Suppression of Propionibacterium acnes-Induced Skin Inflammation by Laurus nobilis Extract and Its Major Constituent Eucalyptol
Abstract
:1. Introduction
2. Results
2.1. Dose Optimization of LNE in Bone Marrow-Derived Macrophages (BMMs).
2.2. Inhibition of P. acnes-Induced Proinflammatory Cytokine Expression by LNE
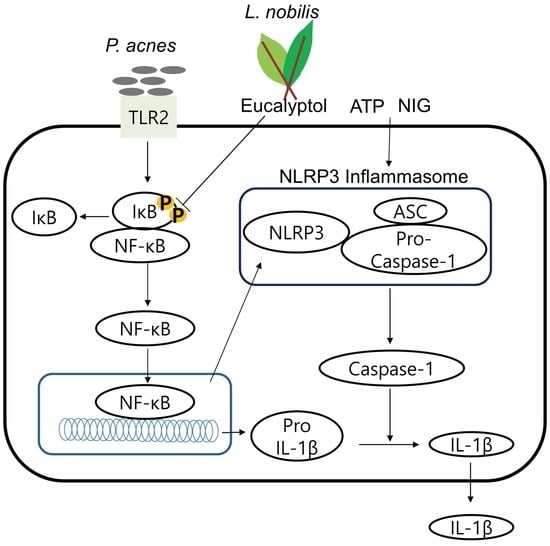
2.3. Inhibition of P. acnes-Induced NF-κB Activation by LNE
2.4. Inhibition of P. acnes-Induced NLRP3 Inflammasome Activation by LNE
2.5. P. acnes-Induced MAPK Activation is not Inhibited by LNE
2.6. Eucalyptol Inhibits P. acnes-Induced Inflammation
2.7. LNE Ameliorates P. acnes-Induced Skin Inflammation in vivo
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. L. nobilis Extract (LNE)
4.3. P. acnes
4.4. Cell Culture
4.5. Western Blot Analysis
4.6. Reporter Gene Analysis
4.7. Cell Counting
4.8. 3-(4,5-Dimethyl-2-Thiazolyl)-2H-Tetrazolium Bromide (MTT) Assay
4.9. ELISA
4.10. Quantitative Real-Time PCR
4.11. In vivo Mouse Model
4.12. Histological Analysis
4.13. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Marples, R.R. The microflora of the face and acne lesions. J. Investig. Derm. 1974, 62, 326–331. [Google Scholar] [CrossRef]
- Leyden, J.J. The evolving role of Propionibacterium acnes in acne. Semin. Cutan. Med. Surg. 2001, 20, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Leeming, J.P.; Holland, K.T.; Cunliffe, W.J. The pathological and ecological significance of microorganisms colonizing acne vulgaris comedones. J. Med. Microbiol. 1985, 20, 11–16. [Google Scholar] [CrossRef]
- Chen, Q.; Koga, T.; Uchi, H.; Hara, H.; Terao, H.; Moroi, Y.; Urabe, K.; Furue, M. Propionibacterium acnes-induced IL-8 production may be mediated by NF-kappaB activation in human monocytes. J. Derm. Sci. 2002, 29, 97–103. [Google Scholar] [CrossRef]
- Jain, A.; Basal, E. Inhibition of Propionibacterium acnes-induced mediators of inflammation by Indian herbs. Phytomedicine 2003, 10, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Webster, G.F. Acne vulgaris. BMJ 2002, 325, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Heymann, W.R. Toll-like receptors in acne vulgaris. J. Am. Acad. Derm. 2006, 55, 691–692. [Google Scholar] [CrossRef]
- Dreno, B.; Thiboutot, D.; Gollnick, H.; Bettoli, V.; Kang, S.; Leyden, J.J.; Shalita, A.; Torres, V. Antibiotic stewardship in dermatology: Limiting antibiotic use in acne. Eur. J. Derm. 2014, 24, 330–334. [Google Scholar]
- Dhawan, S.S. Comparison of 2 clindamycin 1%-benzoyl peroxide 5% topical gels used once daily in the management of acne vulgaris. Cutis 2009, 83, 265–272. [Google Scholar]
- Charakida, A.; Mouser, P.E.; Chu, A.C. Safety and side effects of the acne drug, oral isotretinoin. Expert Opin. Drug Saf. 2004, 3, 119–129. [Google Scholar] [CrossRef]
- Pickert, A.; Raimer, S. An evaluation of dapsone gel 5% in the treatment of acne vulgaris. Expert Opin. Pharm. 2009, 10, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, S.; Viola, G.; Giorgetti, M.; Loi, M.C.; Innocenti, G. Two new sesquiterpene lactones from the leaves of Laurus nobilis. Chem. Pharm. Bull. (Tokyo) 2006, 54, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Statti, G.; Uzunov, D.; Menichini, F. Comparative chemical composition and antioxidant activities of wild and cultivated Laurus nobilis L. leaves and Foeniculum vulgare subsp. piperitum (Ucria) coutinho seeds. Biol. Pharm. Bull. 2006, 29, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Sayyah, M.; Saroukhani, G.; Peirovi, A.; Kamalinejad, M. Analgesic and anti-inflammatory activity of the leaf essential oil of Laurus nobilis Linn. Phytother. Res. 2003, 17, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, B.; Esen, M.; Sangun, M.K.; Coleri, A.; Caliskan, M. Effective antibacterial and antioxidant properties of methanolic extract of Laurus nobilis seed oil. J. Environ. Biol. 2010, 31, 637–641. [Google Scholar] [PubMed]
- Lev, E.; Amar, Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J. Ethnopharmacol. 2000, 72, 191–205. [Google Scholar] [CrossRef]
- Pieroni, A.; Quave, C.L.; Villanelli, M.L.; Mangino, P.; Sabbatini, G.; Santini, L.; Boccetti, T.; Profili, M.; Ciccioli, T.; Rampa, L.G.; et al. Ethnopharmacognostic survey on the natural ingredients used in folk cosmetics, cosmeceuticals and remedies for healing skin diseases in the inland Marches, Central-Eastern Italy. J. Ethnopharmacol. 2004, 91, 331–344. [Google Scholar] [CrossRef]
- Bruni, A.; Ballero, M.; Poli, F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J. Ethnopharmacol. 1997, 57, 97–124. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef]
- Cherrat, L.; Espina, L.; Bakkali, M.; Garcia-Gonzalo, D.; Pagan, R.; Laglaoui, A. Chemical composition and antioxidant properties of Laurus nobilis L. and Myrtus communis L. essential oils from Morocco and evaluation of their antimicrobial activity acting alone or in combined processes for food preservation. J. Sci. Food Agric. 2014, 94, 1197–1204. [Google Scholar] [CrossRef]
- Ozcan, M.; Chalchat, J.C. Effect of different locations on the chemical composition of essential oils of laurel (Laurus nobilis L.) leaves growing wild in Turkey. J. Med. Food 2005, 8, 408–411. [Google Scholar] [CrossRef]
- Nakase, K.; Nakaminami, H.; Takenaka, Y.; Hayashi, N.; Kawashima, M.; Noguchi, N. Relationship between the severity of acne vulgaris and antimicrobial resistance of bacteria isolated from acne lesions in a hospital in Japan. J. Med. Microbiol. 2014, 63, 721–728. [Google Scholar] [CrossRef]
- Jeong, M.S.; Kim, J.Y.; Lee, H.I.; Seo, S.J. Calcitriol May Down-Regulate mRNA Over-Expression of Toll-Like Receptor-2 and -4, LL-37 and Proinflammatory Cytokines in Cultured Human Keratinocytes. Ann. Derm. 2014, 26, 296–302. [Google Scholar] [CrossRef]
- Lee, W.R.; Kim, K.H.; An, H.J.; Kim, J.Y.; Chang, Y.C.; Chung, H.; Park, Y.Y.; Lee, M.L.; Park, K.K. The protective effects of melittin on Propionibacterium acnes-induced inflammatory responses in vitro and in vivo. J. Investig. Derm. 2014, 134, 1922–1930. [Google Scholar] [CrossRef]
- Valins, W.; Amini, S.; Berman, B. The Expression of Toll-like Receptors in Dermatological Diseases and the Therapeutic Effect of Current and Newer Topical Toll-like Receptor Modulators. J. Clin. Aesthet. Derm. 2010, 3, 20–29. [Google Scholar]
- Jugeau, S.; Tenaud, I.; Knol, A.C.; Jarrousse, V.; Quereux, G.; Khammari, A.; Dreno, B. Induction of toll-like receptors by Propionibacterium acnes. Br. J. Derm. 2005, 153, 1105–1113. [Google Scholar] [CrossRef]
- Gurung, P.; Li, B.; Subbarao Malireddi, R.K.; Lamkanfi, M.; Geiger, T.L.; Kanneganti, T.D. Chronic TLR Stimulation Controls NLRP3 Inflammasome Activation through IL-10 Mediated Regulation of NLRP3 Expression and Caspase-8 Activation. Sci. Rep. 2015, 5, 14488. [Google Scholar] [CrossRef] [Green Version]
- Schroder, K.; Zhou, R.; Tschopp, J. The NLRP3 inflammasome: A sensor for metabolic danger? Science 2010, 327, 296–300. [Google Scholar] [CrossRef]
- Neven, B.; Prieur, A.M.; Quartier dit Maire, P. Cryopyrinopathies: Update on pathogenesis and treatment. Nat. Clin. Pr. Rheumatol. 2008, 4, 481–489. [Google Scholar] [CrossRef]
- Bode, J.G.; Ehlting, C.; Haussinger, D. The macrophage response towards LPS and its control through the p38 (MAPK)-STAT3 axis. Cell. Signal. 2012, 24, 1185–1194. [Google Scholar] [CrossRef]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of Essential Oil and Its Biological Activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.R.; de Melo, T.S.; Carvalho, K.M.; de Oliveira, I.B.; Arruda, B.R.; de Castro Brito, G.A.; Rao, V.S.; Santos, F.A. 1,8-cineole (eucalyptol) ameliorates cerulein-induced acute pancreatitis via modulation of cytokines, oxidative stress and NF-kappaB activity in mice. Life Sci. 2013, 92, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lai, Y.; Wang, Y.; Liu, N.; Zhang, F.; Xu, P. 1, 8-Cineol Protect Against Influenza-Virus-Induced Pneumonia in Mice. Inflammation 2016, 39, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, J.; Fang, C.; Tang, F. 1, 8-cineol attenuates LPS-induced acute pulmonary inflammation in mice. Inflammation 2014, 37, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Vaibhav, K.; Javed, H.; Tabassum, R.; Ahmed, M.E.; Khan, M.M.; Khan, M.B.; Shrivastava, P.; Islam, F.; Siddiqui, M.S.; et al. 1, 8-cineole (eucalyptol) mitigates inflammation in amyloid Beta toxicated PC12 cells: Relevance to Alzheimer’s disease. Neurochem. Res. 2014, 39, 344–352. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Lee, W.R.; Kim, K.H.; Kim, J.Y.; Lee, S.J.; Han, S.M.; Lee, K.G.; Lee, C.K.; Park, K.K. Inhibitory effects of bee venom on Propionibacterium acnes-induced inflammatory skin disease in an animal model. Int. J. Mol. Med. 2014, 34, 1341–1348. [Google Scholar] [CrossRef] [Green Version]
- Cal, K.; Sopala, M. Tremendous ex vivo child skin absorption and permeation of eucalyptol. J. Derm. Sci. 2008, 52, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Shin, J.H.; Kim, S.S.; Lee, H.; Yang, S.R.; Seo, S.R. Laurus nobilis leaf extract controls inflammation by suppressing NLRP3 inflammasome activation. J. Cell. Physiol. 2019, 234, 6854–6864. [Google Scholar] [CrossRef] [PubMed]
- Englen, M.D.; Valdez, Y.E.; Lehnert, N.M.; Lehnert, B.E. Granulocyte/macrophage colony-stimulating factor is expressed and secreted in cultures of murine L929 cells. J. Immunol. Methods 1995, 184, 281–283. [Google Scholar] [CrossRef]
- Boltz-Nitulescu, G.; Wiltschke, C.; Holzinger, C.; Fellinger, A.; Scheiner, O.; Gessl, A.; Forster, O. Differentiation of rat bone marrow cells into macrophages under the influence of mouse L929 cell supernatant. J. Leukoc. Biol. 1987, 41, 83–91. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.H.; Shin, J.H.; Kim, S.S.; Joo, J.-H.; Choi, E.; Seo, S.R. Suppression of Propionibacterium acnes-Induced Skin Inflammation by Laurus nobilis Extract and Its Major Constituent Eucalyptol. Int. J. Mol. Sci. 2019, 20, 3510. https://doi.org/10.3390/ijms20143510
Lee EH, Shin JH, Kim SS, Joo J-H, Choi E, Seo SR. Suppression of Propionibacterium acnes-Induced Skin Inflammation by Laurus nobilis Extract and Its Major Constituent Eucalyptol. International Journal of Molecular Sciences. 2019; 20(14):3510. https://doi.org/10.3390/ijms20143510
Chicago/Turabian StyleLee, Eun Hye, Jin Hak Shin, Seon Sook Kim, Ji-Hye Joo, Eunmi Choi, and Su Ryeon Seo. 2019. "Suppression of Propionibacterium acnes-Induced Skin Inflammation by Laurus nobilis Extract and Its Major Constituent Eucalyptol" International Journal of Molecular Sciences 20, no. 14: 3510. https://doi.org/10.3390/ijms20143510