Growth Hormone Deficiency Following Traumatic Brain Injury
Abstract
:1. Introduction
2. Prevalence
3. GH/IGF-1 and the Brain
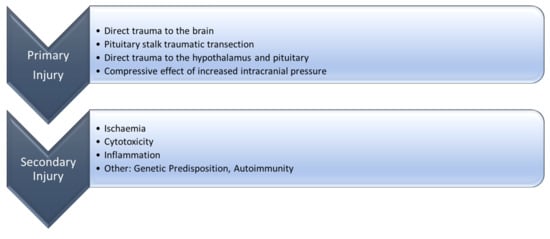
4. Pathophysiology of GHD after TBI
4.1. Molecular Mechanisms of the Growth Hormone Deficiency after Traumatic Brain Injury
4.1.1. Ischemia
4.1.2. Cytotoxicity
4.1.3. Inflammation
4.1.4. Other Possible Mechanisms
5. Signs and Symptoms
6. Mild Traumatic Brain Injury
7. Evidence for Treatment of Post-Traumatic GHD
7.1. Cognition
7.2. Metabolic and Cardiovascular
7.3. Bone
7.4. Quality of Life (QoL)
8. Who and When to Test?
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bondanelli, M.; Ambrosio, M.R.; Zatelli, M.C.; De Marinis, L.; degli Uberti, E.C. Hypopituitarism after traumatic brain injury. Eur. J. Endocrinol. 2005, 152, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 27, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cyran, E. Hypophysenschadigung durch Schadelbasisfraktur. Dtsch. Med. Wochenschr. 1918, 44, 1261. [Google Scholar]
- Kreber, L.A.; Griesbach, G.S.; Ashley, M.J. Detection of Growth Hormone Deficiency in Adults with Chronic Traumatic Brain Injury. J. Neurotrauma 2016, 33, 1607–1613. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J.; Kreitschmann-Andermahr, I.; Ghigo, E.; Stalla, G.K.; Agha, A. Hypothalamopituitary dysfunction following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A systematic review. JAMA 2007, 298, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Olivecrona, Z.; Dahlqvist, P.; Koskinen, L.O. Acute neuro-endocrine profile and prediction of outcome after severe brain injury. Scand. J. Trauma Resusc. Emerg. Med. 2013, 21, 33. [Google Scholar] [CrossRef]
- Tanriverdi, F.; Senyurek, H.; Unluhizarci, K.; Selcuklu, A.; Casanueva, F.F.; Kelestimur, F. High risk of hypopituitarism after traumatic brain injury: A prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J. Clin. Endocrinol. Metab. 2006, 91, 2105–2111. [Google Scholar] [CrossRef]
- Agha, A.; Rogers, B.; Mylotte, D.; Taleb, F.; Tormey, W.; Phillips, J.; Thompson, C.J. Neuroendocrine dysfunction in the acute phase of traumatic brain injury. Clin. Endocrinol. (Oxf.) 2004, 60, 584–591. [Google Scholar] [CrossRef]
- Agha, A.; Rogers, B.; Sherlock, M.; O’Kelly, P.; Tormey, W.; Phillips, J.; Thompson, C.J. Anterior pituitary dysfunction in survivors of traumatic brain injury. J. Clin. Endocrinol. Metab. 2004, 89, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Aimaretti, G.; Ambrosio, M.R.; Di Somma, C.; Gasperi, M.; Cannavò, S.; Scaroni, C.; Fusco, A.; Del Monte, P.; De Menis, E.; Faustini-Fustini, M.; et al. Residual pituitary function after brain injury-induced hypopituitarism: A prospective 12-month study. J. Clin. Endocrinol. Metab. 2005, 90, 6085–6092. [Google Scholar] [CrossRef] [PubMed]
- Moreau, O.K.; Yollin, E.; Merlen, E.; Daveluy, W.; Rousseaux, M. Lasting pituitary hormone deficiency after traumatic brain injury. J. Neurotrauma 2012, 29, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Klose, M.; Juul, A.; Poulsgaard, L.; Kosteljanetz, M.; Brennum, J.; Feldt-Rasmussen, U. Prevalence and predictive factors of post-traumatic hypopituitarism. Clin. Endocrinol. (Oxf.) 2007, 67, 193–201. [Google Scholar] [CrossRef]
- Abadi, M.R.; Ghodsi, M.; Merazin, M.; Roozbeh, H. Pituitary function impairment after moderate traumatic brain injury. Acta Med. Iran. 2011, 49, 438–441. [Google Scholar] [PubMed]
- Bondanelli, M.; De Marinis, L.; Ambrosio, M.R.; Monesi, M.; Valle, D.; Zatelli, M.C.; Fusco, A.; Bianchi, A.; Farneti, M.; Uberti, E.C. Occurrence of pituitary dysfunction following traumatic brain injury. J. Neurotrauma 2004, 21, 685–696. [Google Scholar] [CrossRef]
- Hannon, M.J.; Crowley, R.K.; Behan, L.A.; O’Sullivan, E.P.; O’Brien, M.M.; Sherlock, M.; Rawluk, D.; O’Dwyer, R.; Tormey, W.; Thompson, C.J. Acute glucocorticoid deficiency and diabetes insipidus are common after acute traumatic brain injury and predict mortality. J. Clin. Endocrinol. Metab. 2013, 98, 3229–3237. [Google Scholar] [CrossRef]
- Krahulik, D.; Zapletalova, J.; Frysak, Z.; Vaverka, M. Dysfunction of hypothalamic-hypophysial axis after traumatic brain injury in adults. J. Neurosurg. 2010, 113, 581–584. [Google Scholar] [CrossRef]
- Schneider, H.J.; Schneider, M.; Saller, B.; Petersenn, S.; Uhr, M.; Husemann, B.; von Rosen, F.; Stalla, G.K. Prevalence of anterior pituitary insufficiency 3 and 12 months after traumatic brain injury. Eur. J. Endocrinol. 2006, 154, 259–265. [Google Scholar] [CrossRef]
- Zhai, Q.; Lai, Z.; Roos, P.; Nyberg, F. Characterization of growth hormone binding sites in rat brain. Acta Paediatr. Suppl. 1994, 83, 92–95. [Google Scholar] [CrossRef]
- Lai, Z.; Roos, P.; Zhai, O.; Olsson, Y.; Fhölenhag, K.; Larsson, C.; Nyberg, F. Age-related reduction of human growth hormone-binding sites in the human brain. Brain Res. 1993, 621, 260–266. [Google Scholar] [CrossRef]
- Lun, M.P.; Monuki, E.S.; Lehtinen, M.K. Development and functions of the choroid plexus-cerebrospinal fluid system. Nat. Rev. Neurosci. 2015, 16, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, F. Growth hormone in the brain: Characteristics of specific brain targets for the hormone and their functional significance. Front. Neuroendocr. 2000, 21, 330–348. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Flanagan, J.U.; Langley, R.J.; Hay, M.P.; Perry, J.K. Targeting growth hormone function: Strategies and therapeutic applications. Signal Transduct. Target. 2019, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Lobie, P.E.; Zhu, T.; Graichen, R.; Goh, E. Growth hormone, insulin-like growth factor I and the CNS: Localization, function and mechanism of action. Growth Horm. IGF Res. 2000, 10, S51–S56. [Google Scholar] [CrossRef]
- Devesa, J.; Reimunde, P.; Devesa, P.; Barberá, M.; Arce, V. Growth hormone (GH) and brain trauma. Horm. Behav. 2013, 63, 331–344. [Google Scholar] [CrossRef]
- Svensson, A.L.; Bucht, N.; Hallberg, M.; Nyberg, F. Reversal of opiate-induced apoptosis by human recombinant growth hormone in murine fetus primary hippocampal neuronal cell cultures. Proc. Natl. Acad. Sci. USA 2008, 105, 7304–7308. [Google Scholar] [CrossRef] [PubMed]
- Nylander, E.; Gronbladh, A.; Zelleroth, S.; Diwakarla, S.; Nyberg, F.; Hallberg, M. Growth hormone is protective against acute methadone-induced toxicity by modulating the NMDA receptor complex. Neuroscience 2016, 339, 538–547. [Google Scholar] [CrossRef]
- Devesa, J.; Almengló, C.; Devesa, P. Multiple Effects of Growth Hormone in the Body: Is it Really the Hormone for Growth? Clin. Med. Insights Endocrinol. Diabetes 2016, 9, 47–71. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Estévez, V.; Defterali, Ç.; Vicario-Abejón, C. IGF-I: A Key Growth Factor that Regulates Neurogenesis and Synaptogenesis from Embryonic to Adult Stages of the Brain. Front. Neurosci. 2016, 10, 52. [Google Scholar] [CrossRef] [Green Version]
- Russo, V.C.; Gluckman, P.D.; Feldman, E.L.; Werther, G.A. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr. Rev. 2005, 26, 916–943. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Kastin, A.J. Interactions of IGF-1 with the blood-brain barrier in vivo and in situ. Neuroendocrinology 2000, 72, 171–178. [Google Scholar] [CrossRef] [PubMed]
- O’Kusky, J.; Ye, P. Neurodevelopmental effects of insulin-like growth factor signaling. Front. Neuroendocr. 2012, 33, 230–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trejo, J.L.; Carro, E.; Lopez-Lopez, C.; Torres-Aleman, I. Role of serum insulin-like growth factor I in mammalian brain aging. Growth Horm. IGF Res. 2004, 14 (Suppl. A), S39–S43. [Google Scholar] [CrossRef]
- Aberg, N.D.; Brywe, K.G.; Isgaard, J. Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. Sci. World J. 2006, 6, 53–80. [Google Scholar] [CrossRef]
- Aberg, N.D.; Lind, J.; Isgaard, J.; Georg Kuhn, H. Peripheral growth hormone induces cell proliferation in the intact adult rat brain. Growth Horm. IGF Res. 2010, 20, 264–269. [Google Scholar] [CrossRef]
- Zhang, H.; Han, M.; Zhang, X.; Sun, X.; Ling, F. The effect and mechanism of growth hormone replacement on cognitive function in rats with traumatic brain injury. PLoS ONE 2014, 9, e108518. [Google Scholar] [CrossRef]
- Shin, D.H.; Lee, E.; Kim, J.W.; Kwon, B.S.; Jung, M.K.; Jee, Y.H.; Kim, J.; Bae, S.R.; Chang, Y.P. Protective effect of growth hormone on neuronal apoptosis after hypoxia-ischemia in the neonatal rat brain. Neurosci. Lett. 2004, 354, 64–68. [Google Scholar] [CrossRef]
- Morel, G.R.; León, M.L.; Uriarte, M.; Reggiani, P.C.; Goya, R.G. Therapeutic potential of IGF-I on hippocampal neurogenesis and function during aging. Neurogenesis (Austin) 2016, 4, e1259709. [Google Scholar] [CrossRef] [Green Version]
- Ashpole, N.M.; Sanders, J.E.; Hodges, E.L.; Yan, H.; Sonntag, W.E. Review Growth hormone, insulin-like growth factor-1 and the aging brain. Exp. Gerontol. 2015, 68, 76–81. [Google Scholar] [CrossRef]
- Dusick, J.R.; Wang, C.; Cohan, P.; Swerdloff, R.; Kelly, D.F. Pathophysiology of hypopituitarism in the setting of brain injury. Pituitary 2012, 15, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Bavisetty, S.; Bavisetty, S.; McArthur, D.L.; Dusick, J.R.; Wang, C.; Cohan, P.; Boscardin, W.J.; Swerdloff, R.; Levin, H.; Chang, D.J.; et al. Chronic hypopituitarism after traumatic brain injury: Risk assessment and relationship to outcome. Neurosurgery 2008, 62, 1080. [Google Scholar] [CrossRef] [PubMed]
- Veenith, T.; Goon, S.S.H.; Burnstein, R.M. Molecular mechanisms of traumatic brain injury: The missing link in management. World J. Emerg. Surg. 2009, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, R.N.; Fisher, R.S. Pituitary lesions in craniocerebral injuries. Arch. Pathol 1969, 88, 242–248. [Google Scholar] [PubMed]
- Ceballos, R. Pituitary changes in head trauma (analysis of 102 consecutive cases of head injury). Ala. J. Med. Sci. 1966, 3, 185–198. [Google Scholar]
- Sav, A.; Rotondo, F.; Syro, L.V.; Serna, C.A.; Kovacs, K. Pituitary pathology in traumatic brain injury: A review. Pituitary 2019, 22, 201–211, (ahead of print). [Google Scholar] [CrossRef]
- Xuereb, G.P.; Prichard, M.M.; Daniel, P.M. The arterial supply and venous drainage of the human hypophysis cerebri. Q. J. Exp. Physiol. Cogn. Med. Sci. 1954, 39, 199–217. [Google Scholar] [CrossRef]
- Gorczyca, W.; Hardy, J. Arterial supply of the human anterior pituitary gland. Neurosurgery 1987, 20, 369–378. [Google Scholar] [CrossRef]
- Scranton, R.A.; Baskin, D.S. Impaired pituitary axes following traumatic brain injury. J. Clin. Med. 2015, 4, 1463–1479. [Google Scholar] [CrossRef]
- Popovic, V. GH deficiency as the most common pituitary defect after TBI: Clinical implications. Pituitary 2005, 8, 239–243. [Google Scholar] [CrossRef]
- Maiya, B.; Newcombe, V.; Nortje, J.; Bradley, P.; Bernard, F.; Chatfield, D.; Outtrim, J.; Hutchinson, P.; Matta, B.; Antoun, N.; et al. Magnetic resonance imaging changes in the pituitary gland following acute traumatic brain injury. Intensive Care Med. 2008, 34, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Schneider, H.J.; Sämann, P.G.; Schneider, M.; Croce, C.G.; Corneli, G.; Sievers, C.; Ghigo, E.; Stalla, G.K.; Aimaretti, G. Pituitary imaging abnormalities in patients with and without hypopituitarism after traumatic brain injury. J. Endocrinol. Investig. 2007, 30, RC9–RC12. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, A.; Bellastella, G.; Maiorino, M.I.; Costantino, A.; Cirillo, P.; Longo, M.; Pernice, V.; Bellastella, A.; Esposito, K. The role of autoimmunity in pituitary dysfunction due to traumatic brain injury. Pituitary 2019, 22, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Kovacs, K.; Scheithauer, B.W.; Pfeifer, E.A.; Cusimano, M. Histologic study of the human pituitary gland in acute traumatic brain injury. Brain Inj. 2007, 21, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Bullock, R.; Zauner, A.; Woodward, J.J.; Myseros, J.; Choi, S.C.; Ward, J.D.; Marmarou, A.; Young, H.F. Factors affecting excitatory amino acid release following severe human head injury. J. Neurosurg. 1998, 89, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, B.S.; Stein, D.G. Traumatic Brain Injury Causes Long-term reduction in serum growth hormone and persistent astrocytosis in the cortico-hypothalamo-pituitary axis of adult male rats. J. Neurotrauma 2009, 26, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Evans, C.O.; Hoffman, S.W.; Oyesiku, N.M.; Stein, D.G. Progesterone and allopregnanolone reduce inflammatory cytokines after traumatic brain injury. Exp. Neurol. 2004, 189, 404–412. [Google Scholar] [CrossRef]
- Bach-y-Rita, P. Theoretical basis for brain plasticity after a TBI. Brain Inj. 2003, 17, 643–651. [Google Scholar] [CrossRef]
- Tanriverdi, F.; De Bellis, A.; Bizzarro, A.; Sinisi, A.A.; Bellastella, G.; Pane, E.; Bellastella, A.; Unluhizarci, K.; Selcuklu, A.; Casanueva, F.F.; et al. Antipituitary antibodies after traumatic brain injury: Is head trauma-induced pituitary dysfunction associated with autoimmunity? Eur. J. Endocrinol. 2008, 159, 7–13. [Google Scholar] [CrossRef]
- Tanriverdi, F.; De Bellis, A.; Ulutabanca, H.; Bizzarro, A.; Sinisi, A.A.; Bellastella, G.; Amoresano Paglionico, V.; Dalla Mora, L.; Selcuklu, A.; Unluhizarci, K.; et al. A five-year prospective investigation of anterior pituitary function after traumatic brain injury: Is hypopituitarism long-term after head trauma associated with autoimmunity? J. Neurotrauma 2013, 30, 1426–1433. [Google Scholar] [CrossRef]
- Tanriverdi, F.; Unluhizarci, K.; Karaca, Z.; Casanueva, F.F.; Kelestimur, F. Hypopituitarism due to sports related head trauma and the effects of growth hormone replacement in retired amateur boxers. Pituitary 2010, 13, 111–114. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, A.; Kelestimur, F.; Sinisi, A.A.; Ruocco, G.; Tirelli, G.; Battaglia, M.; Bellastella, G.; Conzo, G.; Tanriverdi, F.; Unluhizarci, K.; et al. Anti-hypothalamus and anti-pituitary antibodies may contribute to perpetuate the hypopituitarism in patients with Sheehan’s syndrome. Eur. J. Endocrinol. 2008, 158, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Cocco, C.; Brancia, C.; Corda, G.; Ferri, G.L. The hypothalamic-pituitary axis and autoantibody related disorders. Int. J. Mol. Sci. 2017, 18, 2322. [Google Scholar] [CrossRef] [PubMed]
- Bennett, E.R.; Reuter-Rice, K.; Laskowitz, D.T. Genetic influences in traumatic brain injury. In Translational Research in Traumatic Brain Injury; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2016. [Google Scholar]
- Tanriverdi, F.; Taheri, S.; Ulutabanca, H.; Caglayan, A.O.; Ozkul, Y.; Dundar, M.; Selcuklu, A.; Unluhizarci, K.; Casanueva, F.F.; Kelestimur, F. Apolipoprotein E3/E3 genotype decreases the risk of pituitary dysfunction after traumatic brain injury due to various causes: Preliminary data. J. Neurotrauma 2008, 25, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Popovic, V.; Pekic, S.; Pavlovic, D.; Maric, N.; Jasovic-Gasic, M.; Djurovic, B.; Medic Stojanoska, M.; Zivkovic, V.; Stojanovic, M.; Doknic, M.; et al. Hypopituitarism as a consequence of traumatic brain injury (TBI) and its possible relation with cognitive disabilities and mental distress. J. Endocrinol. Investig. 2004, 27, 1048–1054. [Google Scholar] [CrossRef]
- Reed, M.L.; Merriam, G.R.; Kargi, A.Y. Adult growth hormone deficiency—Benefits, side effects, and risks of growth hormone replacement. Front. Endocrinol. (Lausanne) 2013, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- Tanriverdi, F.; Unluhizarci, K.; Coksevim, B.; Selcuklu, A.; Casanueva, F.F.; Kelestimur, F. Kickboxing sport as a new cause of traumatic brain injury-mediated hypopituitarism. Clin. Endocrinol. (Oxf.) 2007, 66, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Undurti, A.; Colasurdo, E.A.; Sikkema, C.L.; Schultz, J.S.; Peskind, E.R.; Pagulayan, K.F.; Wilkinson, C.W. Chronic Hypopituitarism Associated with Increased Postconcussive Symptoms Is Prevalent after Blast-Induced Mild Traumatic Brain Injury. Front. Neurol. 2018, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Giuliano, S.; Talarico, S.; Bruno, L.; Nicoletti, F.B.; Ceccotti, C.; Belfiore, A. Growth hormone deficiency and hypopituitarism in adults after complicated mild traumatic brain injury. Endocrine 2017, 58, 115–123. [Google Scholar] [CrossRef]
- Kelestimur, F.; Tanriverdi, F.; Atmaca, H.; Unluhizarci, K.; Selcuklu, A.; Casanueva, F.F. Boxing as a sport activity associated with isolated GH deficiency. J. Endocrinol. Investig. 2004, 27, RC28–RC32. [Google Scholar] [CrossRef]
- Tanriverdi, F.; Kelestimur, F. Pituitary dysfunction following traumatic brain injury: Clinical perspectives. Neuropsychiatr. Dis. Treat. 2015, 11, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Sener, R.N. Diffusion MRI: Apparent diffusion coefficient (ADC) values in the normal brain and a classification of brain disorders based on ADC values. Comput. Med. Imaging Graph. 2001, 25, 299–326. [Google Scholar] [CrossRef]
- Shanmuganathan, K.; Gullapalli, R.P.; Mirvis, S.E.; Roys, S.; Murthy, P. Whole-brain apparent diffusion coefficient in traumatic brain injury: Correlation with Glasgow Coma Scale score. Am. J. Neuroradiol. 2004, 25, 539–544. [Google Scholar] [PubMed]
- Zheng, P.; He, B.; Tong, W.S. Decrease in Pituitary Apparent Diffusion Coefficient in Normal Appearing Brain Correlates with Hypopituitarism Following Traumatic Brain Injury. J. Endocrinol. Investig. 2014, 37, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Madathil, S.K.; Evans, H.N.; Saatman, K.E. Temporal and regional changes in IGF-1/IGF-1R signaling in the mouse brain after traumatic brain injury. J. Neurotrauma 2010, 27, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Walter, H.J.; Berry, M.; Hill, D.J.; Logan, A. Spatial and temporal changes in the insulin-like growth factor (IGF) axis indicate autocrine/paracrine actions of IGF-I within wounds of the rat brain. Endocrinology 1997, 138, 3024–3034. [Google Scholar] [CrossRef]
- Gasco, V.; Caputo, M.; Lanfranco, F.; Ghigo, E.; Grottoli, S. Management of GH treatment in adult GH deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 13–24. [Google Scholar] [CrossRef]
- Falleti, M.G.; Maruffa, P.; Burman, P.; Harris, A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: A meta-analysis of the current literature. Psychoneuroendocrinology 2006, 6, 681–691. [Google Scholar] [CrossRef]
- Park, K.D.; Lim, O.K.; Yoo, C.J.; Kim, Y.W.; Lee, S.; Park, Y.; Lee, J.K. Voxel-based statistical analysis of brain metabolism in patients with growth hormone deficiency after traumatic brain injury. Brain Inj. 2016, 30, 407–413. [Google Scholar] [CrossRef]
- Molitch, M.E.; Clemmons, D.R.; Malozowski, S.; Merriam, G.R.; Vance, M.L. Evaluation and treatment of adult growth hormone deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1587–1609. [Google Scholar] [CrossRef]
- Ho, K.K.; 2007 GH Deficiency Consensus Workshop Participants. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: A statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur. J. Endocrinol. 2007, 157, 695–700. [Google Scholar] [CrossRef]
- Bondanelli, M.; Ambrosio, M.R.; Cavazzini, L.; Bertocchi, A.; Zatelli, M.C.; Carli, A.; Valle, D.; Basaglia, N.; Uberti, E.C. Anterior pituitary function may predict functional and cognitive outcome in patients with traumatic brain injury undergoing rehabilitation. J. Neurotrauma 2007, 24, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Reimunde, P.; Quintana, A.; Castañón, B.; Casteleiro, N.; Vilarnovo, Z.; Otero, A.; Devesa, A.; Otero-Cepeda, X.L.; Devesa, J. Effects of growth hormone (GH) replacement and cognitive rehabilitation in patients with cognitive disorders after traumatic brain injury. Brain Inj. 2011, 25, 65–73. [Google Scholar] [CrossRef]
- Maric, N.P.; Doknic, M.; Pavlovic, D.; Pekic, S.; Stojanovic, M.; Jasovic-Gasic, M.; Popovic, V. Psychiatric and neuropsychological changes in growth hormone-deficient patients after traumatic brain injury in response to growth hormone therapy. J. Endocrinol. Investig. 2010, 33, 770–775. [Google Scholar] [CrossRef] [PubMed]
- Moreau, O.K.; Cortet-Rudelli, C.; Yollin, E.; Merlen, E.; Daveluy, W.; Rousseaux, M. Growth hormone replacement therapy in patients with traumatic brain injury. J. Neurotrauma 2013, 30, 998–1006. [Google Scholar] [CrossRef]
- Woodhouse, L.J.; Mukherjee, A.; Shalet, S.M.; Ezzat, S. The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocr. Rev. 2006, 27, 287–317. [Google Scholar] [CrossRef] [PubMed]
- Beshyah, S.A.; Sharp, P.S.; Gelding, S.V.; Halliday, D.; Johnston, D.G. Whole-body leucine turnover in adults on conventional treatment for hypopituitarism. Acta Endocrinol. (Cph.) 1993, 129, 158–164. [Google Scholar] [CrossRef]
- Mossberg, K.A.; Masel, B.E.; Gilkison, C.R.; Urban, R.J. Aerobic capacity and growth hormone deficiency after traumatic brain injury. J. Clin. Endocrinol. Metab. 2008, 93, 2581–2587. [Google Scholar] [CrossRef]
- Mossberg, K.A.; Durham, W.J.; Zgaljardic, D.J.; Gilkison, C.R.; Danesi, C.P.; Sheffield-Moore, M.; Masel, B.E.; Urban, R.J. Functional changes after recombinant human growth hormone replacement in patients with chronic traumatic brain injury and abnormal growth hormone secretion. J. Neurotrauma 2017, 34, 845–852. [Google Scholar] [CrossRef]
- Bhagia, V.; Gilkison, C.; Fitts, R.H.; Zgaljardic, D.J.; High, W.M.; Masel, B.E.; Urban, R.J.; Mossberg, K.A. Effect of recombinant growth hormone replacement in a growth hormone deficient subject recovering from mild traumatic brain injury: A case report. Brain Inj. 2010, 24, 560–567. [Google Scholar] [CrossRef]
- Klose, M.; Watt, T.; Brennum, J.; Feldt-Rasmussen, U. Posttraumatic hypopituitarism is associated with an unfavorable body composition and lipid profile, and decreased quality of life 12 months after injury. J. Clin. Endocrinol. Metab. 2007, 92, 3861–3868. [Google Scholar] [CrossRef] [PubMed]
- Kreitschmann-Andermahr, I.; Poll, E.M.; Reineke, A.; Gilsbach, J.M.; Brabant, G.; Buchfelder, M.; Fassbender, W.; Faust, M.; Kann, P.H.; Wallaschofski, H. Growth hormone deficient patients after traumatic brain injury-baseline characteristics and benefits after growth hormone replacement-an analysis of the German KIMS database. Growth Horm. IGF Res. 2008, 18, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Gardner, C.J.; Mattsson, A.F.; Daousi, C.; Korbonits, M.; Koltowska-Haggstrom, M.; Cuthbertson, D.J. GH deficiency after traumatic brain injury: Improvement in quality of life with GH therapy: Analysis of the KIMS database. Eur. J. Endocrinol. 2015, 172, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Bülow, B.; Hagmar, L.; Mikoczy, Z.; Nordström, C.H.; Erfurth, E.M. Increased cerebrovascular mortality in patients with hypopituitarism. Clin. Endocrinol. (Oxf.) 1997, 46, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Bengtsson, B.A.; Rosén, T.; Odén, A.; Johannsson, G. Malignant disease and cardiovascular morbidity in hypopituitary adults with or without growth hormone replacement therapy. J. Clin. Endocrinol. Metab. 2004, 89, 3306–3312. [Google Scholar] [CrossRef] [PubMed]
- Wüster, C.; Abs, R.; Bengtsson, B.A.; Bennmarker, H.; Feldt-Rasmussen, U.; Hernberg-Ståhl, E.; Monson, J.P.; Westberg, B.; Wilton, P. The influence of growth hormone deficiency, growth hormone replacement therapy, and other aspects of hypopituitarism on fracture rate and bone mineral density. J. Bone Min. Res. 2001, 16, 398–405. [Google Scholar] [CrossRef]
- O’Halloran, D.J.; Tsatsoulis, A.; Whitehouse, R.W.; Holmes, S.J.; Adams, J.E.; Shalet, S.M. Increased bone density after recombinant human growth hormone (GH) therapy in adults with isolated GH deficiency. J. Clin. Endocrinol. Metab. 1993, 76, 1344–1348. [Google Scholar] [CrossRef]
- Götherström, G.; Bengtsson, B.A.; Bosaeus, I.; Johannsson, G.; Svensson, J. Ten-year GH replacement increases bone mineral density in hypopituitary patients with adult onset GH deficiency. Eur. J. Endocrinol. 2007, 156, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Wallymahmed, M.E.; Foy, P.; MacFarlane, I.A. The quality of life of adults with growth hormone deficiency: Comparison with diabetic patients and control subjects. Clin. Endocrinol. (Oxf.) 1999, 51, 333–338. [Google Scholar] [CrossRef]
- Badia, X.; Lucas, A.; Sanmartí, A.; Roset, M.; Ulied, A. One-year follow-up of quality of life in adults with untreated growth hormone deficiency. Clin. Endocrinol. (Oxf.) 1998, 49, 765–771. [Google Scholar] [CrossRef]
- Kelly, D.F.; McArthur, D.L.; Levin, H.; Swimmer, S.; Dusick, J.R.; Cohan, P.; Wang, C.; Swerdloff, R. Neurobehavioral and quality of life changes associated with growth hormone insufficiency after complicated mild, moderate, or severe traumatic brain injury. J. Neurotrauma 2006, 23, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Appelman-Dijkstra, N.M.; Claessen, K.M.; Roelfsema, F.; Pereira, A.M.; Biermasz, N.R. Long-term effects of recombinant human GH replacement in adults with GH deficiency: A systematic review. Eur. J. Endocrinol. 2013, 169, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.; McKenna, S.P.; Doward, L.C.; Hunt, S.M.; Shalet, S.M. Development of a questionnaire to assess the quality of life of adults with growth hormone deficiency. Endocrinol. Metab. 1995, 2, 63–69. [Google Scholar]
- Gasco, V.; Prodam, F.; Pagano, L.; Grottoli, S.; Belcastro, S.; Marzullo, P.; Beccuti, G.; Ghigo, E.; Aimaretti, G. Hypopituitarism following brain injury: When does it occur and how best to test? Pituitary 2012, 15, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.F.; Gonzalo, I.T.; Cohan, P.; Berman, N.; Swerdloff, R.; Wang, C. Hypopituitarism following traumatic brain injury and aneurysmal subarachnoid hemorrhage: A preliminary report. J. Neurosurg. 2000, 93, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Nemes, O.; Kovacs, N.; Czeiter, E.; Kenyeres, P.; Tarjanyi, Z.; Bajnok, L.; Buki, A.; Doczi, T.; Mezosi, E. Predictors of posttraumatic pituitary failure during long-term follow-up. Hormones (Athens) 2015, 14, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Kgosidialwa, O.; Agha, A. Hypopituitarism post traumatic brain injury (TBI): Review. Ir. J. Med. Sci. 2019. (Ahead of print). [Google Scholar] [CrossRef] [PubMed]
- Glynn, N.; Agha, A. Which patient requires neuroendocrine assessment following traumatic brain injury, when and how? Clin. Endocrinol. (Oxf.) 2013, 78, 17–20. [Google Scholar] [CrossRef]
- Lissett, C.A.; Jönsson, P.; Monson, J.P.; Shalet, S.M. Determinants of IGF-I status in a large cohort of growth hormone-deficient (GHD) subjects: The role of timing of onset of GHD. Clin. Endocrinol. (Oxf.) 2003, 59, 773–778. [Google Scholar] [CrossRef]
- Ghigo, E.; Masel, B.; Aimaretti, G.; Léon-Carrión, J.; Casanueva, F.F.; Dominguez-Morales, M.R.; Elovic, E.; Perrone, K.; Stalla, G.; Thompson, C.; et al. Review Consensus guidelines on screening for hypopituitarism following traumatic brain injury. Brain Inj. 2005, 19, 711–724. [Google Scholar] [CrossRef]
- Agha, A.; Phillips, J.; O’Kelly, P.; Tormey, W.; Thompson, C.J. The natural history of post-traumatic hypopituitarism: Implications for assessment and treatment. Am. J. Med. 2005, 118, 1416. [Google Scholar] [CrossRef] [PubMed]
- Vespa, P.M.; Nuwer, M.R.; Nenov, V.; Ronne-Engstrom, E.; Hovda, D.A.; Bergsneider, M.; Kelly, D.F.; Martin, N.A.; Becker, D.P. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J. Neurosurg. 1999, 91, 750–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuen, K.C. Glucagon stimulation testing in assessing for adult growth hormone deficiency: Current status and future perspectives. ISNR Endocrinol. 2011, 2011, 608056. [Google Scholar] [CrossRef] [PubMed]


| Study | Number of Participants | Severity (GCS) | Median Age at TBI (Range) (Years) | Timing of Testing Post TBI (Days) | GHD (%) |
|---|---|---|---|---|---|
| Olivecrona et al. [8] | 45 | ≤8 | 15–64 | 1 4 | 30 2 |
| Tanriverdi et al. [9] | 52 | 3–15 | 35 (17–65) | 0–1 | 20 |
| Agha et al. [10] | 50 | 8–13 | 37 (15–65) | 7–20 | 18 |
| Study | Number of participants | Severity (GCS) | Test Used to Diagnose GHD | Median Age at TBI (Range) (Years) | Timing of Testing Post TBI (Months) | GHD (%) |
|---|---|---|---|---|---|---|
| Tanriverdi et al. [9] | 52 | 3–15 | GHRH + GHRP-6 | 35 (17–65) | 12 | 37.7 |
| Agha et al. [11] | 102 | 3–13 | ITT Or GHRH test + Arginine | 28 (15–65) | 6–36 | 10.7 |
| Aimaretti et al. [12] | 70 | 3–15 | GHRH + arginine test | 39 | 3 | 38.5 |
| 12 | 38.6 | |||||
| Kozlowski et al. [13] | 55 | 3–15 | - | 36.1 | >12 | 63.6 |
| Klose et al. [14] | 104 | 3–15 | ITT Or GHRH test + Arginine | 41 (18–64) | 13 (10–27) | 15 |
| Abadi et al. [15] | 75 | 9–13 | IGF-1 | 38 (15–54) | 3 | 24 |
| 6 | 9.3 | |||||
| Bondanelli et al. [16] | 50 | 3–15 | GHRH + arginine test | 37.6 (20–87) | 12–64 | 28 |
| Hannon et al. [17] | 32 | <14 | ITT Or GST | - | 6–24 | 18.8 |
| Krahulik et al. [18] | 186 | 3–14 | GHRH test + Arginine Or GST | 36 (18–65) | 12 | 13.5 |
| Schneider et al. [19] | 78 | 3–15 | GHRH test + Arginine | 36 | 12 | 10 |
| Deficient Hormone | Symptoms | Signs |
|---|---|---|
| GH | Poor QoL Decreased energy Low mood | Decreased muscle mass Increased fat mass Altered metabolic profile Decreased exercise capacity Reduced BMD Increased Fractures |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kgosidialwa, O.; Hakami, O.; Zia-Ul-Hussnain, H.M.; Agha, A. Growth Hormone Deficiency Following Traumatic Brain Injury. Int. J. Mol. Sci. 2019, 20, 3323. https://doi.org/10.3390/ijms20133323
Kgosidialwa O, Hakami O, Zia-Ul-Hussnain HM, Agha A. Growth Hormone Deficiency Following Traumatic Brain Injury. International Journal of Molecular Sciences. 2019; 20(13):3323. https://doi.org/10.3390/ijms20133323
Chicago/Turabian StyleKgosidialwa, Oratile, Osamah Hakami, Hafiz Muhammad Zia-Ul-Hussnain, and Amar Agha. 2019. "Growth Hormone Deficiency Following Traumatic Brain Injury" International Journal of Molecular Sciences 20, no. 13: 3323. https://doi.org/10.3390/ijms20133323