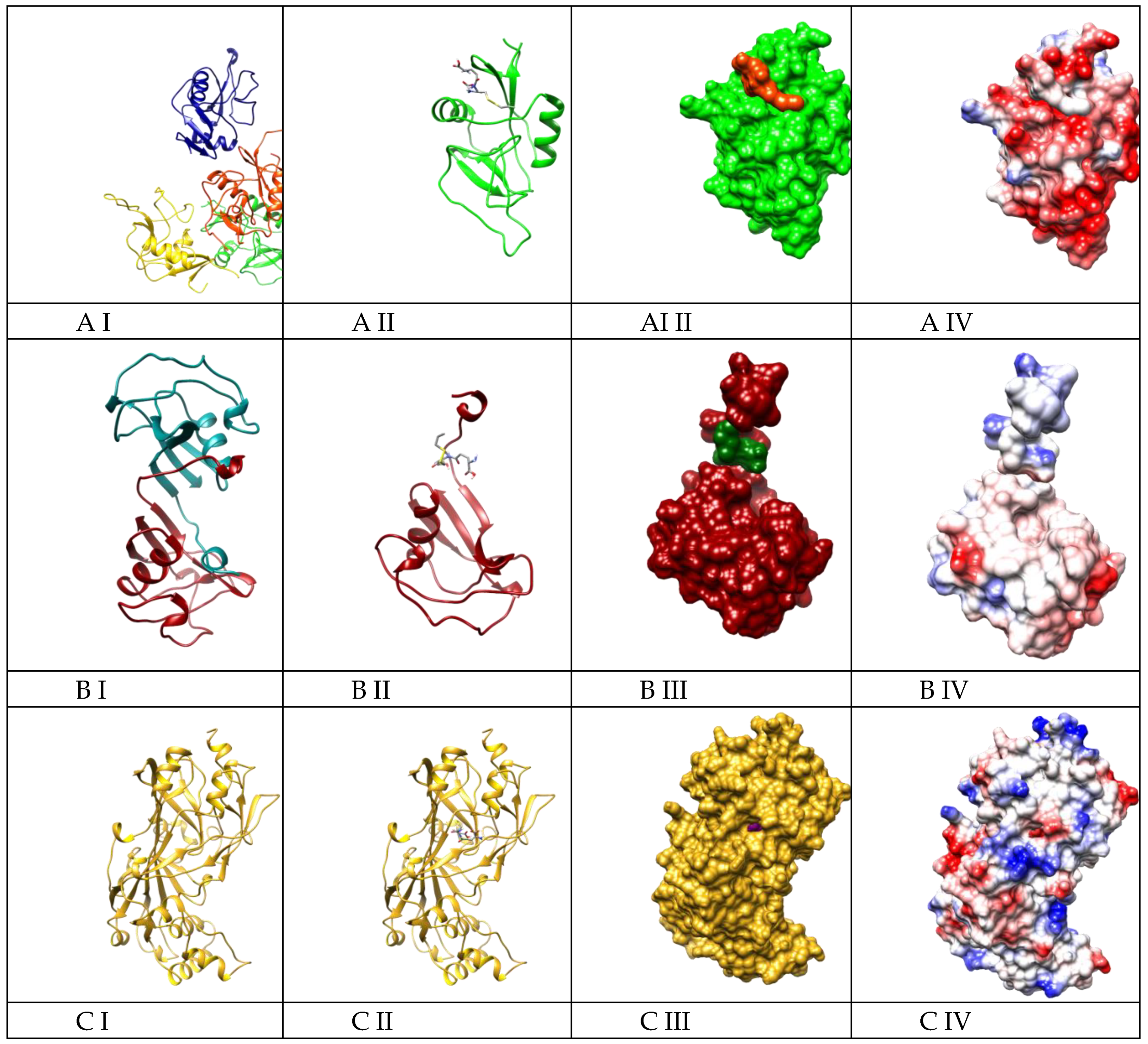
2.4. Molecular Docking Suggested the Most Specificity of S-Allylmercaptoglutathione (GSSA) Binding on Colec12, MARCO, and SCARB1 Scavenger Receptors
3. Discussion
4. Materials and Methods
4.1. In Vivo Studies
4.2. Animals
4.3. Ethics Statement
4.4. Hematology
4.5. Immunoglobulins
4.6. Oxidative Stress, Total Proteins, and Albumin Assay
4.7. In Vitro Testing on CD19+ B Cells
4.8. Molecular Docking
4.9. Allicin and Other Chemicals
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKT | protein kinase B |
| ALB | albumin |
| AP1 | activator protein 1 |
| APP/PS1 | amyloid precursor protein/presenilin 1 |
| BCAP | phosphoinositide 3-kinase adapter protein 1 |
| BCR | B cell receptor |
| BLNK | adaptor protein B cell linker |
| CAT | catalase |
| Colec12 | scavenger receptor with C-type lectin |
| ERK | extracellular-signal-regulated kinase |
| GRA/GRA% | the number and percentage of granulocytes |
| GPX | glutathione peroxidase |
| GSH | reduced glutathione |
| IgA/IgM/IgG | immunoglobulins |
| IkB | NFKB inhibitor alpha |
| IL | interleukin |
| JNK | Janus kinase |
| LPZ | lipopolysaccharides |
| LYM | lymphocytes |
| LYN | tyrosine-protein kinase Lyn |
| MARCO | macrophage receptor with collagenous structure |
| MEK1/2 | mitogen-activated protein kinase kinase 1 and 2 |
| MID | middle cells |
| Msr1 | macrophage scavenger receptor 1 |
| NFkB | nuclear factor kappa B subunit 1 |
| NF-Kb | nuclear factor kB |
| NK | natural killer cells |
| PI3K | phosphatidylinositol-4,5-bisphosphate 3-kinase |
| GSSA | S-allylmercaptoglutathione |
| SCARA | scavenger receptor class A |
| SCARB | scavenger receptor class B |
| SD | standard deviation |
| SOD | superoxide dismutase |
| SOS | son of sevenless/Ras/Rac guanine nucleotide exchange factor 1 |
| SR | scavenger receptor |
| SYK | spleen tyrosine kinase |
| TNFα | tumor necrosis factor α |
| TP | total proteins |
| WBC | white blood cell count |
References
- Trio, P.Z.; You, S.; He, X.; Sakao, K.; Hou, D.E. Chemopreventive functions and molecular mechanisms of garlic organosulfur compounds. Food Funct. 2014, 5, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Vainio, H. Allium vegetables and organosulfur compounds: Do they help prevent cancer? Environ. Health Perspect. 2001, 109, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Breyer, K.E.; Getchell, R.G.; Cornwell, E.R.; Wooster, G.A.; Ketola, H.G.; Bowser, P.R. Efficacy of an extract from garlic, Allium sativum, against infection with the furunculosis bacterium, Aeromonas salmonicida, in rainbow trout, Oncorhynchus mykiss. J. World Aquacult. Soc. 2015, 46, 273–282. [Google Scholar] [CrossRef]
- Tanekhy, M.; Fall, J. Expression of innate immunity genes in kuruma shrimp Marsupenaeus japonicus after in vivo stimulation with garlic extract (allicin). Vet. Med. 2015, 60, 39–47. [Google Scholar] [CrossRef]
- Nya, E.J.; Dawood, Z.; Austin, B. The garlic component, allicin, prevents disease caused by Aeromonas hydrophila in rainbow trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2010, 33, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J.; et al. Allicin and health: A comprehensive review. Trends Food Sci. Technol. 2019, 86, 502–512. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Rabinkov, A.; Miron, T.; Mirelman, D.; Wilchek, M.; Glozman, S.; Yavin, E.; Weiner, L. S-allylmercaptoglutathione: The reaction product of allicin with glutathione possesses SH-modifying and antioxidant properties. Biochim. Biophys. Acta. 2000, 1499, 144–153. [Google Scholar] [CrossRef]
- Liu, K.L.; Chen, H.W.; Wang, R.Y.; Lei, Y.P.; Sheen, L.Y.; Lii, C.K. DATS reduces LPS-induced iNOS expression, NO production, oxidative stress, and NF-κB activation in RAW 264.7 macrophages. J. Agric. Food Chem. 2006, 54, 3472–3478. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, G.; Kaschula, C.H. The immunomodulation and anti-inflammatory effects of garlic organosulfur compounds in cancer chemoprevention. Anti-Cancer Agents Med. Chem. 2014, 14, 233–240. [Google Scholar]
- Lund, T.; Stokke, T.; Olsen, Ø.E.; Fodstad, Ø. Garlic arrests MDA-MB-435 cancer cells in mitosis, phosphorylates the proapoptotic BH3-only protein BimEL and induces apoptosis. Br. J. Cancer 2005, 92, 1773–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augusti, K.T.; Mathew, P.T. Effect of allicin on certain enzymes of liver after a short term feeding to normal rats. Experientia 1975, 31, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Pârvu, M.; Rosca-Casian, O.; Puscas, M.; Groza, G. Antifungal activity of Allium fistulosum L. Contrib. Bot. 2009, 44, 125–129. [Google Scholar]
- Kang, N.S.; Moon, E.Y.; Cho, C.G.; Pyo, S. Immunomodulating effect of garlic component, allicin, on murine peritoneal macrophages. Nutr. Res. 2001, 21, 617–626. [Google Scholar] [CrossRef]
- Son, E.W.; Mo, S.J.; Rhee, D.K.; Pyo, S. Inhibition of ICAM-1 expression by garlic component, allicin, in gamma-irradiated human vascular endothelial cells via downregulation of the JNK signaling pathway. Int. Immunopharmacol. 2006, 6, 1788–1795. [Google Scholar] [CrossRef]
- Li, C.; Lun, W.; Zhao, X.; Lei, S.; Guo, Y.; Ma, J.; Zhi, F. Allicin alleviates inflammation of trinitrobenzensulfonic acid-induced rats abd suppresses P38 and JNK pathways in Caco-2 cells. Mediat. Inflamm. 2015, 2015, 1–11. [Google Scholar]
- Zhang, H.; Wang, P.; Xue, Y.; Liu, L.; Li, Z.; Liu, Y. Allicin ameliorates cognitive impairment in APP/PS1 mice via suppressing oxidative stress by blocking JNK signaling pathways. Tissue Cell 2018, 50, 89–95. [Google Scholar] [CrossRef]
- Seki, K.; Ishikawa, J.; Okada, Y. Contribution of 2-propenesulfenic acid to the antioxidant activity of allicin. J. Food Sci. 2018, 83, 1265–1270. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, D. Allicin suppresses the migration and invasion in cervical cancer cells mainly by inhibiting NRF2. Exp. Med. 2019, 17, 1523–1528. [Google Scholar] [CrossRef]
- Gruhlke, M.C.; Antelmann, H.; Bernhardt, J.; Kloubert, V.; Rink, L.; Slusarenko, A.J. The human allicin-proteome: S-thioallylation of proteins by the garlic defence substance allicin and its biological effects. Free Rad. Biol. Med. 2019, 131, 144–153. [Google Scholar] [CrossRef]
- Feldberg, R.S.; Chang, S.C.; Kotik, A.N.; Nadler, M.; Neuwirth, Z.; Sundstrom, D.C.; Thompson, N.H. In vitro mechanism of inhibition of bacterial cell growth by allicin. Antimicrob. Agents Chemother. 1988, 32, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Arreola, R.; Quintero-Fabián, S.; López-Roa, R.I.; Flores-Gutiérrez, E.O.; Reyes-Grajeda, J.P.; Carrera-Quintanar, L.; Ortuño-Sahagún, D. Immunomodulation and anti-inflammatory effects of garlic compounds. J. Immunol. Res. 2015, 2015, 401630. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, E.A.; Rifkin, I.R.; Hohlbaum, A.M.; Beaudette, B.C.; Shlomchik, M.J.; Marshak-Rothstein, A. Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 2002, 416, 603. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M. Immunobiology: The Immune System in Health and Disease, 6th ed.; Garland Science: New York, NY, USA, 1996; p. 26. [Google Scholar]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Atata, J.A.; Esievo, K.A.N.; Adamu, S.; Abdulsalam, H.; Avazi, D.O.; Ajadi, A.A. Haemato-biochemical studies of dogs with haemorrhage-induced dehydration. Comp. Clin. Path. 2019, 28, 129–135. [Google Scholar] [CrossRef]
- Chen, Q.; Lu, M.; Monks, B.R.; Birnbaum, M.J. Insulin is required to maintain albumin expression by inhibiting forkhead box O1 protein. J. Biol. Chem. 2016, 291, 2371–2378. [Google Scholar] [CrossRef]
- Cavallito, C.J.; Bailey, J.H. Allicin, the antibacterial principle of Allium sativum. II. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Tzianabos, A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef]
- Plüddemann, A.; Neyen, C.; Gordon, S. Macrophage scavenger receptors and host-derived ligands. Methods 2007, 43, 207–217. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. Gauss View. Semichem Inc. Version 5.0. Available online: https://gaussian.com/gaussview6/ (accessed on 27 November 2018).
- Moţ, A.C.; Bischin, C.; Muresan, B.; Parvu, M.; Damian, G.; Vlase, L.; Silaghi-Dumitrescu, R. Antioxidant activity evaluation by physiologically relevant assays based on haemoglobin peroxidase activity and cytochrome c-induced oxidation of liposomes. Nat. Prod. Res. 2015, 24, 1–5. [Google Scholar]
- Buynitsky, T.; Mostofsky, D.I. Restraint stress in biobehavioral research: Recent developments. Neurosci. Biobehav. R. 2009, 33, 1089–1098. [Google Scholar] [CrossRef]
- Toma, V.A.; Farcas, A.D.; Parvu, M.; Silaghi-Dumitrescu, R.; Roman, I. CA3 hippocampal field: Cellular changes and its relation with blood nitro-oxidative stress reveal a balancing function of CA3 area in rats exposed to repeated restraint stress. Brain. Res. Bull. 2017, 130, 10–17. [Google Scholar] [CrossRef]
- Toma, V.A.; Farcas, A.D.; Roman, I.; Sevastre, B.; Hathazi, D.; Scurtu, F.; Damian, G.; Silaghi-Dumitrescu, R. Comparative in vivo effects of hemoglobin-based oxygen carriers (HBOC) with varying prooxidant and physiological reactivity. PLoS ONE 2016, 11, 1–16. [Google Scholar]
- Sagor, A.T.; Chowdhury, M.R.H.; Tabassum, N.; Hossain, H.; Rahman, M.; Alam, A. Supplementation of fresh ucche (Momordicacharantia L. var. muricataWilld) prevented oxidative stress, fibrosis and hepatic damage in CCl4 treated rats. BMC Complement. Altern. Med. 2015, 15, 115. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Villanueva, C.; Kross, R.D. Antioxidant-induced stress. Int. J. Mol. Sci. 2012, 13, 2091–2109. [Google Scholar] [CrossRef]
- Jayanthi, M.K.; Dhar, M. Anti-inflammatory effects of Allium sativum (garlic) in experimental rats. Biomedicine 2011, 31, 84–89. [Google Scholar]
- Dinarello, C.A. The role of the interleukin-1–receptor antagonist in blocking inflammation mediated by interleukin-1. N. Engl. J. Med. 2000, 343, 732–734. [Google Scholar] [CrossRef]
- Jafari, R.A.; Jalali, M.R.; Ghorbanpoor, M.; Saraei, S.M. Effect of dietary garlic on immune response of broiler chicks to live Newcastle Disease vaccine. Pak. J. Biol. Sci. 2008, 11, 1848–1851. [Google Scholar] [CrossRef]
- Shokrollahi, B.; Hesami, S.M.; Baneh, H. The effect of garlic extract on growth, haematology and cell-mediated immune response of newborn goat kids. J. Agric. Rural Dev. Trop. 2016, 117, 225–232. [Google Scholar]
- Hirsch, K.; Danilenko, M.; Giat, J.; Miron, T.; Rabinkov, A.; Wilchek, M.; Mirelman, D.; Levy, J.; Sharoni, Y. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr. Cancer 2000, 38, 245–254. [Google Scholar] [CrossRef]
- Mohamed, E.H.; Baiomy, A.A.A.; Ibrahim, Z.S.; Soliman, M.M. Modulatory effects of levamisole and garlic oil on the immune response of Wistar rats: Biochemical, immunohistochemical, molecular and immunological study. Molec. Med. Rep. 2016, 14, 2755–2763. [Google Scholar] [CrossRef]
- Jördo, E.D.; Wermeling, F.; Chen, Y.; Karlsson, M.C. Scavenger receptors as regulators of natural antibody responses and B cell activation in autoimmunity. Molec. Immunol. 2011, 48, 1307–1318. [Google Scholar] [CrossRef]
- Rahman, K. Effects of garlic on platelet biochemistry and physiology. Mol. Nutr. Food Res. 2007, 51, 1335–1344. [Google Scholar] [CrossRef]
- Arredouani, M.S. Is the scavenger receptor MARCO a new immune checkpoint? Oncoimmunology 2014, 3, e955709. [Google Scholar] [CrossRef] [Green Version]
- De Lima Batista, A.P.; Zahariev, F.; Slowing, I.I.; Braga, A.A.; Ornellas, F.R.; Gordon, M.S. Silanol-assisted carbinolamine formation in an amine-functionalized mesoporous silica Surface: Theoretical investigation by fragmentation methods. J. Phys. Chem. B 2015, 120, 1660–1669. [Google Scholar] [CrossRef]
- Urban, S.; Zieseniss, S.; Werder, M.; Hauser, H.; Budzinski, R.; Engelmann, B. Scavenger receptor BI transfers major lipoprotein-associated phospholipids into the cells. J. Biol. Chem. 2000, 275, 33409–33415. [Google Scholar] [CrossRef]
- Martineau, C.; Kevorkova, O.; Brissette, L.; Moreau, R. Scavenger receptor class B, type I (Scarb1) deficiency promotes osteoblastogenesis but stunts terminal osteocyte differentiation. Physiol. Rep. 2014, 2, 12117. [Google Scholar] [CrossRef]
- Schäfer, G.; Guler, R.; Murray, G.; Brombacher, F.; Brown, G.D. The role of scavenger seceptor B1 in infection with Mycobacterium tuberculosis in a murine model. PLoS ONE 2009, 4, 8448. [Google Scholar] [CrossRef]
- Neyestany, T.R.; Shariatzadeh, N.; Gharavi, A.; Kalayi, A.; Khalaji, N. Physiological dose of lycopene suppressed oxidative stress and enhanced serum levels of immunoglobulin M in patients with Type 2 diabetes mellitus: A possible role in the prevention of long-term complications. J. Endocrinol. Invest. 2007, 30, 833–838. [Google Scholar] [CrossRef]
- Ercal, N.; Neal, R.; Treeratphan, P.; Lutz, P.M.; Hammond, T.C.; Dennery, P.A.; Spitz, D.R. A role for oxidative stress in suppressing serum immunoglobulin levels in lead-exposed Fisher 344 rats. Arch. Environ. Contam. Toxicol. 2000, 39, 251–256. [Google Scholar] [CrossRef]
- Vasquez, M.; Simões, I.; Consuegra-Fernández, M.; Aranda, F.; Lozano, F.; Berraondo, P. Exploiting scavenger receptors in cancer immunotherapy: Lessons from CD5 and SR-B1. Eur. J. Immunol. 2017, 47, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.J. High density lipoprotein: Assembly, structure, cargo, and functions. ISRN Physiol. 2013, 2013, 1–20. [Google Scholar] [CrossRef]
- Fischer-Fodor, E.; Mikláš, R.; Krausz, L.T.; Virag, P.; Moldovan, D.; PerdeSchrepler, M.; Berindan-Neagoe, I.; Devínsky, F.; Miklášová, N. Immunomodulatory potential of palladium(II) complexes with (1E,6E)-1,7-bis (3,4-dimethoxyphenyl) hepta-1,6-diene-3,5-dione. Stud. U. Babes Bol. Che. 2015, 60, 93–100. [Google Scholar]
- Sharma, V.; McNeill, J.H. To scale or not to scale: The principles of dose extrapolation. Br. J. Pharm. 2009, 157, 907–921. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 16, 2785–2791. [Google Scholar] [CrossRef]
- Feinberg, H.; Taylor, M.E.; Weis, W.I. Scavenger receptor C-type lecithin binds to leukocyte cell surface glycan lewis by a novel mechanism. J. Biol. Chem. 2007, 282, 17250–17258. [Google Scholar] [CrossRef]
- Ojala, J.R.; Pikkarainen, T.; Tuuttila, A.; Sandalova, T.; Tryggvason, K. Crystal structure of the cysteine-rich domain of scavenger receptor MARCO reveals the presence of a basic and an acidic cluster that both contribute to ligand recognition. J. Biol. Chem. 2007, 282, 16654–16666. [Google Scholar] [CrossRef]
- Hohenester, E.; Sasaki, T.; Timpl, R. Crystal structure of a scavenger receptor cysteine-rich domain sheds light on an ancient superfamily. Nat. Struc. Biol. 1999, 6, 228–232. [Google Scholar] [CrossRef]
- Boudko, S.P.; Bächinger, H.P. Structural insight for chain selection and stagger control in collagen. Sci. Rep. 2016, 6, 37831. [Google Scholar] [CrossRef] [Green Version]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modeling of protein structures and complexes. Nucleic Acids Res. 2018, 46, 296–303. [Google Scholar] [CrossRef]
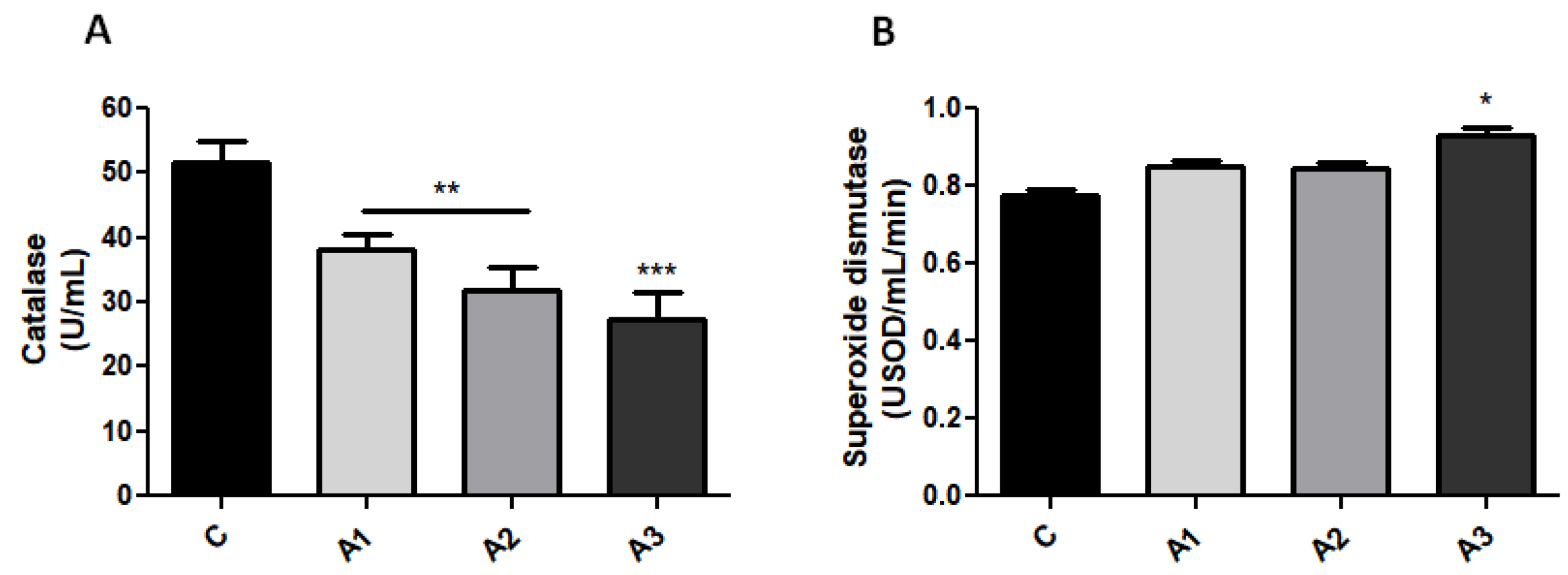
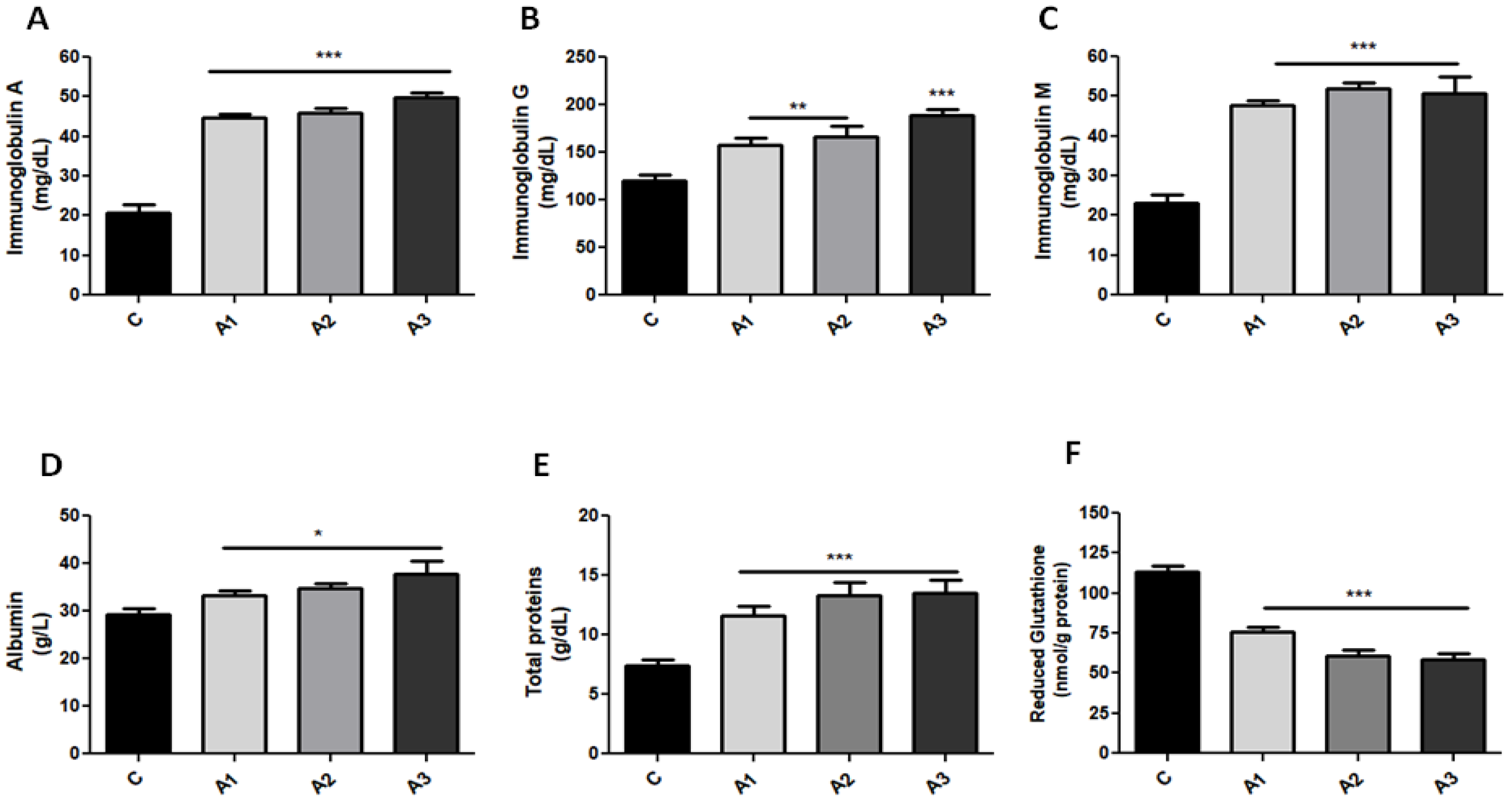
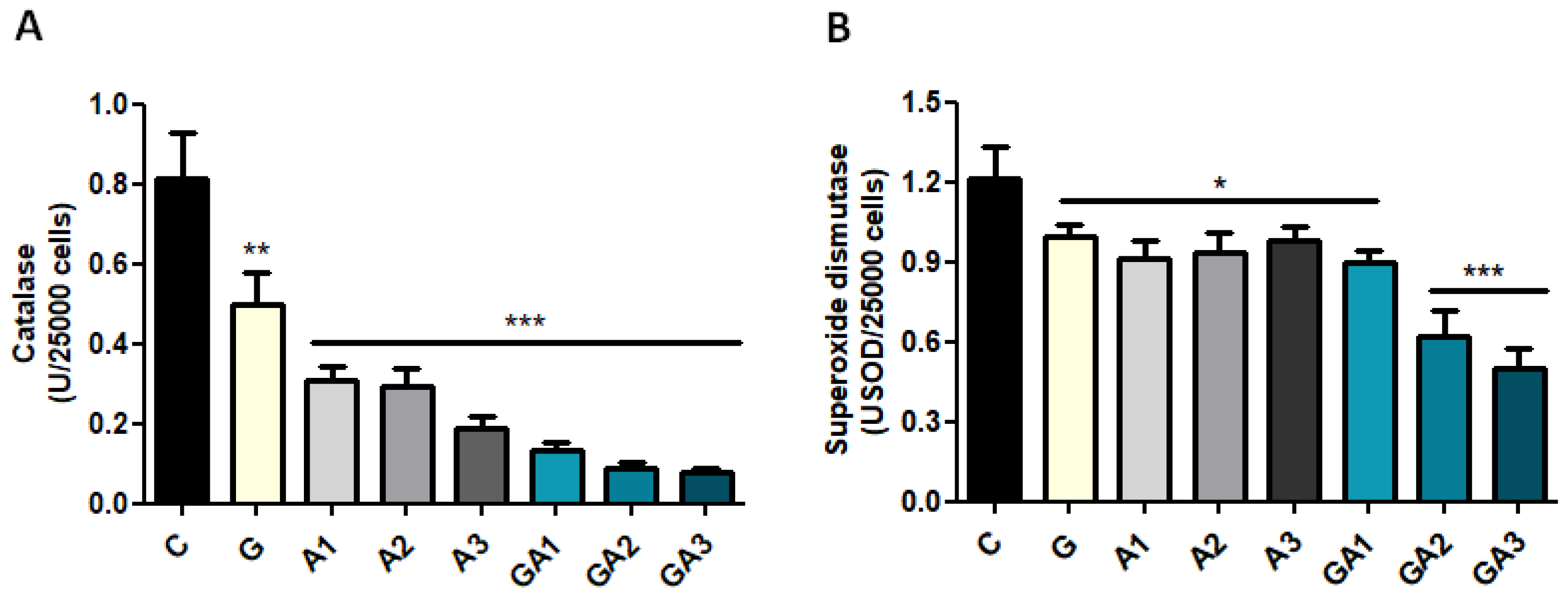
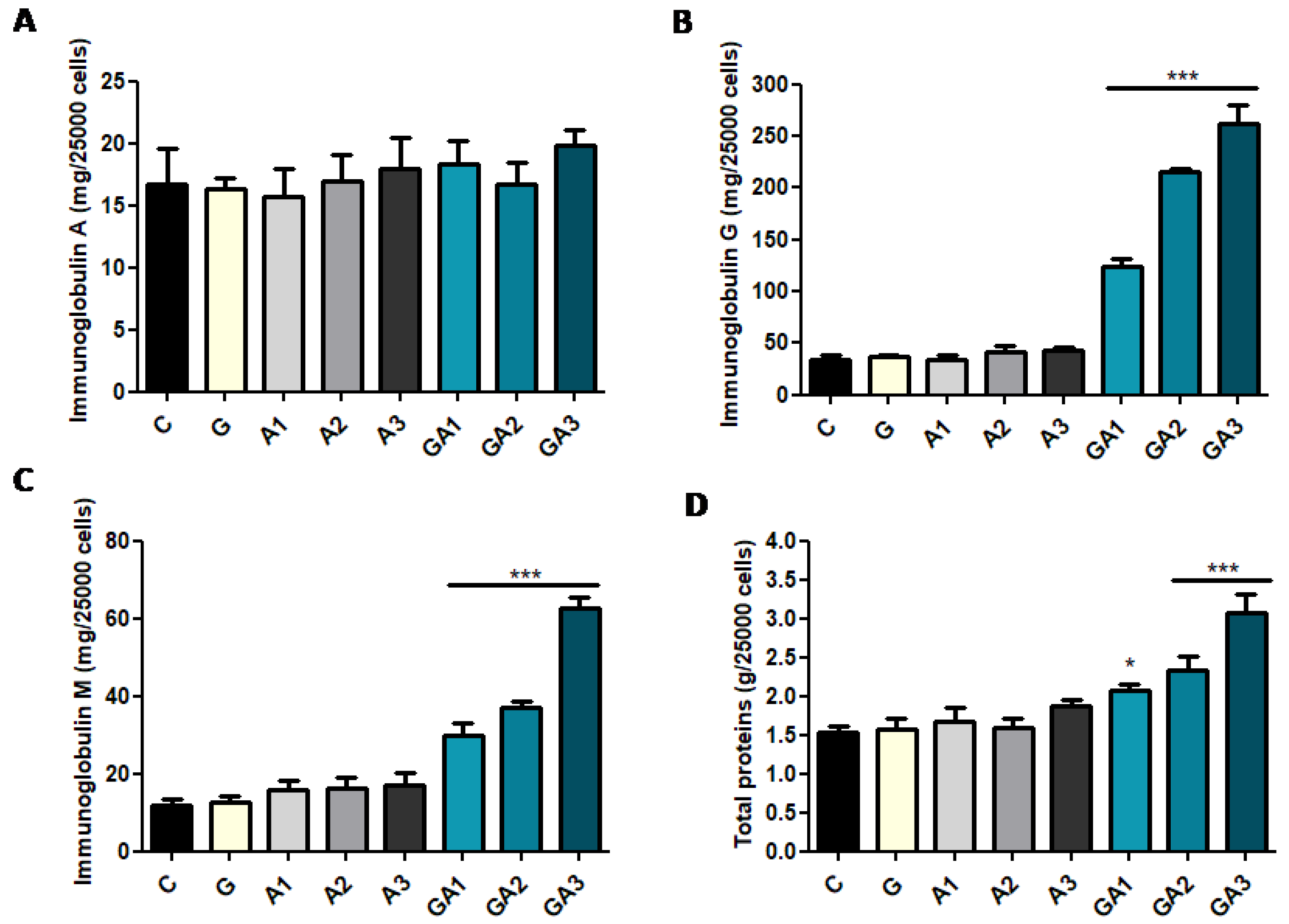


| Parameters | C | A1 Allicin 1.25 mg/kg | A2 Allicin 2.5 mg/kg | A3 Allicin 5 mg/kg |
|---|---|---|---|---|
| WBC (109/L) | 14.5 ± 1.63 | 13.1 ± 1.23 | 13.4 ± 1.02 | 14.2 ± 2.14 |
| LYM (109/L) | 10.1 ± 1.49 | 9.7 ± 1.24 | 10.5 ± 1.02 | 11.0 ± 1.76 |
| MON (109/L) | 0.3 ± 0.10 | 0.2 ± 0.08 | 0.3 ± 0.31 | 0.2 ± 0.09 |
| NEU (109/L) | 2.8 ± 0.13 | 3.2 ± 0.12 | 2.7 ± 0.20 | 2.9 ± 0.45 |
| LYM% | 76.4 ± 1.93 | 73.5 ± 2.80 | 75.1 ± 2.78 | 77.8 ± 2.20 |
| MON% | 2.08 ± 0.85 | 1.57 ± 0.97 | 2.2 ± 2.04 | 1.30 ± 0.47 |
| NEU% | 20.8 ±1.26 | 25.1 ± 1.73 | 21.7 ± 3.35 | 20.9 ± 2.06 |
| Ligand | Type | Binding Energy (kcal/mol) | Geometric Specificity |
|---|---|---|---|
| Colec12 | SCAR A | −6.7 | Very high |
| MARCO | SCAR A | −5.7 | Very high |
| Msr1 | SCAR A | −4.7 | Low |
| SCARA3 | SCAR A | −4.8 | Low |
| SCARA5 | SCAR A | −5.0 | Very low |
| SCARB1 | SCAR B | −5.7 | Very high |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, V.A.; Tigu, A.B.; Farcaș, A.D.; Sevastre, B.; Taulescu, M.; Gherman, A.M.R.; Roman, I.; Fischer-Fodor, E.; Pârvu, M. New Aspects Towards a Molecular Understanding of the Allicin Immunostimulatory Mechanism via Colec12, MARCO, and SCARB1 Receptors. Int. J. Mol. Sci. 2019, 20, 3627. https://doi.org/10.3390/ijms20153627
Toma VA, Tigu AB, Farcaș AD, Sevastre B, Taulescu M, Gherman AMR, Roman I, Fischer-Fodor E, Pârvu M. New Aspects Towards a Molecular Understanding of the Allicin Immunostimulatory Mechanism via Colec12, MARCO, and SCARB1 Receptors. International Journal of Molecular Sciences. 2019; 20(15):3627. https://doi.org/10.3390/ijms20153627
Chicago/Turabian StyleToma, Vlad Al., Adrian Bogdan Tigu, Anca D. Farcaș, Bogdan Sevastre, Marian Taulescu, Ana Maria Raluca Gherman, Ioana Roman, Eva Fischer-Fodor, and Marcel Pârvu. 2019. "New Aspects Towards a Molecular Understanding of the Allicin Immunostimulatory Mechanism via Colec12, MARCO, and SCARB1 Receptors" International Journal of Molecular Sciences 20, no. 15: 3627. https://doi.org/10.3390/ijms20153627
APA StyleToma, V. A., Tigu, A. B., Farcaș, A. D., Sevastre, B., Taulescu, M., Gherman, A. M. R., Roman, I., Fischer-Fodor, E., & Pârvu, M. (2019). New Aspects Towards a Molecular Understanding of the Allicin Immunostimulatory Mechanism via Colec12, MARCO, and SCARB1 Receptors. International Journal of Molecular Sciences, 20(15), 3627. https://doi.org/10.3390/ijms20153627






