Annexins in Adipose Tissue: Novel Players in Obesity
Abstract
:1. Introduction
1.1. Obesity
1.2. Annexins
2. Annexin Expression Patterns in Adipose Tissue and Their Potential Functions in Obesity
2.1. Annexin A1 (AnxA1)
2.2. Annexin A2 (AnxA2)
2.3. Annexin A6 (AnxA6)
2.4. Other Annexins
2.4.1. Annexin A3 (AnxA3)
2.4.2. Annexin A5 (AnxA5)
2.4.3. Annexin A7 (AnxA7)
2.4.4. Annexin A8 (AnxA8)
2.4.5. Other Annexins
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buechler, C.; Schaffler, A. Does global gene expression analysis in type 2 diabetes provide an opportunity to identify highly promising drug targets? Endocr. Metab. Immune Disord. Drug Targets 2007, 7, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2013, 34, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buechler, C.; Krautbauer, S.; Eisinger, K. Adipose tissue fibrosis. World J. Diabetes 2015, 6, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, L.; Kleiner, S.; Wu, J.; Sah, R.; Gupta, R.K.; Banks, A.S.; Cohen, P.; Khandekar, M.J.; Bostrom, P.; Mepani, R.J.; et al. Trpv4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 2012, 151, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, S.; Merlin, J.; Lee, M.K.S.; Murphy, A.J.; Guinamard, R.R. Biology and function of adipose tissue macrophages, dendritic cells and b cells. Atherosclerosis 2018, 271, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K.; Yoneshiro, T.; Nio-Kobayashi, J.; Iwanaga, T.; Miyagawa, M.; Kameya, T.; Nakada, K.; et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes 2009, 58, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Virtanen, K.A.; Lidell, M.E.; Orava, J.; Heglind, M.; Westergren, R.; Niemi, T.; Taittonen, M.; Laine, J.; Savisto, N.J.; Enerback, S.; et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 2009, 360, 1518–1525. [Google Scholar] [CrossRef]
- Krautbauer, S.; Eisinger, K.; Hader, Y.; Buechler, C. Free fatty acids and il-6 induce adipocyte galectin-3 which is increased in white and brown adipose tissues of obese mice. Cytokine 2014, 69, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Veech, R.L. Brown and brite: The fat soldiers in the anti-obesity fight. Front. Physiol. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Worthmann, A.; John, C.; Ruhlemann, M.C.; Baguhl, M.; Heinsen, F.A.; Schaltenberg, N.; Heine, M.; Schlein, C.; Evangelakos, I.; Mineo, C.; et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med. 2017, 23, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Scheja, L.; Heeren, J. Metabolic interplay between white, beige, brown adipocytes and the liver. J. Hepatol. 2016, 64, 1176–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 2017, 13, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, J.; Cereijo, R.; Villarroya, F. An endocrine role for brown adipose tissue? Am. J. Physiol. Endocrinol. Metab. 2013, 305, E567–E572. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.C.; Chiang, Y.T.; Lin, Y.C.; Chang, Y.T.; Lu, C.Y.; Chen, T.Y.; Yeh, C.S. Disruption of lipid raft function increases expression and secretion of monocyte chemoattractant protein-1 in 3t3-l1 adipocytes. PLoS ONE 2016, 11, e0169005. [Google Scholar] [CrossRef]
- Fletcher, R.; Gribben, C.; Ma, X.; Burchfield, J.G.; Thomas, K.C.; Krycer, J.R.; James, D.E.; Fazakerley, D.J. The role of the niemann-pick disease, type c1 protein in adipocyte insulin action. PLoS ONE 2014, 9, e95598. [Google Scholar] [CrossRef]
- Xie, L.; O’Reilly, C.P.; Chapes, S.K.; Mora, S. Adiponectin and leptin are secreted through distinct trafficking pathways in adipocytes. Biochim. Biophys. Acta 2008, 1782, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Buechler, C.; Wanninger, J.; Neumeier, M. Adiponectin, a key adipokine in obesity related liver diseases. World J. Gastroenterol. 2011, 17, 2801–2811. [Google Scholar] [PubMed] [Green Version]
- Cohen, P.; Spiegelman, B.M. Cell biology of fat storage. Mol. Biol. Cell 2016, 27, 2523–2527. [Google Scholar] [CrossRef] [PubMed]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.E.; Morgan, R.O. The annexins. Genome Biol. 2004, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- Gerke, V.; Moss, S.E. Annexins: From structure to function. Physiol. Rev. 2002, 82, 331–371. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T.; Enrich, C. Annexins—Modulators of egf receptor signalling and trafficking. Cell. Signal. 2009, 21, 847–858. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T.; Wason, S.J.; Enrich, C.; Rentero, C. Annexins—Insights from knockout mice. Biol. Chem. 2016, 397, 1031–1053. [Google Scholar] [CrossRef]
- Hayes, M.J.; Rescher, U.; Gerke, V.; Moss, S.E. Annexin-actin interactions. Traffic 2004, 5, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.; Rentero, C.; Cairns, R.; Tebar, F.; Enrich, C.; Grewal, T. Annexins—Scaffolds modulating pkc localization and signaling. Cell. Signal. 2014, 26, 1213–1225. [Google Scholar] [CrossRef]
- Monastyrskaya, K.; Babiychuk, E.B.; Hostettler, A.; Rescher, U.; Draeger, A. Annexins as intracellular calcium sensors. Cell Calcium 2007, 41, 207–219. [Google Scholar] [CrossRef]
- Rentero, C.; Blanco-Munoz, P.; Meneses-Salas, E.; Grewal, T.; Enrich, C. Annexins—Coordinators of cholesterol homeostasis in endocytic pathways. Int. J. Mol. Sci. 2018, 19, 1444. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lizarbe, S.; Lecona, E.; Santiago-Gomez, A.; Olmo, N.; Lizarbe, M.A.; Turnay, J. Structural and lipid-binding characterization of human annexin a13a reveals strong differences with its long a13b isoform. Biol. Chem. 2017, 398, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Neville, M.J.; Edelmann, M.J.; Kessler, B.M.; Karpe, F. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity 2010, 18, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Akasheh, R.T.; Pini, M.; Pang, J.; Fantuzzi, G. Increased adiposity in annexin a1-deficient mice. PLoS ONE 2013, 8, e82608. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Benabdelkamel, H.; Masood, A.; Moustafa, A.; Sallam, R.; Bassas, A.; Duncan, M. Proteomic analysis of mature adipocytes from obese patients in relation to aging. Exp. Gerontol. 2013, 48, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhuo, S.; Zhu, T.; Yao, P.; Yang, M.; Mei, H.; Li, N.; Ma, F.; Wang, J.M.; Chen, S.; et al. Fpr2 deficiency alleviates diet-induced insulin resistance through reducing body weight gain and inhibiting inflammation mediated by macrophage chemotaxis and m1 polarization. Diabetes 2019, 68, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Fredman, G.; Kamaly, N.; Spolitu, S.; Milton, J.; Ghorpade, D.; Chiasson, R.; Kuriakose, G.; Perretti, M.; Farokzhad, O.; Tabas, I. Targeted nanoparticles containing the proresolving peptide ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 2015, 7, 275ra220. [Google Scholar] [CrossRef] [PubMed]
- Kosicka, A.; Cunliffe, A.D.; Mackenzie, R.; Zariwala, M.G.; Perretti, M.; Flower, R.J.; Renshaw, D. Attenuation of plasma annexin a1 in human obesity. FASEB J. 2013, 27, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; D’Acquisto, F. Annexin a1 and glucocorticoids as effectors of the resolution of inflammation. Nat. Rev. Immunol. 2009, 9, 62–70. [Google Scholar] [CrossRef]
- Pietrani, N.T.; Ferreira, C.N.; Rodrigues, K.F.; Perucci, L.O.; Carneiro, F.S.; Bosco, A.A.; Oliveira, M.C.; Pereira, S.S.; Teixeira, A.L.; Alvarez-Leite, J.I.; et al. Proresolving protein annexin a1: The role in type 2 diabetes mellitus and obesity. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 103, 482–489. [Google Scholar] [CrossRef]
- Purvis, G.S.D.; Collino, M.; Loiola, R.A.; Baragetti, A.; Chiazza, F.; Brovelli, M.; Sheikh, M.H.; Collotta, D.; Cento, A.; Mastrocola, R.; et al. Identification of annexina1 as an endogenous regulator of rhoa, and its role in the pathophysiology and experimental therapy of type-2 diabetes. Front. Immunol. 2019, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O. (Re)solving atherosclerosis. Sci. Transl. Med. 2015, 7, 275fs7. [Google Scholar] [CrossRef] [PubMed]
- Warne, J.P.; John, C.D.; Christian, H.C.; Morris, J.F.; Flower, R.J.; Sugden, D.; Solito, E.; Gillies, G.E.; Buckingham, J.C. Gene deletion reveals roles for annexin a1 in the regulation of lipolysis and il-6 release in epididymal adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1264–E1273. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.T.; Nick, H.S.; Frost, S.C. Regulation of annexin i in adipogenesis: Camp-independent action of methylisobutylxanthine. Am. J. Physiol. 1992, 262, C91–C97. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Kim, D.; Jang, J.H.; Ghim, J.; Park, S.; Song, P.; Kwon, Y.; Kim, J.; Hwang, D.; Bae, Y.S.; et al. Proteomic analysis of the palmitate-induced myotube secretome reveals involvement of the annexin a1-formyl peptide receptor 2 (fpr2) pathway in insulin resistance. Mol. Cell. Proteom. MCP 2015, 14, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Biener, Y.; Feinstein, R.; Mayak, M.; Kaburagi, Y.; Kadowaki, T.; Zick, Y. Annexin ii is a novel player in insulin signal transduction. Possible association between annexin ii phosphorylation and insulin receptor internalization. J. Biol. Chem. 1996, 271, 29489–29496. [Google Scholar] [CrossRef] [PubMed]
- Bluher, M.; Wilson-Fritch, L.; Leszyk, J.; Laustsen, P.G.; Corvera, S.; Kahn, C.R. Role of insulin action and cell size on protein expression patterns in adipocytes. J. Biol. Chem. 2004, 279, 31902–31909. [Google Scholar] [CrossRef]
- Bouwman, F.G.; Claessens, M.; van Baak, M.A.; Noben, J.P.; Wang, P.; Saris, W.H.; Mariman, E.C. The physiologic effects of caloric restriction are reflected in the in vivo adipocyte-enriched proteome of overweight/obese subjects. J. Proteome Res. 2009, 8, 5532–5540. [Google Scholar] [CrossRef]
- Bouwman, F.G.; Wang, P.; van Baak, M.; Saris, W.H.; Mariman, E.C. Increased beta-oxidation with improved glucose uptake capacity in adipose tissue from obese after weight loss and maintenance. Obesity 2014, 22, 819–827. [Google Scholar] [CrossRef]
- Caron, D.; Boutchueng-Djidjou, M.; Tanguay, R.M.; Faure, R.L. Annexin a2 is sumoylated on its n-terminal domain: Regulation by insulin. FEBS Lett. 2015, 589, 985–991. [Google Scholar] [CrossRef]
- Huang, J.; Hsia, S.H.; Imamura, T.; Usui, I.; Olefsky, J.M. Annexin ii is a thiazolidinedione-responsive gene involved in insulin-induced glucose transporter isoform 4 translocation in 3t3-l1 adipocytes. Endocrinology 2004, 145, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gomez, Y.; Cruz-Teno, C.; Rangel-Zuniga, O.A.; Peinado, J.R.; Perez-Martinez, P.; Delgado-Lista, J.; Garcia-Rios, A.; Camargo, A.; Vazquez-Martinez, R.; Ortega-Bellido, M.; et al. Effect of dietary fat modification on subcutaneous white adipose tissue insulin sensitivity in patients with metabolic syndrome. Mol. Nutr. Food Res. 2014, 58, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Leggate, M.; Carter, W.G.; Evans, M.J.; Vennard, R.A.; Sribala-Sundaram, S.; Nimmo, M.A. Determination of inflammatory and prominent proteomic changes in plasma and adipose tissue after high-intensity intermittent training in overweight and obese males. J. Appl. Physiol. 2012, 112, 1353–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, P.; Kanaujiya, J.K.; Lochab, S.; Tripathi, S.B.; Sanyal, S.; Behre, G.; Trivedi, A.K. Proteomic analysis of rosiglitazone and guggulsterone treated 3t3-l1 preadipocytes. Mol. Cell. Biochem. 2013, 376, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Raynal, P.; Pollard, H.B.; Cushman, S.W.; Guerre-Millo, M. Unique subcellular distribution of five annexins in resting and insulin-stimulated rat adipose cells. Biochem. Biophys. Res. Commun. 1996, 225, 116–121. [Google Scholar] [CrossRef]
- Rescher, U.; Ludwig, C.; Konietzko, V.; Kharitonenkov, A.; Gerke, V. Tyrosine phosphorylation of annexin a2 regulates rho-mediated actin rearrangement and cell adhesion. J. Cell Sci. 2008, 121, 2177–2185. [Google Scholar] [CrossRef]
- Salameh, A.; Daquinag, A.C.; Staquicini, D.I.; An, Z.; Hajjar, K.A.; Pasqualini, R.; Arap, W.; Kolonin, M.G. Prohibitin/annexin 2 interaction regulates fatty acid transport in adipose tissue. JCI Insight 2016, 1. [Google Scholar] [CrossRef]
- Song, Y.B.; An, Y.R.; Kim, S.J.; Park, H.W.; Jung, J.W.; Kyung, J.S.; Hwang, S.Y.; Kim, Y.S. Lipid metabolic effect of korean red ginseng extract in mice fed on a high-fat diet. J. Sci. Food Agric. 2012, 92, 388–396. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Y.S.; Yin, X.Q.; Yu, G.; Jia, B.L. Anxa2 gene silencing attenuates obesity-induced insulin resistance by suppressing the nf-kappab signaling pathway. Am. J. Physiol. Cell Physiol. 2019, 316, C223–C234. [Google Scholar] [CrossRef]
- Zhao, W.Q.; Chen, G.H.; Chen, H.; Pascale, A.; Ravindranath, L.; Quon, M.J.; Alkon, D.L. Secretion of annexin ii via activation of insulin receptor and insulin-like growth factor receptor. J. Biol. Chem. 2003, 278, 4205–4215. [Google Scholar] [CrossRef]
- Cairns, R.; Alvarez-Guaita, A.; Martinez-Saludes, I.; Wason, S.J.; Hanh, J.; Nagarajan, S.R.; Hosseini-Beheshti, E.; Monastyrskaya, K.; Hoy, A.J.; Buechler, C.; et al. Role of hepatic annexin a6 in fatty acid-induced lipid droplet formation. Exp. Cell Res. 2017, 358, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Cairns, R.; Fischer, A.W.; Blanco-Munoz, P.; Alvarez-Guaita, A.; Meneses-Salas, E.; Egert, A.; Buechler, C.; Hoy, A.J.; Heeren, J.; Enrich, C.; et al. Altered hepatic glucose homeostasis in anxa6-ko mice fed a high-fat diet. PLoS ONE 2018, 13, e0201310. [Google Scholar] [CrossRef] [PubMed]
- Cubells, L.; Vila de Muga, S.; Tebar, F.; Wood, P.; Evans, R.; Ingelmo-Torres, M.; Calvo, M.; Gaus, K.; Pol, A.; Grewal, T.; et al. Annexin a6-induced alterations in cholesterol transport and caveolin export from the golgi complex. Traffic 2007, 8, 1568–1589. [Google Scholar] [CrossRef] [PubMed]
- Enrich, C.; Rentero, C.; de Muga, S.V.; Reverter, M.; Mulay, V.; Wood, P.; Koese, M.; Grewal, T. Annexin a6-linking Ca2+ signaling with cholesterol transport. Biochim. Biophys. Acta 2011, 1813, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Enrich, C.; Rentero, C.; Grewal, T. Annexin a6 in the liver: From the endocytic compartment to cellular physiology. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T.; Evans, R.; Rentero, C.; Tebar, F.; Cubells, L.; de Diego, I.; Kirchhoff, M.F.; Hughes, W.E.; Heeren, J.; Rye, K.A.; et al. Annexin a6 stimulates the membrane recruitment of p120gap to modulate ras and raf-1 activity. Oncogene 2005, 24, 5809–5820. [Google Scholar] [CrossRef]
- Jones, P.G.; Fitzpatrick, S.; Waisman, D.M. Chromaffin granules release calcium on contact with annexin vi: Implications for exocytosis. Biochemistry 1994, 33, 8180–8187. [Google Scholar] [CrossRef] [PubMed]
- Krautbauer, S.; Haberl, E.M.; Eisinger, K.; Pohl, R.; Rein-Fischboeck, L.; Rentero, C.; Alvarez-Guaita, A.; Enrich, C.; Grewal, T.; Buechler, C.; et al. Annexin a6 regulates adipocyte lipid storage and adiponectin release. Mol. Cell. Endocrinol. 2017, 439, 419–430. [Google Scholar] [CrossRef]
- Meier, E.M.; Rein-Fischboeck, L.; Pohl, R.; Wanninger, J.; Hoy, A.J.; Grewal, T.; Eisinger, K.; Krautbauer, S.; Liebisch, G.; Weiss, T.S.; et al. Annexin a6 protein is downregulated in human hepatocellular carcinoma. Mol. Cell. Biochem. 2016, 418, 81–90. [Google Scholar] [CrossRef]
- Monastyrskaya, K.; Babiychuk, E.B.; Hostettler, A.; Wood, P.; Grewal, T.; Draeger, A. Plasma membrane-associated annexin a6 reduces Ca2+ entry by stabilizing the cortical actin cytoskeleton. J. Biol. Chem. 2009, 284, 17227–17242. [Google Scholar] [CrossRef]
- Podszywalow-Bartnicka, P.; Kosiorek, M.; Piwocka, K.; Sikora, E.; Zablocki, K.; Pikula, S. Role of annexin a6 isoforms in catecholamine secretion by pc12 cells: Distinct influence on calcium response. J. Cell. Biochem. 2010, 111, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Rentero, C.; de Muga, S.V.; Alvarez-Guaita, A.; Mulay, V.; Cairns, R.; Wood, P.; Monastyrskaya, K.; Pol, A.; Tebar, F.; et al. Cholesterol transport from late endosomes to the golgi regulates t-snare trafficking, assembly, and function. Mol. Biol. Cell 2011, 22, 4108–4123. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Rentero, C.; Garcia-Melero, A.; Hoque, M.; Vila de Muga, S.; Alvarez-Guaita, A.; Conway, J.R.; Wood, P.; Cairns, R.; Lykopoulou, L.; et al. Cholesterol regulates syntaxin 6 trafficking at trans-golgi network endosomal boundaries. Cell Rep. 2014, 7, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Stogbauer, F.; Weigert, J.; Neumeier, M.; Wanninger, J.; Sporrer, D.; Weber, M.; Schaffler, A.; Enrich, C.; Wood, P.; Grewal, T.; et al. Annexin a6 is highly abundant in monocytes of obese and type 2 diabetic individuals and is downregulated by adiponectin in vitro. Exp. Mol. Med. 2009, 41, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Bluher, M.; Yamamoto, Y.; Norris, A.W.; Berndt, J.; Kralisch, S.; Boucher, J.; Lewis, C.; Kahn, C.R. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl. Acad. Sci. USA 2006, 103, 6676–6681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosch, K.; Kwiatkowski, M.; Hofmann, S.; Schobel, A.; Gruttner, C.; Wurlitzer, M.; Schluter, H.; Herker, E. Quantitative lipid droplet proteome analysis identifies annexin a3 as a cofactor for hcv particle production. Cell Rep. 2016, 16, 3219–3231. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ito, Y.; Sato, A.; Hosono, T.; Niimi, S.; Ariga, T.; Seki, T. Annexin a3 as a negative regulator of adipocyte differentiation. J. Biochem. 2012, 152, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Seok, H.; Park, H.J.; Lee, B.W.; Kim, J.W.; Jung, M.; Lee, S.R.; Park, K.H.; Park, Y.G.; Baik, H.H.; Chung, J.H. Association of annexin a5 polymorphisms with obesity. Biomed. Rep. 2013, 1, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.; Smyth, N.; Ullrich, S.; Yun, F.; Sasse, P.; Hescheler, J.; Fleischmann, B.; Lasek, K.; Brixius, K.; Schwinger, R.H.; et al. Loss of annexin a7 leads to alterations in frequency-induced shortening of isolated murine cardiomyocytes. Mol. Cell. Biol. 2001, 21, 4119–4128. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Lang, P.A.; Shumilina, E.; Qadri, S.M.; Kucherenko, Y.; Kempe, D.S.; Foller, M.; Capasso, A.; Wieder, T.; Gulbins, E.; et al. Enhanced eryptosis of erythrocytes from gene-targeted mice lacking annexin a7. Pflug. Arch. Eur. J. Physiol. 2010, 460, 667–676. [Google Scholar] [CrossRef]
- Luo, D.; Fajol, A.; Umbach, A.T.; Noegel, A.A.; Laufer, S.; Lang, F.; Foller, M. Influence of annexin a7 on insulin sensitivity of cellular glucose uptake. Pflug. Arch. Eur. J. Physiol. 2015, 467, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Mears, D.; Zimliki, C.L.; Atwater, I.; Rojas, E.; Glassman, M.; Leighton, X.; Pollard, H.B.; Srivastava, M. The anx7(+/−) knockout mutation alters electrical and secretory responses to Ca2+-mobilizing agents in pancreatic beta-cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2012, 29, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Atwater, I.; Glasman, M.; Leighton, X.; Goping, G.; Caohuy, H.; Miller, G.; Pichel, J.; Westphal, H.; Mears, D.; et al. Defects in inositol 1,4,5-trisphosphate receptor expression, Ca2+ signaling, and insulin secretion in the anx7(+/−) knockout mouse. Proc. Natl. Acad. Sci. USA 1999, 96, 13783–13788. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, V.; Poeter, M.; Zeuschner, D.; Gerke, V.; Rescher, U. Annexin a8 regulates late endosome organization and function. Mol. Biol. Cell 2008, 19, 5267–5278. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, V.; Ruhe, D.; Gerke, V.; Rescher, U. Annexin a8 displays unique phospholipid and f-actin binding properties. FEBS Lett. 2006, 580, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Heitzig, N.; Brinkmann, B.F.; Koerdt, S.N.; Rosso, G.; Shahin, V.; Rescher, U. Annexin a8 promotes vegf-a driven endothelial cell sprouting. Cell Adhes. Migr. 2017, 11, 275–287. [Google Scholar] [CrossRef]
- Heitzig, N.; Kuhnl, A.; Grill, D.; Ludewig, K.; Schloer, S.; Galla, H.J.; Grewal, T.; Gerke, V.; Rescher, U. Cooperative binding promotes demand-driven recruitment of anxa8 to cholesterol-containing membranes. Biochim. Biophys. Acta. Mol. Cell Biol. Lipids 2018, 1863, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Linder, K.; Arner, P.; Flores-Morales, A.; Tollet-Egnell, P.; Norstedt, G. Differentially expressed genes in visceral or subcutaneous adipose tissue of obese men and women. J. Lipid Res. 2004, 45, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Poeter, M.; Brandherm, I.; Rossaint, J.; Rosso, G.; Shahin, V.; Skryabin, B.V.; Zarbock, A.; Gerke, V.; Rescher, U. Annexin a8 controls leukocyte recruitment to activated endothelial cells via cell surface delivery of cd63. Nat. Commun. 2014, 5, 3738. [Google Scholar] [CrossRef]
- Heinick, A.; Husser, X.; Himmler, K.; Kirchhefer, U.; Nunes, F.; Schulte, J.S.; Seidl, M.D.; Rolfes, C.; Dedman, J.R.; Kaetzel, M.A.; et al. Annexin a4 is a novel direct regulator of adenylyl cyclase type 5. FASEB J. 2015, 29, 3773–3787. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C.; Liu, S.; Qi, H.; Yin, Y.; Liang, R.; Sun, M.Z.; Greenaway, F.T. Annexin a11 in disease. Clin. Chim. Acta Int. J. Clin. Chem. 2014, 431, 164–168. [Google Scholar] [CrossRef] [PubMed]
- D’Acunto, C.W.; Gbelcova, H.; Festa, M.; Ruml, T. The complex understanding of annexin a1 phosphorylation. Cell. Signal. 2014, 26, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J.; Moss, S.E. Annexins and disease. Biochem. Biophys. Res. Commun. 2004, 322, 1166–1170. [Google Scholar] [CrossRef]
- Rescher, U.; Gerke, V. Annexins—Unique membrane binding proteins with diverse functions. J. Cell Sci. 2004, 117, 2631–2639. [Google Scholar] [CrossRef] [PubMed]
- Claria, J.; Dalli, J.; Yacoubian, S.; Gao, F.; Serhan, C.N. Resolvin d1 and resolvin d2 govern local inflammatory tone in obese fat. J. Immunol. 2012, 189, 2597–2605. [Google Scholar] [CrossRef] [PubMed]
- Buechler, C.; Pohl, R.; Aslanidis, C. Pro-resolving molecules-new approaches to treat sepsis? Int. J. Mol. Sci. 2017, 18, 476. [Google Scholar] [CrossRef] [PubMed]
- Claria, J.; Nguyen, B.T.; Madenci, A.L.; Ozaki, C.K.; Serhan, C.N. Diversity of lipid mediators in human adipose tissue depots. Am. J. Physiol. Cell Physiol. 2013, 304, C1141–C1149. [Google Scholar] [CrossRef] [PubMed]
- Veugelers, M.; Cat, B.D.; Muyldermans, S.Y.; Reekmans, G.; Delande, N.; Frints, S.; Legius, E.; Fryns, J.P.; Schrander-Stumpel, C.; Weidle, B.; et al. Mutational analysis of the gpc3/gpc4 glypican gene cluster on xq26 in patients with simpson-golabi-behmel syndrome: Identification of loss-of-function mutations in the gpc3 gene. Hum. Mol. Genet. 2000, 9, 1321–1328. [Google Scholar] [CrossRef]
- Debril, M.B.; Renaud, J.P.; Fajas, L.; Auwerx, J. The pleiotropic functions of peroxisome proliferator-activated receptor gamma. J. Mol. Med. 2001, 79, 30–47. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, Y.; Kar, S.; Kanchi, M.M.; Arora, S.; Kim, J.E.; Koh, P.F.; Yousef, E.; Samy, R.P.; Shanmugam, M.K.; et al. Ppargamma ligand-induced annexin a1 expression determines chemotherapy response via deubiquitination of death domain kinase rip in triple-negative breast cancers. Mol. Cancer Ther. 2017, 16, 2528–2542. [Google Scholar] [CrossRef]
- Sawmynaden, P.; Perretti, M. Glucocorticoid upregulation of the annexin-a1 receptor in leukocytes. Biochem. Biophys. Res. Commun. 2006, 349, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, C.M.; Gomez-Llorente, C.; Tofe, I.; Gil-Campos, M.; Canete, R.; Gil, A. Genome-wide expression in visceral adipose tissue from obese prepubertal children. Int. J. Mol. Sci. 2015, 16, 7723–7737. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.; D’Acquisto, F.; Pederzoli-Ribeil, M.; Lavagno, L.; Flower, R.J.; Witko-Sarsat, V.; Perretti, M. Annexin 1 cleavage in activated neutrophils: A pivotal role for proteinase 3. J. Biol. Chem. 2007, 282, 29998–30004. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, I.; Sutti, S.; Jindal, A.; Vacchiano, M.; Bozzola, C.; Reutelingsperger, C.; Kusters, D.; Bena, S.; Parola, M.; Paternostro, C.; et al. Endogenous annexin a1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology 2014, 60, 531–544. [Google Scholar] [CrossRef]
- Hiramoto, H.; Dansako, H.; Takeda, M.; Satoh, S.; Wakita, T.; Ikeda, M.; Kato, N. Annexin a1 negatively regulates viral rna replication of hepatitis c virus. Acta Med. Okayama 2015, 69, 71–78. [Google Scholar] [PubMed]
- Mauvais-Jarvis, F.; Kulkarni, R.N.; Kahn, C.R. Knockout models are useful tools to dissect the pathophysiology and genetics of insulin resistance. Clin. Endocrinol. 2002, 57, 1–9. [Google Scholar] [CrossRef]
- Feng, R.; Luo, C.; Li, C.; Du, S.; Okekunle, A.P.; Li, Y.; Chen, Y.; Zi, T.; Niu, Y. Free fatty acids profile among lean, overweight and obese non-alcoholic fatty liver disease patients: A case-control study. Lipids Health Disease 2017, 16, 165. [Google Scholar] [CrossRef]
- Arzouni, A.A.; Vargas-Seymour, A.; Rackham, C.L.; Dhadda, P.; Huang, G.C.; Choudhary, P.; Nardi, N.; King, A.J.F.; Jones, P.M. Mesenchymal stromal cells improve human islet function through released products and extracellular matrix. Clin. Sci. 2017, 131, 2835–2845. [Google Scholar] [CrossRef] [Green Version]
- Bharadwaj, A.; Bydoun, M.; Holloway, R.; Waisman, D. Annexin a2 heterotetramer: Structure and function. Int. J. Mol. Sci. 2013, 14, 6259–6305. [Google Scholar] [CrossRef]
- Hedhli, N.; Falcone, D.J.; Huang, B.; Cesarman-Maus, G.; Kraemer, R.; Zhai, H.; Tsirka, S.E.; Santambrogio, L.; Hajjar, K.A. The annexin a2/s100a10 system in health and disease: Emerging paradigms. J. Biomed. Biotechnol. 2012, 2012, 406273. [Google Scholar] [CrossRef]
- Bydoun, M.; Waisman, D.M. On the contribution of s100a10 and annexin a2 to plasminogen activation and oncogenesis: An enduring ambiguity. Future Oncol. 2014, 10, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Hajjar, K.A. Annexin a2 system in human biology: Cell surface and beyond. Semin. Thromb. Hemost. 2013, 39, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hao, J.W.; Wang, X.; Guo, H.; Sun, H.H.; Lai, X.Y.; Liu, L.Y.; Zhu, M.; Wang, H.Y.; Li, Y.F.; et al. Dhhc4 and dhhc5 facilitate fatty acid uptake by palmitoylating and targeting cd36 to the plasma membrane. Cell Rep. 2019, 26, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Pons, M.; Ihrke, G.; Koch, S.; Biermer, M.; Pol, A.; Grewal, T.; Jackle, S.; Enrich, C. Late endocytic compartments are major sites of annexin vi localization in nrk fibroblasts and polarized wif-b hepatoma cells. Exp. Cell Res. 2000, 257, 33–47. [Google Scholar] [CrossRef] [PubMed]
- de Diego, I.; Schwartz, F.; Siegfried, H.; Dauterstedt, P.; Heeren, J.; Beisiegel, U.; Enrich, C.; Grewal, T. Cholesterol modulates the membrane binding and intracellular distribution of annexin 6. J. Biol. Chem. 2002, 277, 32187–32194. [Google Scholar] [CrossRef] [PubMed]
- Futter, C.E.; White, I.J. Annexins and endocytosis. Traffic 2007, 8, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Grewal, T.; Heeren, J.; Mewawala, D.; Schnitgerhans, T.; Wendt, D.; Salomon, G.; Enrich, C.; Beisiegel, U.; Jackle, S. Annexin vi stimulates endocytosis and is involved in the trafficking of low density lipoprotein to the prelysosomal compartment. J. Biol. Chem. 2000, 275, 33806–33813. [Google Scholar] [CrossRef]
- Skrahina, T.; Piljic, A.; Schultz, C. Heterogeneity and timing of translocation and membrane-mediated assembly of different annexins. Exp. Cell Res. 2008, 314, 1039–1047. [Google Scholar] [CrossRef]
- Freye-Minks, C.; Kretsinger, R.H.; Creutz, C.E. Structural and dynamic changes in human annexin vi induced by a phosphorylation-mimicking mutation, t356d. Biochemistry 2003, 42, 620–630. [Google Scholar] [CrossRef]
- Chlystun, M.; Campanella, M.; Law, A.L.; Duchen, M.R.; Fatimathas, L.; Levine, T.P.; Gerke, V.; Moss, S.E. Regulation of mitochondrial morphogenesis by annexin a6. PLoS ONE 2013, 8, e53774. [Google Scholar] [CrossRef]
- Turro, S.; Ingelmo-Torres, M.; Estanyol, J.M.; Tebar, F.; Fernandez, M.A.; Albor, C.V.; Gaus, K.; Grewal, T.; Enrich, C.; Pol, A. Identification and characterization of associated with lipid droplet protein 1: A novel membrane-associated protein that resides on hepatic lipid droplets. Traffic 2006, 7, 1254–1269. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.; Martin, S.; Parton, R.G. Lipid droplet-organelle interactions; sharing the fats. Biochim. Biophys. Acta 2009, 1791, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Pani, B.; Singh, B.B. Lipid rafts/caveolae as microdomains of calcium signaling. Cell Calcium 2009, 45, 625–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kioumourtzoglou, D.; Sadler, J.B.; Black, H.L.; Berends, R.; Wellburn, C.; Bryant, N.J.; Gould, G.W. Studies of the regulated assembly of snare complexes in adipocytes. Biochem. Soc. Trans. 2014, 42, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cheng, D.; Liu, L.; Lv, Z.; Liu, K. Tbc1d15 affects glucose uptake by regulating glut4 translocation. Gene 2019, 683, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Komai, A.M.; Brannmark, C.; Musovic, S.; Olofsson, C.S. Pka-independent camp stimulation of white adipocyte exocytosis and adipokine secretion: Modulations by Ca2+ and atp. J. Physiol. 2014, 592, 5169–5186. [Google Scholar] [CrossRef] [PubMed]
- Kandror, K.V.; Pilch, P.F. The sugar is sirved: Sorting glut4 and its fellow travelers. Traffic 2011, 12, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Lindholm, M.; Krogh, M.; Lucas, S.; Larsson, S.; Osmark, P.; Berger, K.; Boren, J.; Fielding, B.; Frayn, K.; et al. Disturbed cholesterol homeostasis in hormone-sensitive lipase-null mice. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E820–E831. [Google Scholar] [CrossRef] [Green Version]
- Rentero Alfonso, C.; Alvarez-Guaita, A.; Moss, S.E.; Grewal, T.; Enrich, C. Annexin a6 is necessary for liver regeneration and glucose homeostasis in 433 mice. Hepatology 2015, 62, 239A. [Google Scholar]
- Matsuda, M.; Shimomura, I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Rev. Endocr. Metab. Disord. 2014, 15, 1–10. [Google Scholar] [CrossRef]
- Alvarez-Guaita, A.; Vila de Muga, S.; Owen, D.M.; Williamson, D.; Magenau, A.; Garcia-Melero, A.; Reverter, M.; Hoque, M.; Cairns, R.; Cornely, R.; et al. Evidence for annexin a6-dependent plasma membrane remodelling of lipid domains. Br. J. Pharmacol. 2015, 172, 1677–1690. [Google Scholar] [CrossRef] [PubMed]
- von Eckardstein, A. Cholesterol efflux from macrophages and other cells. Curr. Opin. Lipidol. 1996, 7, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Sargolzaei, J.; Chamani, E.; Kazemi, T.; Fallah, S.; Soori, H. The role of adiponectin and adipolin as anti-inflammatory adipokines in the formation of macrophage foam cells and their association with cardiovascular diseases. Clin. Biochem. 2018, 54, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schosserer, M.; Grillari, J.; Wolfrum, C.; Scheideler, M. Age-induced changes in white, brite, and brown adipose depots: A mini-review. Gerontology 2018, 64, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Liu, S.; Guo, C.; Hou, Z.; Sun, M.Z. The role of annexin a3 playing in cancers. Clin. Transl. Oncol. 2013, 15, 106–110. [Google Scholar] [CrossRef]
- Lasrich, D.; Bartelt, A.; Grewal, T.; Heeren, J. Apolipoprotein e promotes lipid accumulation and differentiation in human adipocytes. Exp. Cell Res. 2015, 337, 94–102. [Google Scholar] [CrossRef]
- Boersma, H.H.; Kietselaer, B.L.; Stolk, L.M.; Bennaghmouch, A.; Hofstra, L.; Narula, J.; Heidendal, G.A.; Reutelingsperger, C.P. Past, present, and future of annexin a5: From protein discovery to clinical applications. J. Nucl. Med. 2005, 46, 2035–2050. [Google Scholar]
- Peng, B.; Guo, C.; Guan, H.; Liu, S.; Sun, M.Z. Annexin a5 as a potential marker in tumors. Chim. Acta Int. J. Clin. Chem. 2014, 427, 42–48. [Google Scholar] [CrossRef]
- Dubois, T.; Mira, J.P.; Feliers, D.; Solito, E.; Russo-Marie, F.; Oudinet, J.P. Annexin v inhibits protein kinase c activity via a mechanism of phospholipid sequestration. Biochem. J. 1998, 330, 1277–1282. [Google Scholar] [CrossRef]
- Ghislat, G.; Aguado, C.; Knecht, E. Annexin a5 stimulates autophagy and inhibits endocytosis. J. Cell Sci. 2012, 125, 92–107. [Google Scholar] [CrossRef]
- Bouter, A.; Gounou, C.; Berat, R.; Tan, S.; Gallois, B.; Granier, T.; d’Estaintot, B.L.; Poschl, E.; Brachvogel, B.; Brisson, A.R. Annexin-a5 assembled into two-dimensional arrays promotes cell membrane repair. Nat. Commun. 2011, 2, 270. [Google Scholar] [CrossRef] [PubMed]
- Rand, J.H.; Wu, X.X.; Quinn, A.S.; Taatjes, D.J. The annexin a5-mediated pathogenic mechanism in the antiphospholipid syndrome: Role in pregnancy losses and thrombosis. Lupus 2010, 19, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Selbert, S.; Fischer, P.; Pongratz, D.; Stewart, M.; Noegel, A.A. Expression and localization of annexin vii (synexin) in muscle cells. J. Cell Sci. 1995, 108, 85–95. [Google Scholar] [PubMed]
- Kuijpers, G.A.; Lee, G.; Pollard, H.B. Immunolocalization of synexin (annexin vii) in adrenal chromaffin granules and chromaffin cells: Evidence for a dynamic role in the secretory process. Cell Tissue Res. 1992, 269, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Gerelsaikhan, T.; Vasa, P.K.; Chander, A. Annexin a7 and snap23 interactions in alveolar type ii cells and in vitro: A role for Ca2+ and pkc. Biochim. Biophys. Acta 2012, 1823, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Alesutan, I.; Pakladok, T.; Viereck, R.; Feger, M.; Mia, S.; Schonberger, T.; Noegel, A.A.; Gawaz, M.; Lang, F. Annexin a7 deficiency potentiates cardiac nfat activity promoting hypertrophic signaling. Biochem. Biophys. Res. Commun. 2014, 445, 244–249. [Google Scholar] [CrossRef]
- Leighton, X.; Eidelman, O.; Jozwik, C.; Pollard, H.B.; Srivastava, M. Anxa7-gtpase as tumor suppressor: Mechanisms and therapeutic opportunities. Methods Mol. Biol. 2017, 1513, 23–35. [Google Scholar] [PubMed]
- Garcia-Alonso, V.; Titos, E.; Alcaraz-Quiles, J.; Rius, B.; Lopategi, A.; Lopez-Vicario, C.; Jakobsson, P.J.; Delgado, S.; Lozano, J.; Claria, J. Prostaglandin e2 exerts multiple regulatory actions on human obese adipose tissue remodeling, inflammation, adaptive thermogenesis and lipolysis. PLoS ONE 2016, 11, e0153751. [Google Scholar] [CrossRef]
- Pepinsky, R.B.; Hauptmann, R. Detection of vac-beta (annexin-8) in human placenta. FEBS Lett. 1992, 306, 85–89. [Google Scholar] [CrossRef]
- Sarkar, A.; Yang, P.; Fan, Y.H.; Mu, Z.M.; Hauptmann, R.; Adolf, G.R.; Stass, S.A.; Chang, K.S. Regulation of the expression of annexin viii in acute promyelocytic leukemia. Blood 1994, 84, 279–286. [Google Scholar]
- Reutelingsperger, C.P.; van Heerde, W.; Hauptmann, R.; Maassen, C.; van Gool, R.G.; de Leeuw, P.; Tiebosch, A. Differential tissue expression of annexin viii in human. FEBS Lett. 1994, 349, 120–124. [Google Scholar] [CrossRef]
- Hauptmann, R.; Maurer-Fogy, I.; Krystek, E.; Bodo, G.; Andree, H.; Reutelingsperger, C.P. Vascular anticoagulant beta: A novel human Ca2+/phospholipid binding protein that inhibits coagulation and phospholipase a2 activity. Its molecular cloning, expression and comparison with vac-alpha. Eur. J. Biochem. 1989, 185, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hata, H.; Tatemichi, M.; Nakadate, T. Involvement of annexin a8 in the properties of pancreatic cancer. Mol. Carcinog. 2014, 53, 181–191. [Google Scholar] [CrossRef]
- Iglesias, J.M.; Cairney, C.J.; Ferrier, R.K.; McDonald, L.; Soady, K.; Kendrick, H.; Pringle, M.A.; Morgan, R.O.; Martin, F.; Smalley, M.J.; et al. Annexin a8 identifies a subpopulation of transiently quiescent c-kit positive luminal progenitor cells of the ductal mammary epithelium. PLoS ONE 2015, 10, e0119718. [Google Scholar] [CrossRef] [PubMed]
- Oka, R.; Nakashiro, K.; Goda, H.; Iwamoto, K.; Tokuzen, N.; Hamakawa, H. Annexin a8 is a novel molecular marker for detecting lymph node metastasis in oral squamous cell carcinoma. Oncotarget 2016, 7, 4882–4889. [Google Scholar] [CrossRef]
- Lueck, K.; Carr, A.F.; Stampoulis, D.; Gerke, V.; Rescher, U.; Greenwood, J.; Moss, S.E. Regulation of retinal pigment epithelial cell phenotype by annexin a8. Sci. Rep. 2017, 7, 4638. [Google Scholar] [CrossRef] [PubMed]
- Monastyrskaya, K.; Babiychuk, E.B.; Draeger, A. The annexins: Spatial and temporal coordination of signaling events during cellular stress. Cell. Mol. Life Sci. CMLS 2009, 66, 2623–2642. [Google Scholar] [CrossRef]
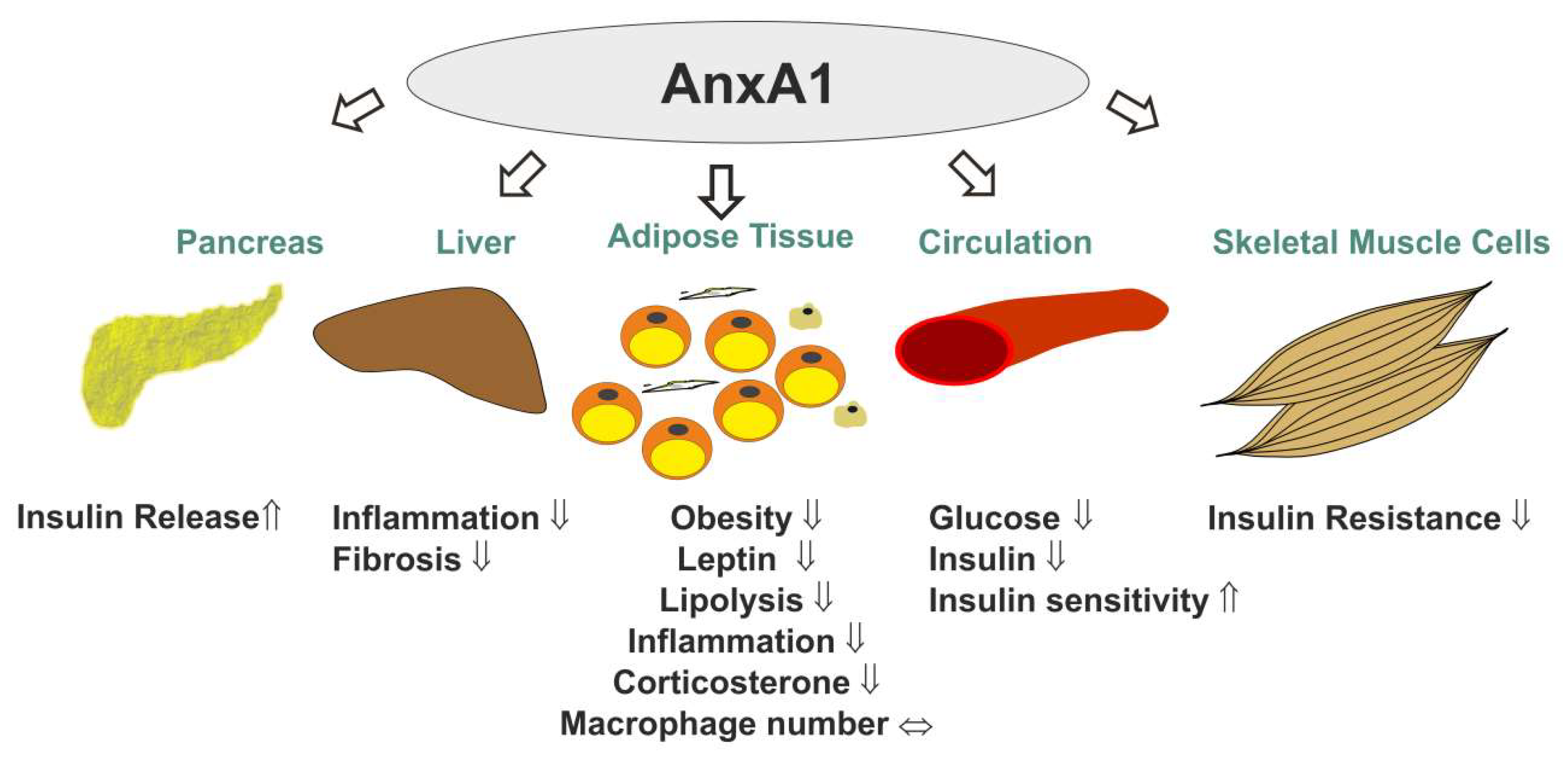

| Name | Structure | Adipose Tissue Expression | Function | References |
|---|---|---|---|---|
| A. Prominent Annexins in Adipose Tissue. | ||||
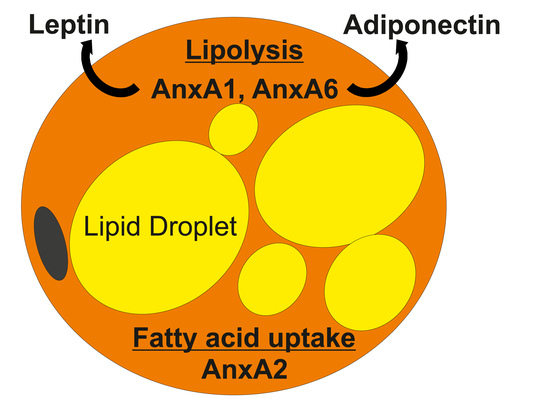
| AnxA1 |  | adipocytes, SV, visceral fat, subcutaneous fat, obesity ↑, HFD ↑, TZDs ↑ | insulin response ↑, obesity ↓, leptin ↓, inflammation ↓ | [33,34,35,36,37,38,39,40,41,42,43,44,45] |
| AnxA2 |  | adipocytes, endothelial cells, macrophages, subcutaneous fat, epididymal fat, mesenteric fat, guggulsterone ↑, TZDs ↑ | GLUT4 translocation, insulin response, glucose uptake, CD36-mediated fatty acid uptake, inflammation ↑, macrophage infiltration ↑, HSL activation | [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] |
| AnxA6 |  | adipocytes, macrophages, subcutaneous fat, perirenal fat, epididymal fat, visceral fat, brown fat, obesity ↑, HFD ↑, oxidative stress ↑ | preadipocyte proliferation ↑, triglyceride storage ↓, adiponectin release ↓, cholesterol-dependent caveolae formation, cholesterol-dependent GLUT4 translocation, cholesterol-dependent adiponectin secretion? | [2,35,47,61,62,63,64,65,66,67,68,69,70,71,72,73,74] |
| B. Other Annexins in Adipose Tissue. | ||||
| AnxA3 |  | adipocytes, SV, subcutaneous fat, intraabdominal fat | adipocyte differentiation ↓, lipid accumulation? | [75,76,77], Geo Profiles; DataSet Record GDS2818 |
| AnxA5 |  | SV, subcutaneous fat, intraabdominal fat | fat deposition, storage or mobilization? | [35,78], Geo Profiles; DataSet Record GDS2818 |
| AnxA7 |  | SV, subcutaneous fat, intraabdominal fat | infiltration of immune cells in dysfunctional adipose tissue? | [79,80,81,82,83], Geo Profiles; DataSet Record GDS2818 |
| AnxA8 |  | adipocytes, SV, subcutaneous fat, intraabdominal fat | cholesterol-dependent caveolae formation, cholesterol-dependent GLUT4 translocation, cholesterol-dependent adiponectin secretion? | [84,85,86,87,88,89], Geo Profiles; DataSet Record GDS2818 |
| C. Insufficiently Studied Annexins in Adipose Tissue. | ||||
| AnxA4 |  | N/A | lipolysis? | [90] |
| AnxA9 |  | N/A | ? | |
| AnxA10 |  | adipocytes, SV, subcutanous fat, intraabdominal fat | ? | |
| AnxA11 |  | adipocytes, SV, subcutanous fat, intraabdominal fat | fatty acid release, adipokine secretion? | [91] |
| AnxA13a |  | N/A | ? | |
| AnxA13b |  | N/A | ? | |
 ,
,  and
and  indicate higher, lower and unchanged mRNA levels, respectively, in adipocytes relative to stromal vascular cells (SVC) or in subcutaneous (sc) fat compared to intraabdominal (intra) fat. The mRNA expression data for AnxA4, AnxA9 and AnxA13 in fat tissue were not available.
indicate higher, lower and unchanged mRNA levels, respectively, in adipocytes relative to stromal vascular cells (SVC) or in subcutaneous (sc) fat compared to intraabdominal (intra) fat. The mRNA expression data for AnxA4, AnxA9 and AnxA13 in fat tissue were not available.
 ,
,  and
and  indicate higher, lower and unchanged mRNA levels, respectively, in adipocytes relative to stromal vascular cells (SVC) or in subcutaneous (sc) fat compared to intraabdominal (intra) fat. The mRNA expression data for AnxA4, AnxA9 and AnxA13 in fat tissue were not available.
indicate higher, lower and unchanged mRNA levels, respectively, in adipocytes relative to stromal vascular cells (SVC) or in subcutaneous (sc) fat compared to intraabdominal (intra) fat. The mRNA expression data for AnxA4, AnxA9 and AnxA13 in fat tissue were not available.| Subcutaneous Fat Adipocyte/SVC | Intrabdominal Fat Adipocyte/SVC | Adipocytes Sc/Intra | SVC Sc/Intra | |
|---|---|---|---|---|
| AnxA3 |  |  |  |  |
| AnxA5 |  |  |  |  |
| AnxA7 |  |  |  |  |
| AnxA8 |  |  |  |  |
| AnxA10 |  |  |  |  |
| AnxA11 |  |  |  |  |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grewal, T.; Enrich, C.; Rentero, C.; Buechler, C. Annexins in Adipose Tissue: Novel Players in Obesity. Int. J. Mol. Sci. 2019, 20, 3449. https://doi.org/10.3390/ijms20143449
Grewal T, Enrich C, Rentero C, Buechler C. Annexins in Adipose Tissue: Novel Players in Obesity. International Journal of Molecular Sciences. 2019; 20(14):3449. https://doi.org/10.3390/ijms20143449
Chicago/Turabian StyleGrewal, Thomas, Carlos Enrich, Carles Rentero, and Christa Buechler. 2019. "Annexins in Adipose Tissue: Novel Players in Obesity" International Journal of Molecular Sciences 20, no. 14: 3449. https://doi.org/10.3390/ijms20143449