Genome-Wide Identification and Expression Profiling of the Polygalacturonase (PG) and Pectin Methylesterase (PME) Genes in Grapevine (Vitis vinifera L.)
Abstract
:1. Introduction
2. Results
2.1. Identification of PGs and PMEs in Grapevine
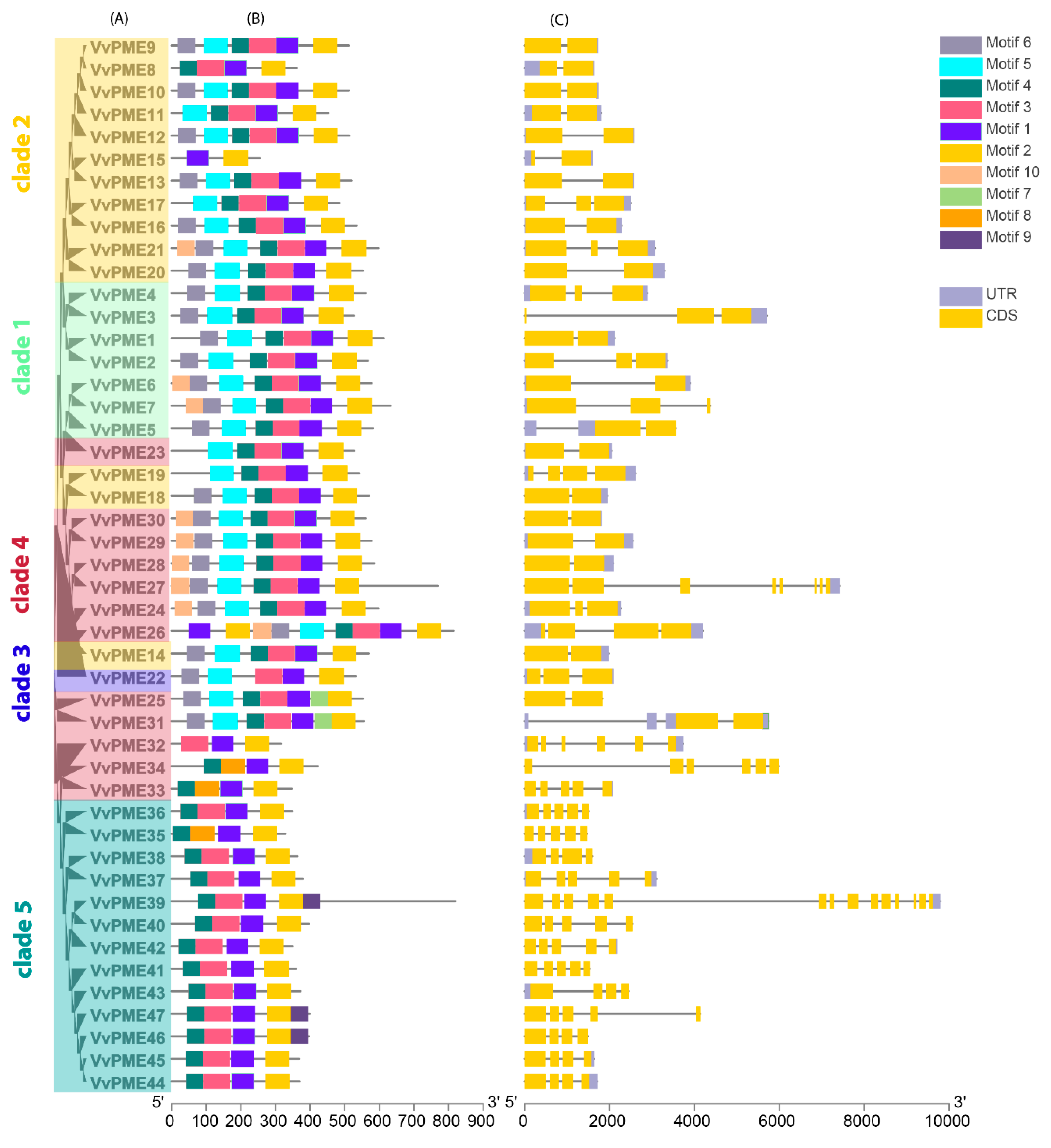
2.2. Phylogenetic and Collinearity Relationships, Motif Compositions and Genomic Structure of PGs and PMEs in Grapevine
2.3. Chromosomal Localization and Gene Duplication Analysis of PG and PME Genes
2.4. Gene Ontology Enrichment (GO) and Cis-Regulatory Elements in Grapevine
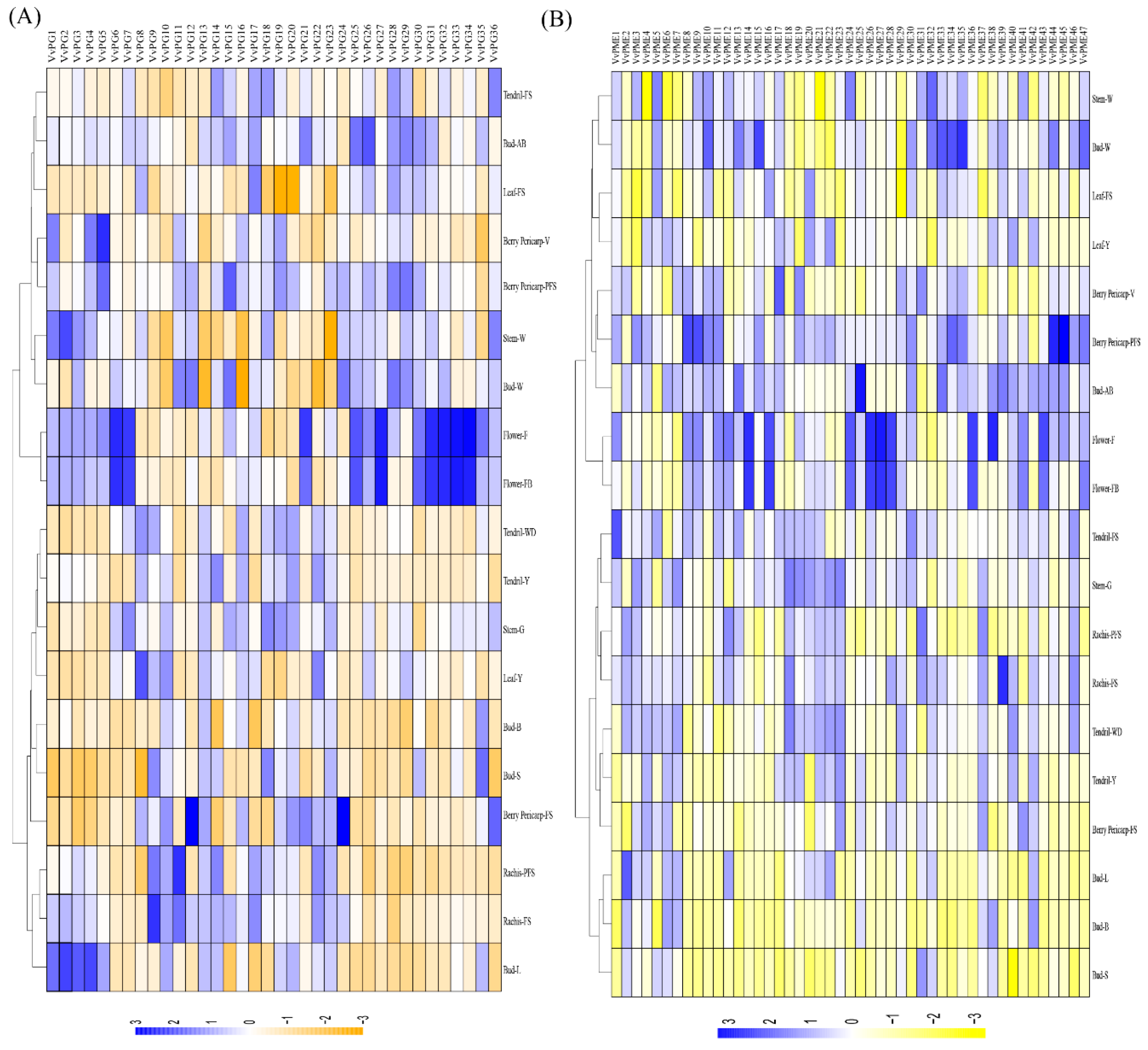
2.5. Tanscriptional Profiling of PGs and PMEs in Different Organs and Developmental Stages in Grapevine
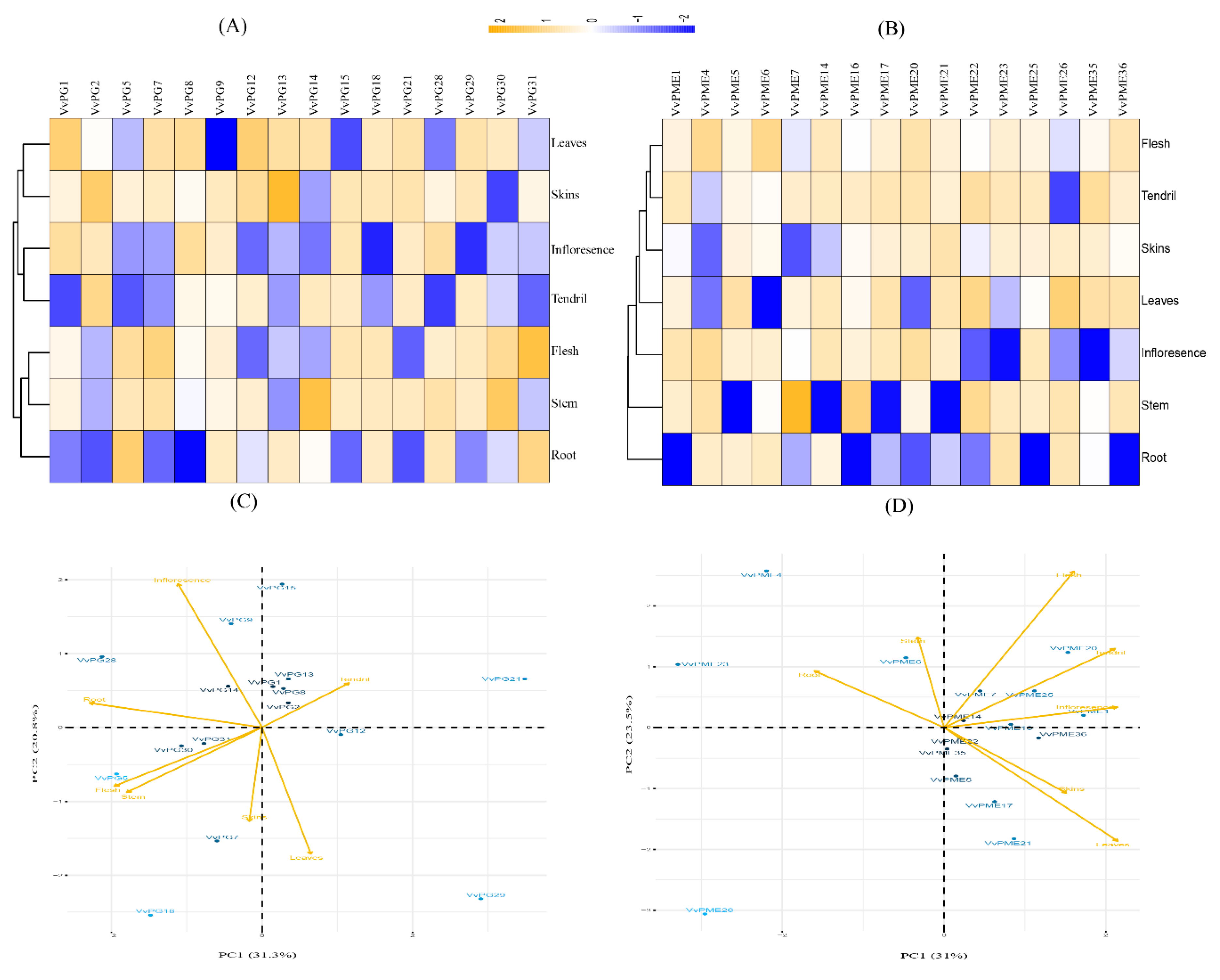
2.6. qRT-PCR Analysis of the Candidate PG and PME Genes in Various Organs of Grapevine
3. Discussion
4. Materials and Methods
4.1. Mining of Grapevine PGs and PMEs
4.2. Phylogenetic Analysis of PGs and PMEs
4.3. Ratio of Synonymous (Ks) and Non-synonymous (Ka) for duplicated genes
4.4. Gene Structure, Conserved Motifs Analysis, and Physicochemical Parameters of PG and PME Proteins
4.5. Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomics (KEGG) and Cis-Elements Predictions of PGs and PMEs
4.6. Chromosomal Location and Collinearity Analysis
4.7. Principal Component Analysis (PCA)
4.8. Plant Material and Methods
4.9. RNA Isolation and Expression Profiling of PGs and PMEs in Grapevine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PGs | Polygalacturonase |
| PMEs | Pectin methylesterase |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| RT-PCR | Real time-PCR |
| PCA | Principal Component Analysis |
References
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T.; et al. Toward a systems approach to understanding plant cell walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef] [PubMed]
- Keegstra, K. Plant cell walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.B.; Orchard, J.; Marín-Rodríguez, M.C. Pectate lyases, cell wall degradation and fruit softening. J. Exp. Bot. 2002, 53, 2115–2119. [Google Scholar]
- Peng, G.; Wu, J.; Lu, W.; Li, J. A polygalacturonase gene clustered into clade e involved in lychee fruitlet abscission. Sci. Hortic. 2013, 150, 244–250. [Google Scholar] [CrossRef]
- Elliott, K.A.; González-Carranza, Z.H.; Roberts, J.A. Expression of polygalacturonases and evidence to support their role during cell separation processes in Arabidopsis thaliana. J. Exp. Bot. 2007, 58, 3719–3730. [Google Scholar]
- Ogawa, M.; Kay, P.; Wilson, S.; Swain, S.M. Arabidopsis dehiscence zone polygalacturonase1 (adpg1), adpg2, and quartet2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 2009, 21, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.G.; Schröder, R.; Hallett, I.C.; Cohen, D.; MacRae, E.A. Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol. 2002, 129, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.K.C.; Catalá, C.; Gonzalez-Carranza, Z.H.; Roberts, J.A. Cell wall disassembly. Annu. Plant Rev. 2018, 8, 264–324. [Google Scholar]
- Torki, M.; Mandaron, P.; Thomas, F.; Quigley, F.; Mache, R.; Falconet, D. Differential expression of a polygalacturonase gene family in Arabidopsis thaliana. Mol. Gen. Genet. 1999, 261, 948–952. [Google Scholar]
- Sander, L.; Child, R.; Ulvskov, P.; Albrechtsen, M.; Borkhardt, B. Analysis of a dehiscence zone endo-polygalacturonase in oilseed rape (Brassica napus) and Arabidopsis thaliana: Evidence for roles in cell separation in dehiscence and abscission zones, and in stylar tissues during pollen tube growth. Plant Mol. Biol. 2001, 46, 469–479. [Google Scholar] [CrossRef]
- Chen, H.; Shao, H.; Fan, S.; Ma, J.; Zhang, D.; Han, M. Identification and phylogenetic analysis of the polygalacturonase gene family in apple. Hortic. Plant J. 2016, 2, 241–252. [Google Scholar] [CrossRef]
- Wakasa, Y.; Kudo, H.; Ishikawa, R.; Akada, S.; Senda, M.; Niizeki, M.; Harada, T. Low expression of an endopolygalacturonase gene in apple fruit with long-term storage potential. Postharvest Biol. Technol. 2006, 39, 193–198. [Google Scholar] [CrossRef]
- Sekine, D.; Munemura, I.; Gao, M.; Mitsuhashi, W.; Toyomasu, T.; Murayama, H. Cloning of cdnas encoding cell-wall hydrolases from pear (Pyrus communis) fruit and their involvement in fruit softening and development of melting texture. Physiol. Plant. 2006, 126, 163–174. [Google Scholar] [CrossRef]
- Deytieux-Belleau, C.; Vallet, A.; Doneche, B.; Geny, L. Pectin methylesterase and polygalacturonase in the developing grape skin. Plant Physiol. Biochem. 2008, 46, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Pose, S.; Paniagua, C.; Cifuentes, M.; Blanco-Portales, R.; Quesada, M.A.; Mercado, J.A. Insights into the effects of polygalacturonase fapg1 gene silencing on pectin matrix disassembly, enhanced tissue integrity, and firmness in ripe strawberry fruits. J. Exp. Bot. 2013, 64, 3803–3815. [Google Scholar] [CrossRef] [PubMed]
- Hiwasa, K.; Kinugasa, Y.; Amano, S.; Hashimoto, A.; Nakano, R.; Inaba, A.; Kubo, Y. Ethylene is required for both the initiation and progression of softening in pear (Pyrus communis L.) fruit. J. Exp. Bot. 2003, 54, 771–779. [Google Scholar] [CrossRef]
- Asif, M.H.; Nath, P. Expression of multiple forms of polygalacturonase gene during ripening in banana fruit. Plant Physiol. Biochem. 2005, 43, 177–184. [Google Scholar] [CrossRef]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef]
- Pelloux, J.; Rusterucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef]
- Kagan-Zur, V.; Tieman, D.M.; Marlow, S.J.; Handa, A.K. Differential regulation of polygalacturonase and pectin methylesterase gene expression during and after heat stress in ripening tomato (Lycopersicon esculentum mill.) fruits. Plant Mol. Biol. 1995, 29, 1101–1110. [Google Scholar] [CrossRef]
- Bosch, M.; Cheung, A.Y.; Hepler, P.K. Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol 2005, 138, 1334–1346. [Google Scholar] [CrossRef]
- Almeida, D.P.F.; Huber, D.J. In vivo pectin solubility in ripening and chill-injured tomato fruit. Plant Sci. 2008, 174, 174–182. [Google Scholar] [CrossRef]
- Giovannoni, J.J.; DellaPenna, D.; Bennett, A.B.; Fischer, R.L. Expression of a chimeric polygalacturonase gene in transgenic rin (ripening inhibitor) tomato fruit results in polyuronide degradation but not fruit softening. Plant Cell 1989, 1, 53–63. [Google Scholar] [PubMed]
- Kim, J.; Shiu, S.H.; Thoma, S.; Li, W.H.; Patterson, S.E. Patterns of expansion and expression divergence in the plant polygalacturonase gene family. Genome Biol. 2006, 7, R87. [Google Scholar] [CrossRef]
- Duan, W.; Huang, Z.; Song, X.; Liu, T.; Liu, H.; Hou, X.; Li, Y. Comprehensive analysis of the polygalacturonase and pectin methylesterase genes in Brassica rapa shed light on their different evolutionary patterns. Sci. Rep. 2016, 6, 25107. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shang, H.; Ge, Q.; Zou, C.; Cai, J.; Wang, D.; Fan, S.; Zhang, Z.; Deng, X.; Tan, Y.; et al. Genome-wide identification, phylogeny, and expression analysis of pectin methylesterases reveal their major role in cotton fiber development. BMC Genom. 2016, 17, 1000. [Google Scholar] [CrossRef]
- Ke, X.; Wang, H.; Li, Y.; Zhu, B.; Zang, Y.; He, Y.; Cao, J.; Zhu, Z.; Yu, Y. Genome-wide identification and analysis of polygalacturonase genes in Solanum lycopersicum. Int. J. Mol. Sci. 2018, 19, 2290. [Google Scholar] [CrossRef]
- Yu, Y.; Liang, Y.; Lv, M.; Wu, J.; Lu, G.; Cao, J. Genome-wide identification and characterization of polygalacturonase genes in cucumis sativus and citrullus lanatus. Plant Physiol. Biochem. 2014, 74, 263–275. [Google Scholar] [CrossRef]
- Grimplet, J.; Adam-Blondon, A.F.; Bert, P.F.; Bitz, O.; Cantu, D.; Davies, C.; Delrot, S.; Pezzotti, M.; Rombauts, S.; Cramer, G.R. The grapevine gene nomenclature system. BMC Genom. 2014, 15, 1077. [Google Scholar] [CrossRef]
- Hurles, M. Gene duplication: The genomic trade in spare parts. PLoS Biol. 2004, 2, E206. [Google Scholar] [CrossRef]
- Fasoli, M.; Dal Santo, S.; Zenoni, S.; Tornielli, G.B.; Farina, L.; Zamboni, A.; Porceddu, A.; Venturini, L.; Bicego, M.; Murino, V.; et al. The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 2012, 24, 3489–3505. [Google Scholar] [CrossRef] [PubMed]
- Hadfield, K.A.; Bennett, A.B. Polygalacturonases: Many genes in search of a function. Plant Physiol 1998, 117, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Hu, C.M. Genome-wide identification, classification, and expression divergence of glutathione-transferase family in Brassica rapa under multiple hormone treatments. BioMed Res. Int. 2018, 2018, 6023457. [Google Scholar] [CrossRef] [PubMed]
- Juretic, N.; Hoen, D.R.; Huynh, M.L.; Harrison, P.M.; Bureau, T.E. The evolutionary fate of mule-mediated duplications of host gene fragments in rice. Genome Res. 2005, 15, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Z.; Vang, S.; Yu, J.; Wong, G.K.-S.; Wang, J. Correlation between ka/ks and ks is related to substitution model and evolutionary lineage. J. Mol. Evol. 2009, 68, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Conery, J.S. The evolutionary fate and consequences of duplicate genes. Science 2000, 290, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Shazadee, H.; Khan, N. Identification and expression profiling of protein phosphatases (pp2c) gene family in Gossypium hirsutum L. Int. J. Mol. Sci. 2019, 20, 1395. [Google Scholar] [CrossRef] [PubMed]
- Moghe, G.D.; Shiu, S.H. The causes and molecular consequences of polyploidy in flowering plants. Ann. N. Y. Acad. Sci. 2014, 1320, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Prince, V.E.; Pickett, F.B. Splitting pairs: The diverging fates of duplicated genes. Nat. Rev. Genet. 2002, 3, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.S.; Khan, N.; Pervaiz, T.; Zhongjie, L.; Nasim, M.; Jogaiah, S.; Mushtaq, N.; Jiu, S.; Jinggui, F. Genome-wide identification, evolution, and molecular characterization of the pp2c gene family in woodland strawberry. Gene 2019, 702, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yu, Y.; Cui, J.; Lyu, M.; Xu, L.; Cao, J. A comparative analysis of the evolution, expression, and cis-regulatory element of polygalacturonase genes in grasses and dicots. Funct. Integr. Genom. 2016, 16, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Liu, H.J.; Wang, X.R.; Zeng, Q.Y. Molecular evolution and expression divergence of the populus polygalacturonase supergene family shed light on the evolution of increasingly complex organs in plants. New Phytol. 2013, 197, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-H.; Citovsky, V. Systemic movement of a tobamovirus requires host cell pectin methylesterase. Plant J. 2003, 35, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raiola, A.; Lionetti, V.; Elmaghraby, I.; Immerzeel, P.; Mellerowicz, E.J.; Salvi, G.; Cervone, F.; Bellincampi, D. Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol. Plant-Microbe Interact. 2011, 24, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Solecka, D.; Zebrowski, J.; Kacperska, A. Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann. Bot. 2008, 101, 521–530. [Google Scholar] [CrossRef] [PubMed]
- An, S.H.; Sohn, K.H.; Choi, H.W.; Hwang, I.S.; Lee, S.C.; Hwang, B.K. Pepper pectin methylesterase inhibitor protein capmei1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 2008, 228, 61–78. [Google Scholar] [CrossRef]
- Mundaya, N.J.; Owen, S.D.W.; Iain, M.; Alan, T.C.; David, H.; Balakrishnan, P. Analysis of seaweed extract-induced transcriptome leads to identification of a negative regulator of salt tolerance in Arabidopsis. Hortsci. Horts 2012, 47, 704–709. [Google Scholar]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Letunic, I.; Doerks, T.; Bork, P. Smart 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef]
- Edgar, R.C. Muscle: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. Mega7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xia, R.; Chen, H.; He, Y. Tbtools, a toolkit for biologists integrating various hts-data handling tools with a user-friendly interface. bioRxiv 2018, 289660. [Google Scholar] [CrossRef]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. Meme suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. Plantcare, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.S.; Jogaiah, S.; Pervaiz, T.; Yanxue, Z.; Khan, N.; Fang, J. Physiological and transcriptional variations inducing complex adaptive mechanisms in grapevine by salt stress. Environ. Exp. Bot. 2019, 162, 455–467. [Google Scholar] [CrossRef]
- Khan, N.; Hu, C.-M.; Amjad Khan, W.; Naseri, E.; Ke, H.; Huijie, D.; Hou, X. Evolution and expression divergence of e2 gene family under multiple abiotic and phytohormones stresses in Brassica rapa. Biomed Res. Int. 2018, 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Hu, C.-M.; Khan, W.A.; Wang, W.; Ke, H.; Huijie, D.; Zhishuo, Z.; Hou, X. Genome-wide identification, classification, and expression pattern of homeobox gene family in Brassica rapa under various stresses. Sci. Rep. 2018, 8, 16265. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, L.; Fang, X.; Chen, L.; Cui, L.; Fang, J. Genome-wide analysis of autophagy-related genes (args) in grapevine and plant tolerance to copper stress. Planta 2018, 247, 1449–1463. [Google Scholar] [CrossRef]
- Khan, N.; Ke, H.; Hu, C.-M.; Naseri, E.; Haider, M.S.; Ayaz, A.; Amjad Khan, W.; Wang, J.; Hou, X. Genome-wide identification, evolution, and transcriptional profiling of pp2c gene family in Brassica rapa. Biomed Res. Int. 2019, 2019, 15. [Google Scholar] [CrossRef]




| Gene 1 | Gene 2 | Ks | Ka | Ka/Ks | Selection Pressure | Gene Duplications |
|---|---|---|---|---|---|---|
| Between PG genes | ||||||
| VvPG1 | VvPG2 | 0.029 | 0.022 | 0.77 | Purifying Selection | Tandem |
| VvPG3 | VvPG4 | 0.034 | 0.016 | 0.47 | Purifying Selection | Tandem |
| VvPG6 | VvPG25 | 0.852 | 0.579 | 0.68 | Purifying Selection | Tandem |
| VvPG28 | VvPG31 | 0.738 | 0.693 | 0.94 | Purifying Selection | Tandem |
| VvPG33 | VvPG34 | 0.663 | 0.197 | 0.30 | Purifying Selection | Tandem |
| VvPG8 | VvPG9 | 0.5 | 0.458 | 0.92 | Purifying Selection | Dispersed |
| VvPG12 | VvPG13 | 0.626 | 0.58 | 0.93 | Purifying Selection | Dispersed |
| VvPG16 | VvPG17 | 0.879 | 0.472 | 0.54 | Purifying Selection | Dispersed |
| VvPG20 | VvPG24 | 1.56 | 0.419 | 0.27 | Purifying Selection | Dispersed |
| VvPG29 | VvPG30 | 0.522 | 0.628 | 1.20 | Positive Selection | Dispersed |
| VvPG5 | VvPG7 | 0.376 | 0.409 | 1.09 | Positive Selection | WGD or Segmental |
| VvPG10 | VvPG11 | 1.266 | 0.231 | 0.18 | Purifying Selection | WGD or Segmental |
| VvPG14 | VvPG15 | 0.212 | 0.343 | 1.62 | Positive Selection | WGD or Segmental |
| VvPG18 | VvPG21 | 0.515 | 0.522 | 1.01 | Positive Selection | WGD or Segmental |
| VvPG22 | VvPG23 | 1.401 | 0.152 | 0.11 | Purifying Selection | WGD or Segmental |
| Between PME genes | ||||||
| VvPME2 | VvPME3 | 1.312 | 0.421 | 0.32 | Purifying Selection | Dispersed |
| VvPME5 | VvPME16 | 0.527 | 0.625 | 1.19 | Positive Selection | Dispersed |
| VvPME18 | VvPME19 | 1.102 | 0.442 | 0.40 | Purifying Selection | Dispersed |
| VvPME20 | VvPME21 | 0.881 | 0.464 | 0.53 | Purifying Selection | Dispersed |
| VvPME22 | VvPME23 | 0.446 | 0.681 | 1.53 | Positive Selection | Dispersed |
| VvPME27 | VvPME28 | 1.042 | 0.334 | 0.32 | Purifying Selection | Dispersed |
| VvPME35 | VvPME36 | 0.565 | 0.697 | 1.23 | Positive Selection | Dispersed |
| VvPME1 | VvPME4 | 0.798 | 0.438 | 0.55 | Purifying Selection | Tandem |
| VvPME8 | VvPME10 | 0.18 | 0.019 | 0.11 | Purifying Selection | Tandem |
| VvPME11 | VvPME12 | 0.879 | 0.086 | 0.10 | Purifying Selection | Tandem |
| VvPME13 | VvPME15 | 1.035 | 0.171 | 0.17 | Purifying Selection | Tandem |
| VvPME17 | VvPME25 | 0.64 | 0.631 | 0.99 | Purifying Selection | Tandem |
| VvPME6 | VvPME7 | 0.271 | 0.41 | 1.51 | Positive Selection | WGD or Segmental |
| VvPME14 | VvPME26 | 0.304 | 0.44 | 1.45 | Positive Selection | WGD or Segmental |
| VvPME33 | VvPME34 | 1.284 | 0.209 | 0.16 | Purifying Selection | WGD or Segmental |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.; Fatima, F.; Haider, M.S.; Shazadee, H.; Liu, Z.; Zheng, T.; Fang, J. Genome-Wide Identification and Expression Profiling of the Polygalacturonase (PG) and Pectin Methylesterase (PME) Genes in Grapevine (Vitis vinifera L.). Int. J. Mol. Sci. 2019, 20, 3180. https://doi.org/10.3390/ijms20133180
Khan N, Fatima F, Haider MS, Shazadee H, Liu Z, Zheng T, Fang J. Genome-Wide Identification and Expression Profiling of the Polygalacturonase (PG) and Pectin Methylesterase (PME) Genes in Grapevine (Vitis vinifera L.). International Journal of Molecular Sciences. 2019; 20(13):3180. https://doi.org/10.3390/ijms20133180
Chicago/Turabian StyleKhan, Nadeem, Fizza Fatima, Muhammad Salman Haider, Hamna Shazadee, Zhongjie Liu, Ting Zheng, and Jinggui Fang. 2019. "Genome-Wide Identification and Expression Profiling of the Polygalacturonase (PG) and Pectin Methylesterase (PME) Genes in Grapevine (Vitis vinifera L.)" International Journal of Molecular Sciences 20, no. 13: 3180. https://doi.org/10.3390/ijms20133180





