Tubulin in Platelets: When the Shape Matters
Abstract
:1. Introduction
2. Platelet Cytoskeleton
| Tubulin | Molecules per Platelet (Human) | Molecules per Platelet (Mouse) |
|---|---|---|
| α-Tubulin | 597,500 total | 400,075 total |
| α1A | <500 | - |
| α1B | - | 54,936 |
| α1C | 174,000 | 25,217 |
| α3C | 110,000 | - |
| α4A | 185,000 | 295,013 |
| α8 | 128,000 | 24,882 |
| β-Tubulin | 752,900 total | 568,722 total |
| β1 | 144,000 | 246,000 |
| β2A | - | 34,850 |
| β2B | - | 284 |
| β2C | 200,000 | - |
| β3 | 78,800 | - |
| β4 | 96,000 | 227,095 |
| β5 | 115,000 | 59,134 |
| β6 | 80,000 | 1,359 |
| β8 | 39,100 | - |
| γ-Tubulin | 2,300 | 796 |
3. The role of Tubulin in Platelet Formation
4. Microtubule Organization in Resting Platelets and Upon Activation
5. Tubulin Post-Translational Modifications in Platelets
6. Implications of Tubulin Genetic Variability in Pathological and Physiological Platelet Function
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Thon, J.N.; Italiano, J.E. Platelets: Production, Morphology and Ultrastructure. In Handbook of Experimental Pharmacology; Gresele, P., Born, G.V.R., Patrono, C., Page, C.P., Eds.; Springer Berlin Heidelberg: Heidelberg, Germany, 2012; Volume 210, pp. 3–22. ISBN 978-3-642-29422-8. [Google Scholar]
- Rivera, J.; Lozano, M.L.; Navarro-Núñez, L.; Vicente, V. Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 2009, 94, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Seifert, J.; Rheinlaender, J.; Lang, F.; Gawaz, M.; Schäffer, T.E. Thrombin-induced cytoskeleton dynamics in spread human platelets observed with fast scanning ion conductance microscopy. Sci. Rep. 2017, 7, 4810. [Google Scholar] [CrossRef] [PubMed]
- Thon, J.N.; Macleod, H.; Begonja, A.J.; Zhu, J.; Lee, K.-C.; Mogilner, A.; Hartwig, J.H.; Italiano, J.E. Microtubule and cortical forces determine platelet size during vascular platelet production. Nat. Commun. 2012, 3, 852:1–852:9. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cormier, A.; Gigant, B.; Knossow, M. Insight into the GTPase Activity of Tubulin from Complexes with Stathmin-like Domains. Biochemistry 2007, 46, 10595–10602. [Google Scholar] [CrossRef] [PubMed]
- McKean, P.G.; Vaughan, S.; Gull, K. The extended tubulin superfamily. J. Cell Sci. 2001, 114, 2723–2733. [Google Scholar] [PubMed]
- Zhao, Z.; Liu, H.; Luo, Y.; Zhou, S.; An, L.; Wang, C.; Jin, Q.; Zhou, M.; Xu, J.-R. Molecular evolution and functional divergence of tubulin superfamily in the fungal tree of life. Sci. Rep. 2014, 4, 6746. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Natarajan, K.; Curiel, J.; Janke, C.; Liu, J. The emerging role of the tubulin code: From the tubulin molecule to neuronal function and disease. Cytoskeleton 2016, 73, 521–550. [Google Scholar] [CrossRef]
- Burkhart, J.M.; Vaudel, M.; Gambaryan, S.; Radau, S.; Walter, U.; Martens, L.; Geiger, J.; Sickmann, A.; Zahedi, R.P. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 2012, 120, e73–e82. [Google Scholar] [CrossRef] [Green Version]
- Zeiler, M.; Moser, M.; Mann, M. Copy Number Analysis of the Murine Platelet Proteome Spanning the Complete Abundance Range. Mol. Cell. Proteom. 2014, 13, 3435–3445. [Google Scholar] [CrossRef] [Green Version]
- Schwer, H.D.; Lecine, P.; Tiwari, S.; Italiano, J.E.; Hartwig, J.H.; Shivdasani, R.A. A lineage-restricted and divergent β-tubulin isoform is essential for the biogenesis, structure and function of blood platelets. Curr. Biol. 2001, 11, 579–586. [Google Scholar] [CrossRef]
- Cerecedo, D. Platelet cytoskeleton and its hemostatic role. Blood Coagul. Fibrinolysis 2013, 24, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Burley, K.; Westbury, S.K.; Mumford, A.D. TUBB1 variants and human platelet traits. Platelets 2018, 29, 209–211. [Google Scholar] [CrossRef]
- Serrano, L.; Avila, J.; Maccioni, R.B. Controlled proteolysis of tubulin by subtilisin: localization of the site for MAP2 interaction. Biochemistry 1984, 23, 4675–4681. [Google Scholar] [CrossRef] [PubMed]
- Cross, D.; Dominguez, J.; Maccioni, R.B.; Avila, J. MAP-1 and MAP-2 binding sites at the C-terminus of beta-tubulin. Studies with synthetic tubulin peptides. Biochemistry 1991, 30, 4362–4366. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.A.; Sheetz, M.P. Steric inhibition of cytoplasmic dynein and kinesin motility by MAP2. Cell Motil. Cytoskeleton 1993, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Strassel, C.; Magiera, M.M.; Dupuis, A.; Batzenschlager, M.; Hovasse, A.; Pleines, I.; Guéguen, P.; Eckly, A.; Moog, S.; Mallo, L.; et al. An essential role for α4A-tubulin in platelet biogenesis. Life Sci. Alliance 2019, 2, e201900309:1–e201900309:13. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, S.; Brouhard, G.J. A microtubule bestiary: Structural diversity in tubulin polymers. Mol. Biol. Cell 2017, 28, 2924–2931. [Google Scholar] [CrossRef]
- Xu, Z.; Afzelius, B.A. Early changes in the substructure of the marginal bundle in human blood platelets responding to adenosine diphosphate. J. Ultrastruct. Mol. Struct. Res. 1988, 99, 254–260. [Google Scholar] [CrossRef]
- Akhmanova, A.; Steinmetz, M.O. Control of microtubule organization and dynamics: Two ends in the limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef]
- Akhmanova, A.; Steinmetz, M.O. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 2008, 9, 309–322. [Google Scholar] [CrossRef]
- Hayashi, I.; Wilde, A.; Mal, T.K.; Ikura, M. Structural basis for the activation of microtubule assembly by the EB1 and p150Glued complex. Mol. Cell 2005, 19, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Manna, T.; Honnappa, S.; Steinmetz, M.O.; Wilson, L. Suppression of microtubule dynamic instability by the +TIP protein EB1 and its modulation by the CAP-Gly domain of p150glued. Biochemistry 2008, 47, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Mimori-Kiyosue, Y.; Grigoriev, I.; Lansbergen, G.; Sasaki, H.; Matsui, C.; Severin, F.; Galjart, N.; Grosveld, F.; Vorobjev, I.; Tsukita, S.; et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 2005, 168, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.G. The Structure of Resting and Activated Platelets. In Platelets; Michelson, A.D., Ed.; Elsevier: Cambridge, MA, USA, 2019; pp. 47–77. ISBN 9780128134566. [Google Scholar]
- Patel, S.R.; Richardson, J.L.; Schulze, H.; Kahle, E.; Galjart, N.; Drabek, K.; Shivdasani, R.A.; Hartwig, J.H.; Italiano, J.E. Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes. Blood 2005, 106, 4076–4085. [Google Scholar] [CrossRef] [PubMed]
- Diagouraga, B.; Grichine, A.; Fertin, A.; Wang, J.; Khochbin, S.; Sadoul, K. Motor-driven marginal band coiling promotes cell shape change during platelet activation. J. Cell Biol. 2014, 204, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, F.; Kauskot, A.; Kurowska, M.; Goudin, N.; Munoz, I.; Bordet, J.-C.; Huang, J.-D.; Bryckaert, M.; Fischer, A.; Borgel, D.; et al. Kinesin-1 Is a New Actor Involved in Platelet Secretion and Thrombus Stability. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1037–1051. [Google Scholar] [CrossRef] [Green Version]
- Akhmanova, A.; Hoogenraad, C.C. Microtubule Minus-End-Targeting Proteins. Curr. Biol. 2015, 25, R162–R171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, R.; John, A. Microtubule-associated proteins as direct crosslinkers of actin filaments and microtubules. IUBMB Life 2015, 67, 395–403. [Google Scholar] [CrossRef]
- Kunert, S.; Meyer, I.; Fleischhauer, S.; Wannack, M.; Fiedler, J.; Shivdasani, R.A.; Schulze, H. The microtubule modulator RanBP10 plays a critical role in regulation of platelet discoid shape and degranulation. Blood 2009, 114, 5532–5540. [Google Scholar] [CrossRef] [Green Version]
- Strassel, C.; Moog, S.; Mallo, L.; Eckly, A.; Freund, M.; Gachet, C.; Lanza, F. Microtubule plus-end tracking Adenopolyposis Coli negatively regulates proplatelet formation. Sci. Rep. 2018, 8, 15808:1–15808:10. [Google Scholar] [CrossRef]
- Zuidscherwoude, M.; Green, H.L.H.; Thomas, S.G. Formin proteins in megakaryocytes and platelets: regulation of actin and microtubule dynamics. Platelets 2019, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A.; Molina, A.; Rodrigues-Ferreira, S.; Honoré, S.; Nahmias, C. Regulation of end-binding protein EB1 in the control of microtubule dynamics. Cell. Mol. Life Sci. 2017, 74, 2381–2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzi, S.; Lordier, L.; Debili, N.; Raslova, H.; Vainchenker, W. Megakaryocyte and polyploidization. Exp. Hematol. 2018, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Italiano, J.E.; Hartwig, J.H. Megakaryocyte Development and Platelet Formation. In Platelets; Michelson, A.D., Ed.; Elsevier: Cambridge, MA, USA, 2013; pp. 27–49. ISBN 9780123878373. [Google Scholar]
- Edelstein, L.C.; Simon, L.M.; Montoya, R.T.; Holinstat, M.; Chen, E.S.; Bergeron, A.; Kong, X.; Nagalla, S.; Mohandas, N.; Cohen, D.E.; et al. Racial differences in human platelet PAR4 reactivity reflect expression of PCTP and miR-376c. Nat. Med. 2013, 19, 1609–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machlus, K.R.; Italiano, J.E. Megakaryocyte Development and Platelet Formation. In Platelets; Michelson, A.D., Ed.; Elsevier: Cambridge, MA, USA, 2019; pp. 25–46. ISBN 9780128134566. [Google Scholar]
- Tablin, F.; Castro, M.; Leven, R.M. Blood platelet formation in vitro. The role of the cytoskeleton in megakaryocyte fragmentation. J. Cell Sci. 1990, 97, 59–70. [Google Scholar] [PubMed]
- Basciano, P.A.; Matakas, J.; Pecci, A.; Civaschi, E.; Cagioni, C.; Bompiani, N.; Burger, P.; Christos, P.; Snyder, J.P.; Bussel, J.; et al. β-1 tubulin R307H SNP alters microtubule dynamics and affects severity of a hereditary thrombocytopenia. J. Thromb. Haemost. 2015, 13, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Goulas, C.; Pillois, X. A new mutation in TUBB1 associated with thrombocytopenia confirms that C-terminal part of β1-tubulin plays a role in microtubule assembly. Clin. Genet. 2017, 91, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Stoupa, A.; Adam, F.; Kariyawasam, D.; Strassel, C.; Gawade, S.; Szinnai, G.; Kauskot, A.; Lasne, D.; Janke, C.; Natarajan, K.; et al. TUBB1 mutations cause thyroid dysgenesis associated with abnormal platelet physiology. EMBO Mol. Med. 2018, 10, e9569:1–e9569:18. [Google Scholar] [CrossRef]
- Maly, I.V.; Vorobjev, I.A. Centrosome-dependent anisotropic random walk of cytoplasmic vesicles. Cell Biol. Int. 2002, 26, 791–799. [Google Scholar] [CrossRef]
- Richardson, J.L.; Shivdasani, R.A.; Boers, C.; Hartwig, J.H.; Italiano, J.E. Mechanisms of organelle transport and capture along proplatelets during platelet production. Blood 2005, 106, 4066–4075. [Google Scholar] [CrossRef] [PubMed]
- Corash, L.; Costa, J.L.; Shafer, B.; Donlon, J.A.; Murphy, D. Heterogeneity of human whole blood platelet subpopulations. III. Density-dependent differences in subcellular constituents. Blood 1984, 64, 185–193. [Google Scholar] [PubMed]
- Corash, L.; Tan, H.; Gralnick, H.R. Heterogeneity of human whole blood platelet subpopulations. I. Relationship between buoyant density, cell volume, and ultrastructure. Blood 1977, 49, 71–87. [Google Scholar] [PubMed]
- Patel-Hett, S.; Richardson, J.L.; Schulze, H.; Drabek, K.; Isaac, N.A.; Hoffmeister, K.; Shivdasani, R.A.; Bulinski, J.C.; Galjart, N.; Hartwig, J.H.; et al. Visualization of microtubule growth in living platelets reveals a dynamic marginal band with multiple microtubules. Blood 2008, 111, 4605–4616. [Google Scholar] [CrossRef] [Green Version]
- Freson, K.; De Vos, R.; Wittevrongel, C.; Thys, C.; Defoor, J.; Vanhees, L.; Vermylen, J.; Peerlinck, K.; Van Geet, C. The TUBB1 Q43P functional polymorphism reduces the risk of cardiovascular disease in men by modulating platelet function and structure. Blood 2005, 106, 2356–2362. [Google Scholar] [CrossRef] [Green Version]
- Magiera, M.M.; Singh, P.; Gadadhar, S.; Janke, C. Tubulin Posttranslational Modifications and Emerging Links to Human Disease. Cell 2018, 173, 1323–1327. [Google Scholar] [CrossRef] [Green Version]
- Janke, C. The tubulin code: molecular components, readout mechanisms, and functions. J. Cell Biol. 2014, 206, 461–472. [Google Scholar] [CrossRef]
- Webster, D.R.; Borisy, G.G. Microtubules are acetylated in domains that turn over slowly. J. Cell Sci. 1989, 92, 57–65. [Google Scholar] [PubMed]
- Gundersen, G.G.; Kalnoski, M.H.; Bulinski, J.C. Distinct populations of microtubules: Tyrosinated and nontyrosinated alpha tubulin are distributed differently in vivo. Cell 1984, 38, 779–789. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.-J. Tubulin acetylation: responsible enzymes, biological functions and human diseases. Cell. Mol. Life Sci. 2015, 72, 4237–4255. [Google Scholar] [CrossRef]
- Janke, C.; Montagnac, G. Causes and Consequences of Microtubule Acetylation. Curr. Biol. 2017, 27, R1287–R1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadoul, K.; Wang, J.; Diagouraga, B.; Vitte, A.-L.; Buchou, T.; Rossini, T.; Polack, B.; Xi, X.; Matthias, P.; Khochbin, S. HDAC6 controls the kinetics of platelet activation. Blood 2012, 120, 4215–4218. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.O.; Slater, A.; Maclachlan, A.; Nicolson, P.L.R.; Pike, J.A.; Yule, J.; Thomas, S.G.; Morgan, N.V. Post-translational polymodification of the C-terminal tail of TUBB1 regulates motor protein processivity in platelet production and function. bioRxiv 2019, 1–13. [Google Scholar] [CrossRef]
- Van Dijk, J.; Bompard, G.; Cau, J.; Kunishima, S.; Rabeharivelo, G.; Mateos-Langerak, J.; Cazevieille, C.; Cavelier, P.; Boizet-Bonhoure, B.; Delsert, C.; et al. Microtubule polyglutamylation and acetylation drive microtubule dynamics critical for platelet formation. BMC Biol. 2018, 16, 116:1–116:17. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Núñez, L.; Lozano, M.L.; Rivera, J.; Corral, J.; Roldán, V.; González-Conejero, R.; Iniesta, J.A.; Montaner, J.; Vicente, V.; Martínez, C. The association of the beta1-tubulin Q43P polymorphism with intracerebral hemorrhage in men. Haematologica 2007, 92, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Núñez, L.; Roldán, V.; Lozano, M.L.; Rivera, J.; Marin, F.; Vicente, V.; Martínez, C. TUBB1 Q43P polymorphism doesnot protectagainst acutecoronarysyndrome andpremature myocardial infarction. Thromb. Haemost. 2008, 100, 1211–1213. [Google Scholar] [CrossRef]
- Haley, K.M.; Recht, M.; McCarty, O.J.T. Neonatal platelets: mediators of primary hemostasis in the developing hemostatic system. Pediatr. Res. 2014, 76, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Ferrer-Marin, F.; Stanworth, S.; Josephson, C.; Sola-Visner, M. Distinct differences in platelet production and function between neonates and adults: implications for platelet transfusion practice. Transfusion 2013, 53, 2814–2821. [Google Scholar] [CrossRef]
- White, J.G.; Burris, S.M. Morphometry of platelet internal contraction. Am. J. Pathol. 1984, 115, 412–417. [Google Scholar]
- Caparrós-Pérez, E.; Teruel-Montoya, R.; Palma-Barquero, V.; Torregrosa, J.M.; Blanco, J.E.; Delgado, J.L.; Lozano, M.L.; Vicente, V.; Sola-Visner, M.; Rivera, J.; et al. Down Regulation of the Munc18b-syntaxin-11 Complex and β1-tubulin Impairs Secretion and Spreading in Neonatal Platelets. Thromb. Haemost. 2017, 117, 2079–2091. [Google Scholar] [CrossRef]
- White, J.G. Platelet Structure. In Platelets; Michelson, A.D., Ed.; Elsevier: Cambridge, MA, USA, 2013; pp. 45–73. ISBN 9780123878373. [Google Scholar]
- Navarro-Núñez, L.; Teruel, R.; Antón, A.I.; Nurden, P.; Martínez-Martínez, I.; Lozano, M.L.; Rivera, J.; Corral, J.; Mezzano, D.; Vicente, V.; et al. Rare homozygous status of P43 β1-tubulin polymorphism causes alterations in platelet ultrastructure. Thromb. Haemost. 2011, 105, 855–863. [Google Scholar] [CrossRef]
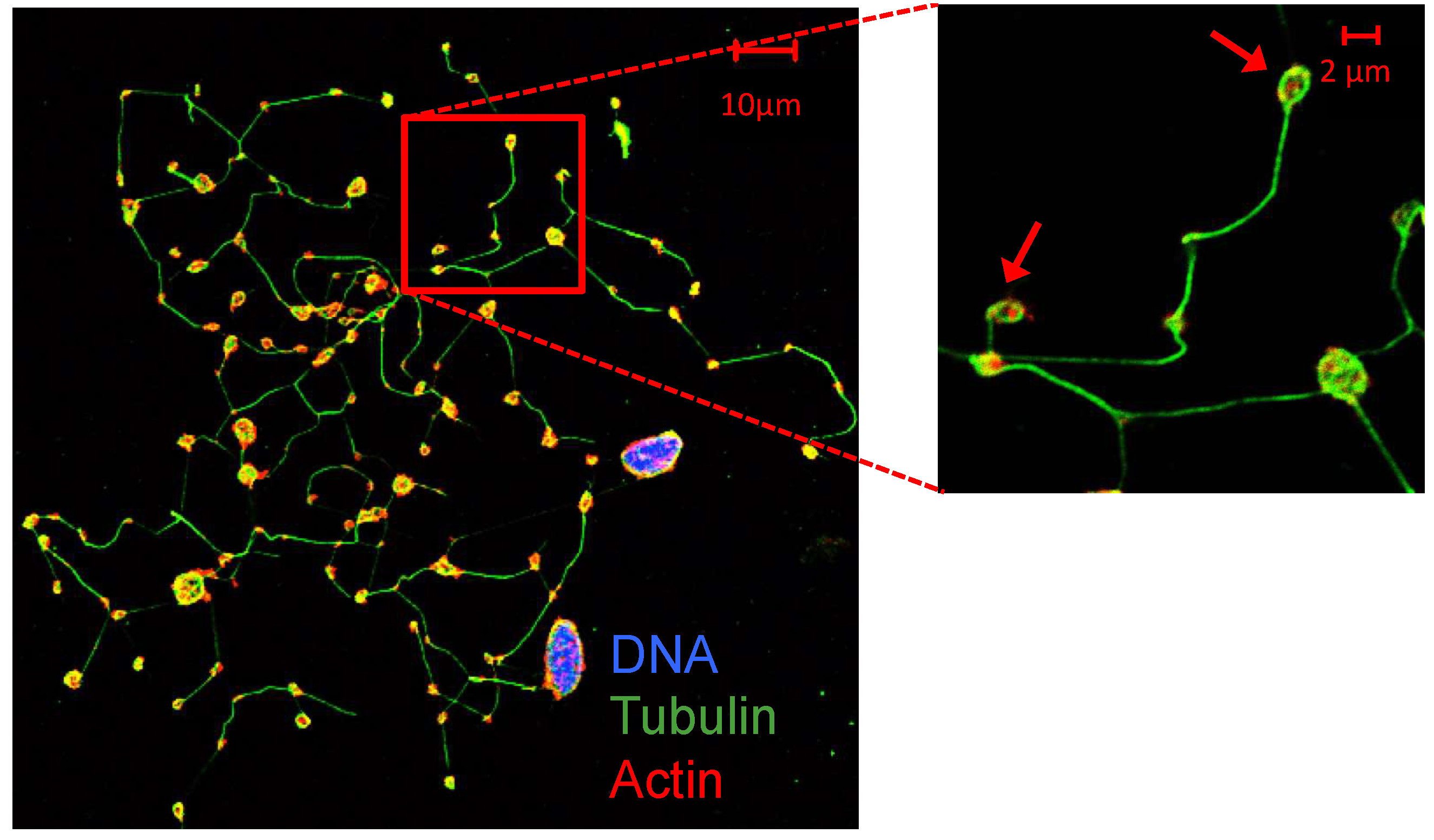
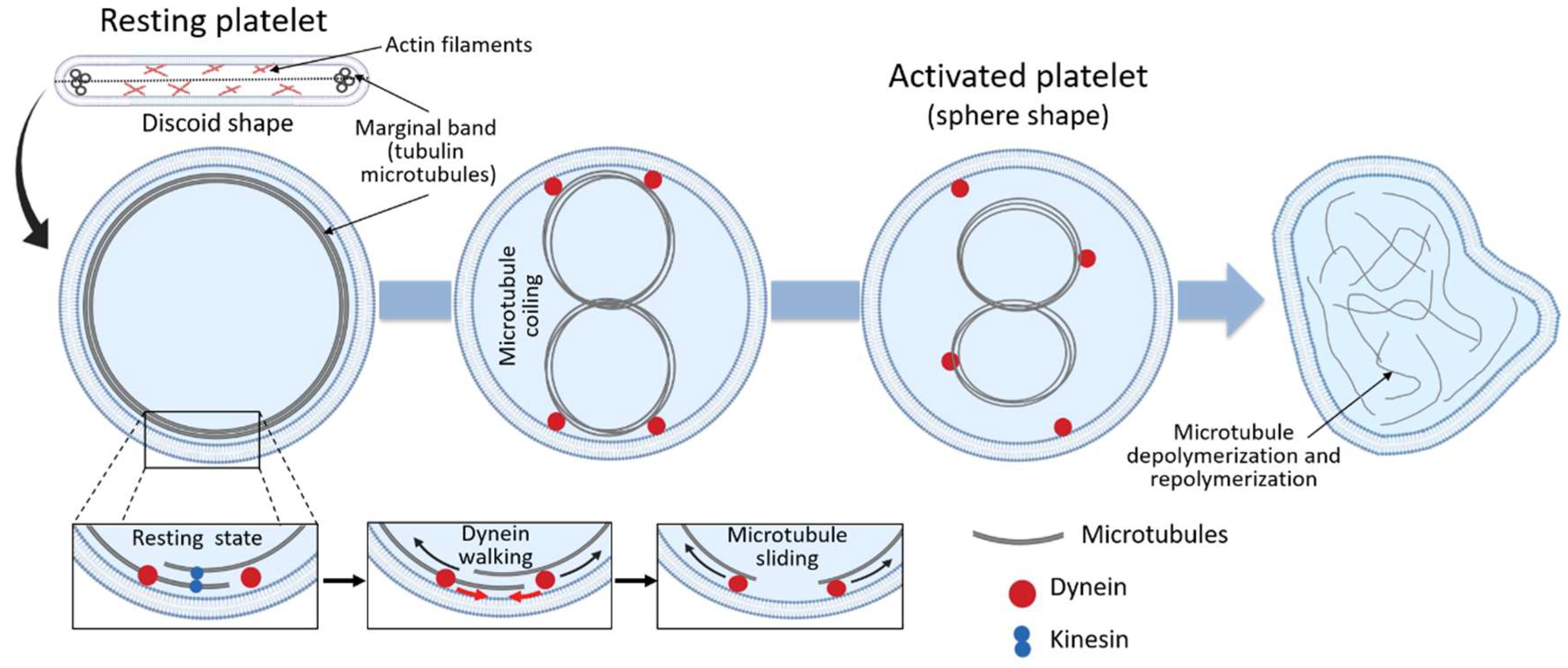
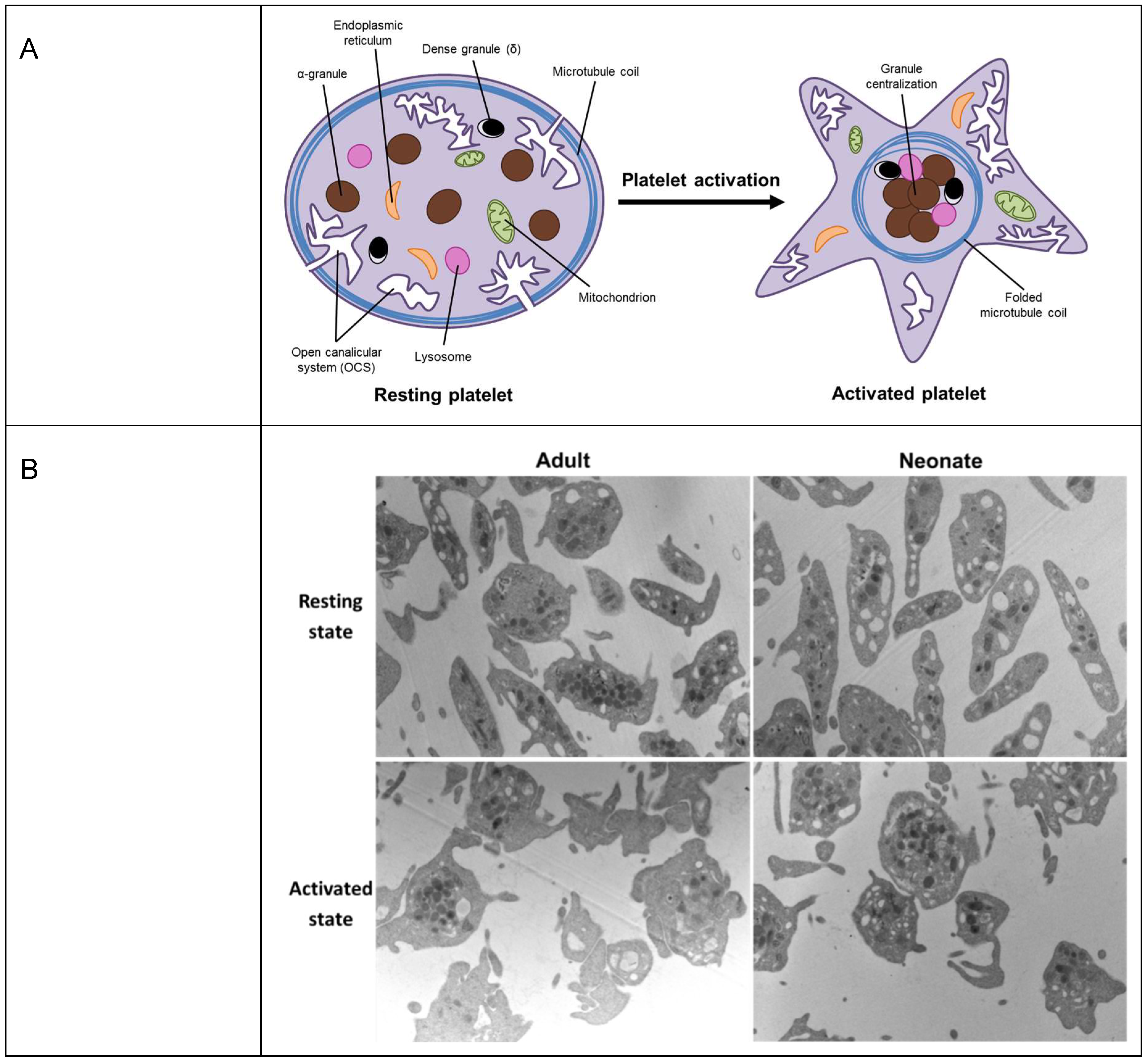
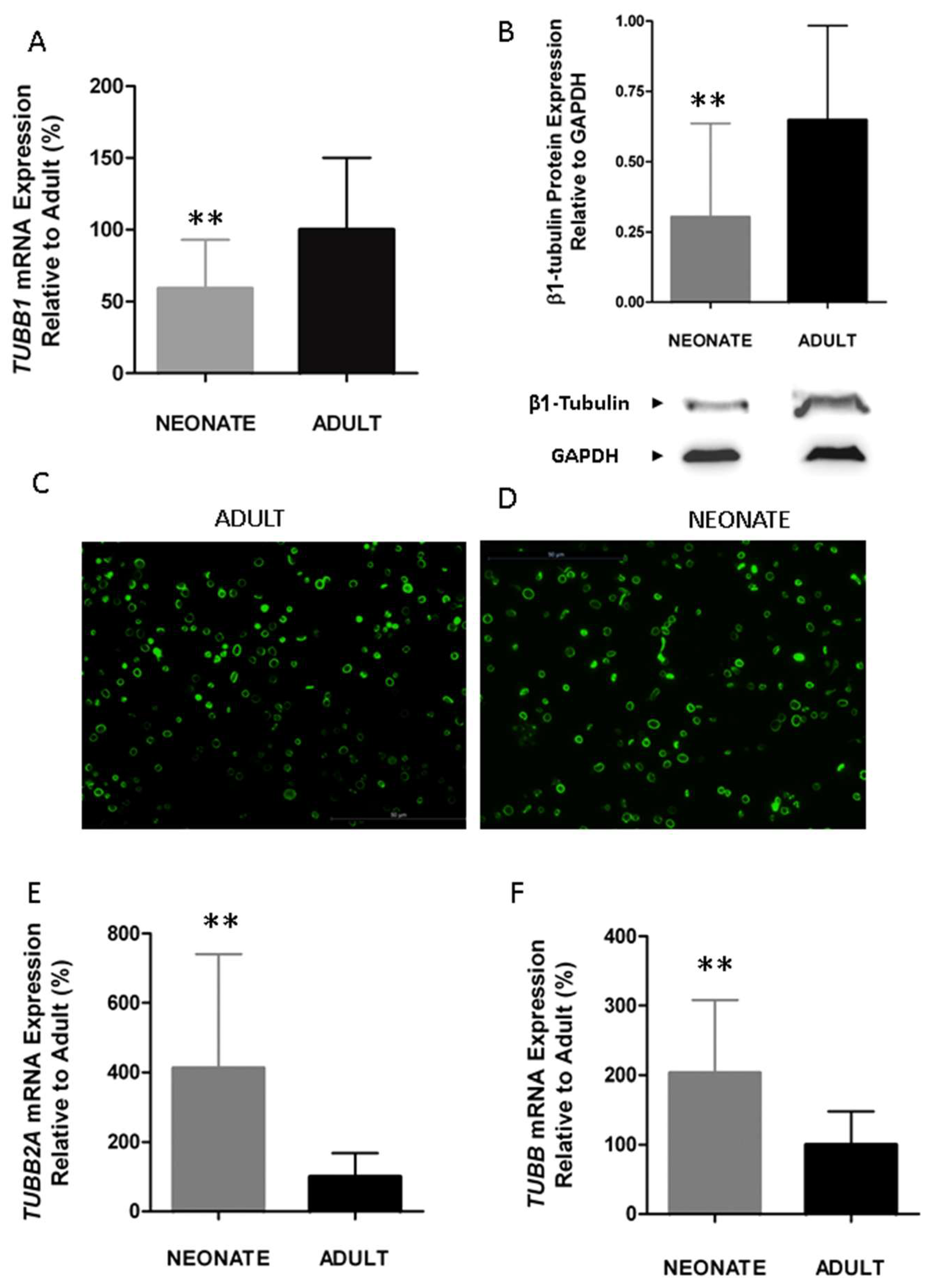
| Protein | Principal Function in Platelets |
|---|---|
| Tubulin α, β, γ | The microtubule coil helps to maintain the resting platelet discoid shape. Upon activation, the microtubule coil centralizes, allowing shape change, granule release and spreading. |
| Dynein | “A subunit of the microtubule motor complex that drives the sliding of the microtubule coil during platelet activation”. |
| Dynactin | A subunit of the microtubule motor complex that increases the processivity of the dynein motor. |
| Kinesin 1 and 4 | “The movement of granules/organelles along microtubules. Antagonizes dynein motor action in resting platelets”. |
| Microtubule-associated proteins (MAPs) 1 and 4 | “Involved in microtubule assembly. Its roles in platelets are unknown”. |
| End-binding protein (EB) | “Binds to the microtubule plus ends to regulate microtubule activity, through post-translational modifications in EB protein itself [34].”. |
| CLIP-associating protein (Clasp) | “Possibly plays a role in microtubule organization”. |
| Ran-binding protein (RanBP) | “The regulation of microtubule coil organization and contraction”. |
| Stathmin | “Microtubule disassembly. No confirmed role in platelet activation”. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuenca-Zamora, E.J.; Ferrer-Marín, F.; Rivera, J.; Teruel-Montoya, R. Tubulin in Platelets: When the Shape Matters. Int. J. Mol. Sci. 2019, 20, 3484. https://doi.org/10.3390/ijms20143484
Cuenca-Zamora EJ, Ferrer-Marín F, Rivera J, Teruel-Montoya R. Tubulin in Platelets: When the Shape Matters. International Journal of Molecular Sciences. 2019; 20(14):3484. https://doi.org/10.3390/ijms20143484
Chicago/Turabian StyleCuenca-Zamora, Ernesto José, Francisca Ferrer-Marín, José Rivera, and Raúl Teruel-Montoya. 2019. "Tubulin in Platelets: When the Shape Matters" International Journal of Molecular Sciences 20, no. 14: 3484. https://doi.org/10.3390/ijms20143484





