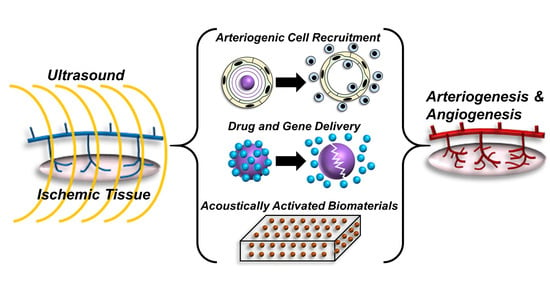
Applications of Ultrasound to Stimulate Therapeutic Revascularization
Abstract
:1. Therapeutic Vascular Remodeling
2. Ultrasound Technology: Basic Principles and Contrast Agents
3. Ultrasound Activation of Microbubbles to Facilitate Angiogenesis and Arteriogenesis
4. Ultrasound and Microbubbles to Deliver Genes, Molecules, or Cells to Facilitate Angiogenesis and Arteriogenesis
5. Activation of Implanted Biomaterials with Ultrasound to Elicit Vascular Remodeling
6. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Carmeliet, P. Blood vessels and nerves: Common signals, pathways and diseases. Nat. Rev. Genet. 2003, 4, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Poredos, P.; Poredos, P. Peripheral arterial occlusive disease and perioperative risk. Int. Angiol. 2018, 37, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lind, B.; Morcos, O.; Ferral, H.; Chen, A.; Aquisto, T.; Lee, S.; Lee, C.J. Endovascular Strategies in the Management of Acute Limb Ischemia. Vasc. Spec. Int. 2019, 35, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Sprengers, R.W.; Teraa, M.; Moll, F.L.; de Wit, G.A.; van der Graaf, Y.; Verhaar, M.C. Quality of life in patients with no-option critical limb ischemia underlines the need for new effective treatment. J. Vasc. Surg. 2010, 52, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Teraa, M.; Conte, M.S.; Moll, F.L.; Verhaar, M.C. Critical Limb Ischemia: Current Trends and Future Directions. J. Am. Heart Assoc. 2016, 5, e002938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, T.D.; Satran, D.; Jolicoeur, E.M. Treatment of refractory angina in patients not suitable for revascularization. Nat. Rev. Cardiol. 2014, 11, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, H.; Maeda, K.; Morishita, R.; Iguchi, S.; Nishikawa, T.; Takami, Y.; Kikuchi, Y.; Saito, Y.; Tamai, K.; Ogihara, T.; et al. Novel Autologous Cell Therapy in Ischemic Limb Disease Through Growth Factor Secretion by Cultured Adipose Tissue–Derived Stromal Cells. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2542–2547. [Google Scholar] [CrossRef]
- Biscetti, F.; Bonadia, N.; Nardella, E.; Cecchini, A.L.; Landolfi, R.; Flex, A. The Role of the Stem Cells Therapy in the Peripheral Artery Disease. Int. J. Mol. Sci. 2019, 20, 2233. [Google Scholar] [CrossRef]
- Litwinowicz, R.; Kapelak, B.; Sadowski, J.; Kędziora, A.; Bartus, K. The use of stem cells in ischemic heart disease treatment. Pol. J. Cardio-Thorac. Surg. 2018, 15, 196–199. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cell therapy for peripheral artery disease. Curr. Opin. Pharmacol. 2018, 39, 27–34. [Google Scholar] [CrossRef]
- Oduk, Y.; Zhu, W.; Kannappan, R.; Zhao, M.; Borovjagin, A.V.; Oparil, S.; Zhang, J.J. VEGF nanoparticles repair the heart after myocardial infarction. Am. J. Physiol. Circ. Physiol. 2018, 314, H278–H284. [Google Scholar] [CrossRef] [PubMed]
- Marushima, A.; Nieminen, M.; Kremenetskaia, I.; Gianni-Barrera, R.; Woitzik, J.; von Degenfeld, G.; Banfi, A.; Vajkoczy, P.; Hecht, N. Balanced single-vector co-delivery of VEGF/PDGF-BB improves functional collateralization in chronic cerebral ischemia. J. Cereb. Blood Flow Metab. 2019. [Google Scholar] [CrossRef] [PubMed]
- Formiga, F.R.; Pelacho, B.; Garbayo, E.; Abizanda, G.; Gavira, J.J.; Simon-Yarza, T.; Mazo, M.; Formiga, F.R.; Tamayo, E.; Jauquicoa, C.; et al. Sustained release of VEGF through PLGA microparticles improves vasculogenesis and tissue remodeling in an acute myocardial ischemia–reperfusion model. J. Control. Release 2010, 147, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tjwa, M.; Moons, L.; Fons, P.; Noel, A.; Ny, A.; Zhou, J.M.; Lennartsson, J.; Li, H.; Luttun, A.; et al. Revascularization of ischemic tissues by PDGF-CC via effects on endothelial cells and their progenitors. J. Clin. Investig. 2005, 115, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.N.; Chleboun, J.O.; Sellers, P.; Sleigh, M.; Muir, J. The Role of PDGF-BB on the Development of the Collateral Circulation after Acute Arterial Occlusion. Growth Factors 1994, 10, 299–306. [Google Scholar] [CrossRef]
- Van Royen, N.; Piek, J.J.; Legemate, D.A.; Schaper, W.; Oskam, J.; Atasever, B.; Voskuil, M.; Ubbink, D.; Schirmer, S.H.; Buschmann, I.; et al. Design of the START-trial: STimulation of ARTeriogenesis using subcutaneous application of GM-CSF as a new treatment for peripheral vascular disease. A randomized, double-blind, placebo-controlled trial. Vasc. Med. 2003, 8, 191–196. [Google Scholar] [CrossRef] [Green Version]
- Marra, S.; Scacciatella, P.; Usmiani, T.; D’Amico, M.; Giorgi, M.; Andriani, M.; Baccega, M.; Boccadoro, M.; Omedè, P.; Sanavio, F.; et al. Concurrent G-CSF and GM-CSF administration for the induction of bone marrow-derived cell mobilization in patients with acute myocardial infarction: A pilot study evaluating feasibility, safety and efficacy. EuroIntervention 2006, 1, 425–431. [Google Scholar]
- JOST, M.M.; Ninci, E.; Meder, B.; Kempf, C.; Van Royen, N.; Hua, J.; Berger, B.; Hoefer, I.; Modolell, M.; Buschmann, I. Divergent effects of GM-CSF and TGFβ1 on bone marrow-derived macrophage arginase-1 activity, MCP-1 expression, and matrix metalloproteinase-12: A potential role during arteriogenesis. FASEB J. 2003, 17, 2281–2283. [Google Scholar] [CrossRef]
- Banfi, A.; von Degenfeld, G.; Gianni-Barrera, R.; Reginato, S.; Merchant, M.J.; McDonald, D.M.; Blau, H.M. Therapeutic angiogenesis due to balanced single-vector delivery of VEGF and PDGF-BB. FASEB J. 2012, 26, 2486–2497. [Google Scholar] [CrossRef]
- Ylä-Herttuala, S.; Bridges, C.; Katz, M.G.; Korpisalo, P. Angiogenic gene therapy in cardiovascular diseases: Dream or vision? Eur. Heart J. 2017, 38, 1365–1371. [Google Scholar] [CrossRef]
- Cooke, J.P.; Losordo, D.W. Modulating the vascular response to limb ischemia: Angiogenic and cell therapies. Circ. Res. 2015, 116, 1561–1578. [Google Scholar] [CrossRef] [PubMed]
- Heuslein, J.L.; Gorick, C.M.; Song, J.; Price, R.J. DNA methyltransferase 1-dependent DNA hypermethylation constrains arteriogenesis by augmenting shear stress set point. J. Am. Heart Assoc. 2017, 6, e007673. [Google Scholar] [CrossRef] [PubMed]
- Heuslein, J.L.; Gorick, C.M.; McDonnell, S.P.; Song, J.; Annex, B.H.; Price, R.J. Exposure of Endothelium to Biomimetic Flow Waveforms Yields Identification of miR-199a-5p as a Potent Regulator of Arteriogenesis. Mol. Ther. Nucleic Acids 2018, 12, 829–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuslein, J.L.; McDonnell, S.P.; Song, J.; Annex, B.H.; Price, R.J. MicroRNA-146a Regulates Perfusion Recovery in Response to Arterial Occlusion via Arteriogenesis. Front. Bioeng. Biotechnol. 2018, 6, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heuslein, J.L.; Gorick, C.M.; Price, R.J. Epigenetic regulators of the revascularization response to chronic arterial occlusion. Cardiovasc. Res. 2019, 115, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Skyba, D.M.; Firschke, C.; Jayaweera, A.R.; Lindner, J.R.; Kaul, S. Interactions between microbubbles and ultrasound: In vitro and in vivo observations. J. Am. Coll. Cardiol. 1997, 29, 1081–1088. [Google Scholar] [CrossRef]
- Wei, K.; Jayaweera, A.R.; Firoozan, S.; Linka, A.; Skyba, D.M.; Kaul, S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion. Circulation 1998, 97, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Ay, T.; Havaux, X.; van Camp, G.; Campanelli, B.; Gisellu, G.; Pasquet, A.; Denef, J.F.; Melin, J.A.; Vanoverschelde, J.L. Destruction of contrast microbubbles by ultrasound: Effects on myocardial function, coronary perfusion pressure, and microvascular integrity. Circulation 2001, 104, 461–466. [Google Scholar] [CrossRef]
- Li, P.; Cao, L.; Dou, C.-Y.; Armstrong, W.F.; Miller, D. Impact of myocardial contrast echocardiography on vascular permeability: An in vivo dose response study of delivery mode, pressure amplitude and contrast dose. Ultrasound Med. Biol. 2003, 29, 1341–1349. [Google Scholar] [CrossRef]
- Skyba, D.M.; Price, R.J.; Linka, A.Z.; Skalak, T.C.; Kaul, S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation 1998, 98, 290–293. [Google Scholar] [CrossRef]
- Price, R.J.; Skyba, D.M.; Kaul, S.; Skalak, T.C. Delivery of Colloidal Particles and Red Blood Cells to Tissue Through Microvessel Ruptures Created by Targeted Microbubble Destruction With Ultrasound. Circulation 1998, 98, 1264–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Qi, M.; Kaul, S.; Price, R.J. Stimulation of arteriogenesis in skeletal muscle by microbubble destruction with ultrasound. Circulation 2002, 106, 1550–1555. [Google Scholar] [CrossRef] [PubMed]
- Miller, D. Overview of experimental studies of biological effects of medical ultrasound caused by gas body activation and inertial cavitation. Prog. Biophys. Mol. Biol. 2007, 93, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Helfield, B.; Chen, X.; Watkins, S.C.; Villanueva, F.S. Biophysical insight into mechanisms of sonoporation. Proc. Natl. Acad. Sci. USA 2016, 113, 9983–9988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.-S.; Brayman, A.A.; Matula, T.J.; Crum, L.A.; Miller, M.W. The pulse length-dependence of inertial cavitation dose and hemolysis. Ultrasound Med. Biol. 2003, 29, 739–748. [Google Scholar] [CrossRef]
- Chen, W.-S.; Brayman, A.A.; Matula, T.J.; Crum, L.A. Inertial cavitation dose and hemolysis produced in vitro with or without Optison. Ultrasound Med. Biol. 2003, 29, 725–737. [Google Scholar] [CrossRef]
- Dalecki, D.; Raeman, C.H.; Child, S.Z.; Cox, C.; Francis, C.W.; Meltzer, R.S.; Carstensen, E.L. Hemolysis in vivo from exposure to pulsed ultrasound. Ultrasound Med. Biol. 1997, 23, 307–313. [Google Scholar] [CrossRef]
- Poliachik, S.L.; Chandler, W.L.; Mourad, P.D.; Bailey, M.R.; Bloch, S.; Cleveland, R.O.; Kaczkowski, P.; Keilman, G.; Porter, T.; Crum, L.A. Effect of high-intensity focused ultrasound on whole blood with and without microbubble contrast agent. Ultrasound Med. Biol. 1999, 25, 991–998. [Google Scholar] [CrossRef]
- Raymond, S.B.; Skoch, J.; Hynynen, K.; Bacskai, B.J. Multiphoton Imaging of Ultrasound/Optison Mediated Cerebrovascular Effects in vivo. J. Cereb. Blood Flow Metab. 2007, 27, 393–403. [Google Scholar] [CrossRef]
- Basta, G.; Venneri, L.; Lazzerini, G.; Pasanisi, E.; Pianelli, M.; Vesentini, N.; del Turco, S.; Kusmic, C.; Picano, E. In vitro modulation of intracellular oxidative stress of endothelial cells by diagnostic cardiac ultrasound. Cardiovasc. Res. 2003, 58, 156–161. [Google Scholar] [CrossRef] [Green Version]
- Bertuglia, S.; Giusti, A.; Picano, E. Effects of diagnostic cardiac ultrasound on oxygen free radical production and microvascular perfusion during ischemia reperfusion. Ultrasound Med. Biol. 2004, 30, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Misík, V.; Riesz, P. Effect of gas-containing microspheres and echo contrast agents on free radical formation by ultrasound. Free Radic. Biol. Med. 1998, 25, 605–612. [Google Scholar] [CrossRef]
- Stride, E.; Saffari, N. The potential for thermal damage posed by microbubble ultrasound contrast agents. Ultrasonics 2004, 42, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.; Wu, S.-K.; Li, Z.; Goertz, D.E.; Hynynen, K. Microbubble-assisted MRI-guided focused ultrasound for hyperthermia at reduced power levels. Int. J. Hyperth. 2018, 35, 599–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klotz, A.R.; Lindvere, L.; Stefanovic, B.; Hynynen, K. Temperature change near microbubbles within a capillary network during focused ultrasound. Phys. Med. Biol. 2010, 55, 1549–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collis, J.; Manasseh, R.; Liovic, P.; Tho, P.; Ooi, A.; Petkovic-Duran, K.; Zhu, Y. Cavitation microstreaming and stress fields created by microbubbles. Ultrasonics 2010, 50, 273–279. [Google Scholar] [CrossRef]
- Kooiman, K.; Vos, H.J.; Versluis, M.; de Jong, N. Acoustic behavior of microbubbles and implications for drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 28–48. [Google Scholar] [CrossRef]
- Kim, J.; Lindsey, B.D.; Chang, W.-Y.; Dai, X.; Stavas, J.M.; Dayton, P.A.; Jiang, X. Intravascular forward-looking ultrasound transducers for microbubble-mediated sonothrombolysis. Sci. Rep. 2017, 7, 3454. [Google Scholar] [CrossRef]
- Chen, H.; Brayman, A.A.; Kreider, W.; Bailey, M.R.; Matula, T.J. Observations of Translation and Jetting of Ultrasound-Activated Microbubbles in Mesenteric Microvessels. Ultrasound Med. Biol. 2011, 37, 2139–2148. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Cottler, P.S.; Klibanov, A.L.; Kaul, S.; Price, R.J. Microvascular remodeling and accelerated hyperemia blood flow restoration in arterially occluded skeletal muscle exposed to ultrasonic microbubble destruction. Am. J. Physiol. Circ. Physiol. 2004, 287, H2754–H2761. [Google Scholar] [CrossRef]
- Chappell, J.C.; Klibanov, A.L.; Price, R.J. Ultrasound-microbubble-induced neovascularization in mouse skeletal muscle. Ultrasound Med. Biol. 2005, 31, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.C.; Song, J.; Klibanov, A.L.; Price, R.J. Ultrasonic Microbubble Destruction Stimulates Therapeutic Arteriogenesis via the CD18-Dependent Recruitment of Bone Marrow–Derived Cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.; Ohmori, K.; Takeuchi, H.; Shinomiya, K.; Namba, T.; Kondo, I.; Kiyomoto, H.; Kohno, M. Treatment of Ischemic Limbs Based on Local Recruitment of Vascular Endothelial Growth Factor-Producing Inflammatory Cells with Ultrasonic Microbubble Destruction. J. Am. Coll. Cardiol. 2005, 46, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; Sarwate, S.; Miller, R.J.; O’Brien, W.D. A temporal study of ultrasound contrast agent-induced changes in capillary density. J. Ultrasound Med. 2010, 29, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.A.; O’Brien, W.D., Jr. The angiogenic response is dependent on ultrasound contrast agent concentration. Vasc. Cell 2012, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Dörner, J.; Struck, R.; Zimmer, S.; Peigney, C.; Duerr, G.D.; Dewald, O.; Kim, S.C.; Malan, D.; Bettinger, T.; Nickenig, G.; et al. Ultrasound-Mediated Stimulation of Microbubbles after Acute Myocardial Infarction and Reperfusion Ameliorates Left-Ventricular Remodeling in Mice via Improvement of Borderzone Vascularization. PLoS ONE 2013, 8, e56841. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Non-invasive opening of BBB by focused ultrasound. Acta Neurochir. Suppl. 2003, 86, 555–558. [Google Scholar] [PubMed]
- Hynynen, K.; McDannold, N.; Sheikov, N.A.; Jolesz, F.A.; Vykhodtseva, N. Local and reversible blood–brain barrier disruption by noninvasive focused ultrasound at frequencies suitable for trans-skull sonications. Neuroimage 2005, 24, 12–20. [Google Scholar] [CrossRef]
- Timbie, K.F.; Mead, B.P.; Price, R.J. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J. Control. Release 2015, 219, 61–75. [Google Scholar] [CrossRef]
- Curley, C.T.; Sheybani, N.D.; Bullock, T.N.; Price, R.J. Focused Ultrasound Immunotherapy for Central Nervous System Pathologies: Challenges and Opportunities. Theranostics 2017, 7, 3608–3623. [Google Scholar] [CrossRef]
- McMahon, D.; Hynynen, K. Acute Inflammatory Response Following Increased Blood-Brain Barrier Permeability Induced by Focused Ultrasound is Dependent on Microbubble Dose. Theranostics 2017, 7, 3989–4000. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.; Mah, E.; Hynynen, K. Angiogenic response of rat hippocampal vasculature to focused ultrasound-mediated increases in blood-brain barrier permeability. Sci. Rep. 2018, 8, 12178. [Google Scholar] [CrossRef] [PubMed]
- Shohet, R.V.; Chen, S.; Zhou, Y.T.; Wang, Z.; Meidell, R.S.; Unger, R.H.; Grayburn, P.A. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation 2000, 101, 2554–2556. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian, R.; Chen, S.; Frenkel, P.A.; Grayburn, P.A.; Shohet, R.V. Ultrasound-Targeted Microbubble Destruction Can Repeatedly Direct Highly Specific Plasmid Expression to the Heart. Circulation 2003, 108, 1022–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korpanty, G.; Chen, S.; Shohet, R.V.; Ding, J.; Yang, B.; Frenkel, P.A.; Grayburn, P.A. Targeting of VEGF-mediated angiogenesis to rat myocardium using ultrasonic destruction of microbubbles. Gene Ther. 2005, 12, 1305–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Huang, C.-W.; Wu, J.; Chen, K.-J.; Li, S.-H.; Weisel, R.D.; Rakowski, H.; Sung, H.-W.; Li, R.-K. The use of cationic microbubbles to improve ultrasound-targeted gene delivery to the ischemic myocardium. Biomaterials 2013, 34, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yan, F.; Ma, J.; Zhang, J.; Liu, L.; Guan, L.; Zheng, H.; Li, T.; Liang, D.; Mu, Y. Ultrasound-Targeted Microbubble Destruction-Mediated Co-Delivery of Cxcl12 (Sdf-1alpha) and Bmp2 Genes for Myocardial Repair. J. Biomed. Nanotechnol. 2019, 15, 1299–1312. [Google Scholar] [CrossRef] [PubMed]
- Kondo, I.; Ohmori, K.; Oshita, A.; Takeuchi, H.; Fuke, S.; Shinomiya, K.; Noma, T.; Namba, T.; Kohno, M. Treatment of Acute Myocardial Infarction by Hepatocyte Growth Factor Gene Transfer. J. Am. Coll. Cardiol. 2004, 44, 644–653. [Google Scholar] [CrossRef]
- Christiansen, J.P.; French, B.A.; Klibanov, A.L.; Kaul, S.; Lindner, J.R. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med. Biol. 2003, 29, 1759–1767. [Google Scholar] [CrossRef]
- Burke, C.W.; Suk, J.S.; Kim, A.J.; Hsiang, Y.-H.J.; Klibanov, A.L.; Hanes, J.; Price, R.J. Markedly enhanced skeletal muscle transfection achieved by the ultrasound-targeted delivery of non-viral gene nanocarriers with microbubbles. J. Control. Release 2012, 162, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Hsiang, Y.-H.; Song, J.; Price, R.J. The partitioning of nanoparticles to endothelium or interstitium during ultrasound-microbubble-targeted delivery depends on peak-negative pressure. J. Nanopart. Res. 2015, 17, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Chappell, J.C.; Qi, M.; VanGieson, E.J.; Kaul, S.; Price, R.J. Influence of injection site, microvascular pressureand ultrasound variables on microbubble-mediated delivery of microspheres to muscle. J. Am. Coll. Cardiol. 2002, 39, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Leong-Poi, H.; Kuliszewski, M.A.; Lekas, M.; Sibbald, M.; Teichert-Kuliszewska, K.; Klibanov, A.L.; Stewart, D.J.; Lindner, J.R. Therapeutic Arteriogenesis by Ultrasound-Mediated VEGF165 Plasmid Gene Delivery to Chronically Ischemic Skeletal Muscle. Circ. Res. 2007, 101, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Taniyama, Y.; Tachibana, K.; Hiraoka, K.; Aoki, M.; Yamamoto, S.; Matsumoto, K.; Nakamura, T.; Ogihara, T.; Kaneda, Y.; Morishita, R. Development of safe and efficient novel nonviral gene transfer using ultrasound: Enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 2002, 9, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.H.; Brayman, A.A.; Loeb, K.R.; Ye, P.; Zhou, L.; Mourad, P.; Crum, L.A. Ultrasound Enhances Gene Delivery of Human Factor IX Plasmid. Hum. Gene Ther. 2005, 16, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.P.; Brayman, A.A.; Chen, L.; Miao, C.H. Ultrasound with microbubbles enhances gene expression of plasmid DNA in the liver via intraportal delivery. Gene Ther. 2008, 15, 1147–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noble, M.L.; Kuhr, C.S.; Graves, S.S.; Loeb, K.R.; Sun, S.S.; Keilman, G.W.; Morrison, K.P.; Paun, M.; Storb, R.F.; Miao, C.H. Ultrasound-targeted Microbubble Destruction-mediated Gene Delivery Into Canine Livers. Mol. Ther. 2013, 21, 1687–1694. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Noble, M.; Sun, S.; Chen, L.; Brayman, A.A.; Miao, C.H. Efficient Microbubble- and Ultrasound-Mediated Plasmid DNA Delivery into a Specific Rat Liver Lobe via a Targeted Injection and Acoustic Exposure Using a Novel Ultrasound System. Mol. Pharm. 2012, 9, 2187–2196. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Shen, Z.; Chen, L.; Brayman, A.A.; Miao, C.H. Explorations of high-intensity therapeutic ultrasound and microbubble-mediated gene delivery in mouse liver. Gene Ther. 2011, 18, 1006–1014. [Google Scholar] [CrossRef]
- Manta, S.; Renault, G.; Delalande, A.; Couture, O.; Lagoutte, I.; Seguin, J.; Lager, F.; Houzé, P.; Midoux, P.; Bessodes, M.; et al. Cationic microbubbles and antibiotic-free miniplasmid for sustained ultrasound–mediated transgene expression in liver. J. Control. Release 2017, 262, 170–181. [Google Scholar] [CrossRef]
- Anderson, C.D.; Moisyadi, S.; Avelar, A.; Walton, C.B.; Shohet, R.V. Ultrasound-targeted hepatic delivery of factor IX in hemophiliac mice. Gene Ther. 2016, 23, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Raju, B.I.; Leyvi, E.; Seip, R.; Sethuraman, S.; Luo, X.; Bird, A.; Li, S.; Koeberl, D. Enhanced gene expression of systemically administered plasmid DNA in the liver with therapeutic ultrasound and microbubbles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xia, G.; Zhang, Y.; Dong, L.; He, B.; Sun, J. Attenuation of hepatic fibrosis through ultrasound-microbubble-mediated HGF gene transfer in rats. Clin. Imaging 2013, 37, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.-K.Y.; Pham, B.; Zong, Y.; Perez, C.; Maris, D.O.; Hemphill, A.; Miao, C.H.; Matula, T.J.; Mourad, P.D.; Wei, H.; et al. Microbubbles and ultrasound increase intraventricular polyplex gene transfer to the brain. J. Control. Release 2016, 231, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamura, M.; Sato, N.; Taniyama, Y.; Yamamoto, S.; Endoh, M.; Kurinami, H.; Aoki, M.; Ogihara, T.; Kaneda, Y.; Morishita, R. Development of efficient plasmid DNA transfer into adult rat central nervous system using microbubble-enhanced ultrasound. Gene Ther. 2004, 11, 1532–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-Y.; Hsieh, H.-Y.; Pitt, W.G.; Huang, C.-Y.; Tseng, I.-C.; Yeh, C.-K.; Wei, K.-C.; Liu, H.-L. Focused ultrasound-induced blood-brain barrier opening for non-viral, non-invasive, and targeted gene delivery. J. Control. Release 2015, 212, 1–9. [Google Scholar] [CrossRef]
- Fan, C.-H.; Lin, C.-Y.; Liu, H.-L.; Yeh, C.-K. Ultrasound targeted CNS gene delivery for Parkinson’s disease treatment. J. Control. Release 2017, 261, 246–262. [Google Scholar] [CrossRef]
- Stavarache, M.A.; Petersen, N.; Jurgens, E.M.; Milstein, E.R.; Rosenfeld, Z.B.; Ballon, D.J.; Kaplitt, M.G. Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound. J. Neurosurg. 2019, 130, 989–998. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Deng, J.; Xie, Z.; Wang, F.; Chen, S.; Lei, B.; Liao, P.; Huang, N.; Wang, Z.; Wang, Z.; et al. Effective Gene Transfer into Central Nervous System Following Ultrasound-Microbubbles-Induced Opening of the Blood-Brain Barrier. Ultrasound Med. Biol. 2012, 38, 1234–1243. [Google Scholar] [CrossRef]
- Huang, Q.; Deng, J.; Wang, F.; Chen, S.; Liu, Y.; Wang, Z.; Wang, Z.; Cheng, Y. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Exp. Neurol. 2012, 233, 350–356. [Google Scholar] [CrossRef]
- Hsu, P.-H.; Wei, K.C.; Huang, C.Y.; Wen, C.J.; Yen, T.C.; Liu, C.L.; Lin, Y.T.; Chen, J.C.; Shen, C.R.; Liu, H.L. Noninvasive and Targeted Gene Delivery into the Brain Using Microbubble-Facilitated Focused Ultrasound. PLoS ONE 2013, 8, e57682. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-B.; Yang, L.; Wu, J.; Sun, L.; Wu, J.; Tian, H.; Weisel, R.D.; Li, R.-K. Reduced Ischemic Injury After Stroke in Mice by Angiogenic Gene Delivery Via Ultrasound-Targeted Microbubble Destruction. J. Neuropathol. Exp. Neurol. 2014, 73, 548–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xhima, K.; Nabbouh, F.; Hynynen, K.; Aubert, I.; Tandon, A. Noninvasive delivery of an α-synuclein gene silencing vector with magnetic resonance-guided focused ultrasound. Mov. Disord. 2018, 33, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.P.; Kim, N.; Miller, G.W.; Hodges, D.; Mastorakos, P.; Klibanov, A.L.; Mandell, J.W.; Hirsh, J.; Suk, J.S.; Hanes, J.; et al. Novel Focused Ultrasound Gene Therapy Approach Noninvasively Restores Dopaminergic Neuron Function in a Rat Parkinson’s Disease Model. Nano Lett. 2017, 17, 3533–3542. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.; Huang, Y.; Querbes, W.; Sah, D.W.; Hynynen, K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J. Control. Release 2012, 163, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Thévenot, E.; Jordão, J.F.; O’Reilly, M.A.; Markham, K.; Weng, Y.-Q.; Foust, K.D.; Kaspar, B.K.; Hynynen, K.; Aubert, I. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther. 2012, 23, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.P.; Mastorakos, P.; Suk, J.S.; Klibanov, A.L.; Hanes, J.; Price, R.J. Targeted gene transfer to the brain via the delivery of brain-penetrating DNA nanoparticles with focused ultrasound. J. Control. Release 2016, 223, 109–117. [Google Scholar] [CrossRef]
- Chappell, J.C.; Price, R.J. Targeted Therapeutic Applications of Acoustically Active Microspheres in the Microcirculation. Microcirculation 2006, 13, 57–70. [Google Scholar] [CrossRef]
- Babischkin, J.S.; Aberdeen, G.W.; Lindner, J.R.; Bonagura, T.W.; Pepe, G.J.; Albrecht, E.D. Vascular Endothelial Growth Factor Delivery to Placental Basal Plate Promotes Uterine Artery Remodeling in the Primate. Endocrinology 2019. [Google Scholar] [CrossRef]
- Yuan, Q.; Huang, J.; Chu, B.; Li, X.; Li, X.; Si, L. A targeted high-efficiency angiogenesis strategy as therapy for myocardial infarction. Life Sci. 2012, 90, 695–702. [Google Scholar] [CrossRef]
- Sun, Y.; Jin, K.; Xie, L.; Childs, J.; Mao, X.O.; Logvinova, A.; Greenberg, D.A. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J. Clin. Investig. 2003, 111, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Bao, X.J.; Wang, R.Z.; Li, G.L.; Gao, J.; Ma, S.H.; Wei, J.J.; Feng, M.; Zhao, Y.J.; Ma, W.B.; et al. Postacute Ischemia Vascular Endothelial Growth Factor Transfer by Transferrin-Targeted Liposomes Attenuates Ischemic Brain Injury after Experimental Stroke in Rats. Hum. Gene Ther. 2011, 22, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Y.; Meng, Q.; Li, S.; Yao, W.; Hu, G.; Li, Z.; Wang, R. Recombinant Adeno-Associated Virus Serotype 1-Vascular Endothelial Growth Factor Promotes Neurogenesis and Neuromigration in the Subventricular Zone and Rescues Neuronal Function in Ischemic Rats. Neurosurgery 2009, 65, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, S.; Morishita, R.; Hayashi, K.; Yamamoto, K.; Nakagami, H.; Kaneda, Y.; Sakai, N.; Ogihara, T. Inhibition of intimal hyperplasia after balloon injury in rat carotid artery model using cis-element ‘decoy’ of nuclear factor-kB binding site as a novel molecular strategy. Gene Ther. 2001, 8, 1635–1642. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, H.; Suzuki, J.; Ogawa, M.; Taniyama, Y.; Morishita, R.; Isobe, M. Ultrasound-Microbubble-Mediated NF-κB Decoy Transfection Attenuates Neointimal Formation after Arterial Injury in Mice. J. Vasc. Res. 2006, 43, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Asai, T.; Shimizu, M.; Aoki, M.; Hashiya, N.; Sakonjo, H.; Makino, H.; Kaneda, Y.; Ogihara, T.; Morishita, R. Inhibition of NFκB activation using cis-element ‘decoy’ of NFκB binding site reduces neointimal formation in porcine balloon-injured coronary artery model. Gene Ther. 2003, 10, 356–364. [Google Scholar] [CrossRef]
- Suzuki, J.; Ogawa, M.; Takayama, K.; Taniyama, Y.; Morishita, R.; Hirata, Y.; Nagai, R.; Isobe, M. Ultrasound-Microbubble–Mediated Intercellular Adhesion Molecule-1 Small Interfering Ribonucleic Acid Transfection Attenuates Neointimal Formation After Arterial Injury in Mice. J. Am. Coll. Cardiol. 2010, 55, 904–913. [Google Scholar] [CrossRef] [PubMed]
- Shintani, S.; Murohara, T.; Ikeda, H.; Ueno, T.; Sasaki, K.; Duan, J.; Imaizumi, T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation 2001, 103, 897–903. [Google Scholar] [CrossRef]
- Tateishi-Yuyama, E.; Matsubara, H.; Murohara, T.; Ikeda, U.; Shintani, S.; Masaki, H.; Amano, K.; Kishimoto, Y.; Yoshimoto, K.; Akashi, H.; et al. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet 2002, 360, 427–435. [Google Scholar] [CrossRef]
- Imada, T.; Tatsumi, T.; Mori, Y.; Nishiue, T.; Yoshida, M.; Masaki, H.; Okigaki, M.; Kojima, H.; Nozawa, Y.; Nishiwaki, Y.; et al. Targeted Delivery of Bone Marrow Mononuclear Cells by Ultrasound Destruction of Microbubbles Induces Both Angiogenesis and Arteriogenesis Response. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2128–2134. [Google Scholar] [CrossRef] [Green Version]
- Zen, K.; Okigaki, M.; Hosokawa, Y.; Adachi, Y.; Nozawa, Y.; Takamiya, M.; Tatsumi, T.; Urao, N.; Tateishi, K.; Takahashi, T.; et al. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J. Mol. Cell. Cardiol. 2006, 40, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Wong, J.; Griffin, B.; Ellis, S.G.; Porter, T.; Sen, S.; Thomas, J.D. Ten-fold augmentation of endothelial uptake of vascular endothelial growth factor with ultrasound after systemic administration. J. Am. Coll. Cardiol. 2000, 35, 1678–1686. [Google Scholar] [CrossRef] [Green Version]
- Miyake, Y.; Ohmori, K.; Yoshida, J.; Ishizawa, M.; Mizukawa, M.; Yukiiri, K.; Kohno, M. Granulocyte Colony-Stimulating Factor Facilitates the Angiogenesis Induced by Ultrasonic Microbubble Destruction. Ultrasound Med. Biol. 2007, 33, 1796–1804. [Google Scholar] [CrossRef] [PubMed]
- Chappell, J.C.; Song, J.; Burke, C.W.; Klibanov, A.L.; Price, R.J. Targeted delivery of nanoparticles bearing fibroblast growth factor-2 by ultrasonic microbubble destruction for therapeutic arteriogenesis. Small 2008, 4, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Rychak, J.J.; Li, B.; Acton, S.T.; Leppänen, A.; Cummings, R.D.; Ley, K.; Klibanov, A.L. Selectin Ligands Promote Ultrasound Contrast Agent Adhesion under Shear Flow. Mol. Pharm. 2006, 3, 516–524. [Google Scholar] [CrossRef]
- Rychak, J.J.; Klibanov, A.L.; Ley, K.F.; Hossack, J.A. Enhanced Targeting of Ultrasound Contrast Agents Using Acoustic Radiation Force. Ultrasound Med. Biol. 2007, 33, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Takalkar, A.M.; Klibanov, A.L.; Rychak, J.J.; Lindner, J.R.; Ley, K. Binding and detachment dynamics of microbubbles targeted to P-selectin under controlled shear flow. J. Control. Release 2004, 96, 473–482. [Google Scholar] [CrossRef]
- Rychak, J.J.; Lindner, J.R.; Ley, K.; Klibanov, A.L. Deformable gas-filled microbubbles targeted to P-selectin. J. Control. Release 2006, 114, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Klibanov, A.L.; Rychak, J.J.; Yang, W.C.; Alikhani, S.; Li, B.; Acton, S.; Lindner, J.R.; Ley, K.; Kaul, S. Targeted ultrasound contrast agent for molecular imaging of inflammation in high-shear flow. Contrast Media Mol. Imaging 2006, 1, 259–266. [Google Scholar] [CrossRef]
- Lindner, J.R.; Song, J.; Christiansen, J.; Klibanov, A.L.; Xu, F.; Ley, K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation 2001, 104, 2107–2112. [Google Scholar] [CrossRef]
- Shentu, W.; Yan, C.; Liu, C.; Qi, R.; Wang, Y.; Huang, Z.; Zhou, L.; You, X. Use of cationic microbubbles targeted to P-selectin to improve ultrasound-mediated gene transfection of hVEGF165 to the ischemic myocardium. J. Zhejiang Univ. B 2018, 19, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Fokong, S.; Fragoso, A.; Rix, A.; Curaj, A.; Wu, Z.; Lederle, W.; Iranzo, O.; Gätjens, J.; Kiessling, F.; Palmowski, M. Ultrasound Molecular Imaging of E-Selectin in Tumor Vessels Using Poly n-Butyl Cyanoacrylate Microbubbles Covalently Coupled to a Short Targeting Peptide. Investig. Radiol. 2013, 48, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Spivak, I.; Rix, A.; Schmitz, G.; Fokong, S.; Iranzo, O.; Lederle, W.; Kiessling, F. Low-Dose Molecular Ultrasound Imaging with E-Selectin-Targeted PBCA Microbubbles. Mol. Imaging Biol. 2016, 18, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Leng, X.; Wang, J.; Carson, A.; Chen, X.; Fu, H.; Ottoboni, S.; Wagner, W.R.; Villanueva, F.S. Ultrasound detection of myocardial ischemic memory using an E-selectin targeting peptide amenable to human application. Mol. Imaging 2014, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Weller, G.E.R.; Villanueva, F.S.; Tom, E.M.; Wagner, W.R. Targeted ultrasound contrast agents: In vitro assessment of endothelial dysfunction and multi-targeting to ICAM-1 and sialyl Lewisx. Biotechnol. Bioeng. 2005, 92, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-P.; Luo, L.-H.; Wu, F.-L. Evaluation of renal tissue ischemia-reperfusion injury with ultrasound radiation force and targeted microbubbles. Nan Fang Yi Ke Da Xue Xue Bao 2017, 37, 402–406. [Google Scholar] [PubMed]
- Moccetti, F.; Weinkauf, C.C.; Davidson, B.P.; Belcik, J.T.; Marinelli, E.R.; Unger, E.; Lindner, J.R. Ultrasound Molecular Imaging of Atherosclerosis Using Small-Peptide Targeting Ligands Against Endothelial Markers of Inflammation and Oxidative Stress. Ultrasound Med. Biol. 2018, 44, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Samiotaki, G.; Acosta, C.; Wang, S.; Konofagou, E.E. Enhanced delivery and bioactivity of the neurturin neurotrophic factor through focused ultrasound-mediated blood-brain barrier opening in vivo. J. Cereb. Blood Flow Metab. 2015, 35, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Unnikrishnan, S.; Herbst, E.B.; Klibanov, A.L.; Mauldin, F.W.; Hossack, J.A. Ultrasound Molecular Imaging of Inflammation in Mouse Abdominal Aorta. Investig. Radiol. 2017, 52, 499–506. [Google Scholar] [CrossRef]
- Phillips, L.C.; Klibanov, A.L.; Wamhoff, B.R.; Hossack, J.A. Intravascular ultrasound detection and delivery of molecularly targeted microbubbles for gene delivery. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Daeichin, V.; Kooiman, K.; Skachkov, I.; Bosch, J.G.; Theelen, T.L.; Steiger, K.; Needles, A.; Janssen, B.J.; Daemen, M.J.; van der Steen, A.F.; et al. Quantification of Endothelial αvβ3 Expression with High-Frequency Ultrasound and Targeted Microbubbles: In Vitro and In Vivo Studies. Ultrasound Med. Biol. 2016, 42, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Wang, W.; Wen, J.; Lin, L.; Exner, A.A.; Guan, P.; Chen, X. Dual-Targeted Microbubbles Specific to Integrin αvβ3 and Vascular Endothelial Growth Factor Receptor 2 for Ultrasonography Evaluation of Tumor Angiogenesis. Ultrasound Med. Biol. 2018, 44, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Xu, X.; Chen, Y.; Deng, Z.; Liu, H.; Xu, J.; Zhou, J.; Tan, G.; Wu, J.; Zheng, H. A Lipopeptide-Based αvβ3 Integrin-Targeted Ultrasound Contrast Agent for Molecular Imaging of Tumor Angiogenesis. Ultrasound Med. Biol. 2015, 41, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Pochon, S.; Tardy, I.; Bussat, P.; Bettinger, T.; Brochot, J.; von Wronski, M.; Passantino, L.; Schneider, M. BR55: A Lipopeptide-Based VEGFR2-Targeted Ultrasound Contrast Agent for Molecular Imaging of Angiogenesis. Investig. Radiol. 2010, 45, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Eschbach, R.S.; Clevert, D.A.; Hirner-Eppeneder, H.; Ingrisch, M.; Moser, M.; Schuster, J.; Tadros, D.; Schneider, M.; Kazmierczak, P.M.; Reiser, M.; et al. Contrast-Enhanced Ultrasound with VEGFR2-Targeted Microbubbles for Monitoring Regorafenib Therapy Effects in Experimental Colorectal Adenocarcinomas in Rats with DCE-MRI and Immunohistochemical Validation. PLoS ONE 2017, 12, e0169323. [Google Scholar] [CrossRef]
- Baetke, S.C.; Rix, A.; Tranquart, F.; Schneider, R.; Lammers, T.; Kiessling, F.; Lederle, W. Squamous Cell Carcinoma Xenografts: Use of VEGFR2-targeted Microbubbles for Combined Functional and Molecular US to Monitor Antiangiogenic Therapy Effects. Radiology 2016, 278, 430–440. [Google Scholar] [CrossRef] [Green Version]
- Chang, E.-L.; Ting, C.Y.; Hsu, P.H.; Lin, Y.C.; Liao, E.C.; Huang, C.Y.; Chang, Y.C.; Chan, H.L.; Chiang, C.S.; Liu, H.L.; et al. Angiogenesis-targeting microbubbles combined with ultrasound-mediated gene therapy in brain tumors. J. Control. Release 2017, 255, 164–175. [Google Scholar] [CrossRef]
- Liu, C.; Yan, F.; Xu, Y.; Zheng, H.; Sun, L. In Vivo Molecular Ultrasound Assessment of Glioblastoma Neovasculature with Endoglin-Targeted Microbubbles. Contrast Media Mol. Imaging 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Zhou, Y.; Gu, H.; Xu, Y.; Li, F.; Kuang, S.; Wang, Z.; Zhou, X.; Ma, H.; Li, P.; Zheng, Y.; et al. Targeted Antiangiogenesis Gene Therapy Using Targeted Cationic Microbubbles Conjugated with CD105 Antibody Compared with Untargeted Cationic and Neutral Microbubbles. Theranostics 2015, 5, 399–417. [Google Scholar] [CrossRef]
- Kripfgans, O.D.; Fowlkes, J.B.; Miller, D.L.; Eldevik, O.P.; Carson, P.L. Acoustic droplet vaporization for therapeutic and diagnostic applications. Ultrasound Med. Biol. 2000, 26, 1177–1189. [Google Scholar] [CrossRef]
- Kripfgans, O.D.; Fabiilli, M.L.; Carson, P.L.; Fowlkes, J.B. On the acoustic vaporization of micrometer-sized droplets. J. Acoust. Soc. Am. 2004, 116, 272–281. [Google Scholar] [CrossRef]
- Dayton, P.A.; Zhao, S.; Bloch, S.H.; Schumann, P.; Penrose, K.; Matsunaga, T.O.; Zutshi, R.; Doinikov, A.; Ferrara, K.W. Application of ultrasound to selectively localize nanodroplets for targeted imaging and therapy. Mol. Imaging 2006, 5, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Shpak, O.; Stricker, L.; Versluis, M.; Lohse, D. The role of gas in ultrasonically driven vapor bubble growth. Phys. Med. Biol. 2013, 58, 2523–2535. [Google Scholar] [CrossRef] [PubMed]
- Sheeran, P.S.; Wong, V.P.; Luois, S.; McFarland, R.J.; Ross, W.D.; Feingold, S.; Matsunaga, T.O.; Dayton, P.A. Decafluorobutane as a Phase-Change Contrast Agent for Low-Energy Extravascular Ultrasonic Imaging. Ultrasound Med. Biol. 2011, 37, 1518–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.-T.; Lin, Y.-C.; Yeh, C.-K. Mechanical bioeffects of acoustic droplet vaporization in vessel-mimicking phantoms. Ultrason. Sonochem. 2014, 21, 1866–1874. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.-L.; Lin, Y.-K.; Chi, C.-H.; Huang, T.-H.; Fang, J.-Y. Development and Evaluation of Perfluorocarbon Nanobubbles for Apomorphine Delivery. J. Pharm. Sci. 2009, 98, 3735–3747. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-Y.; Hung, C.-F.; Hua, S.-C.; Hwang, T.-L. Acoustically active perfluorocarbon nanoemulsions as drug delivery carriers for camptothecin: Drug release and cytotoxicity against cancer cells. Ultrasonics 2009, 49, 39–46. [Google Scholar] [CrossRef]
- Fabiilli, M.L.; Lee, J.A.; Kripfgans, O.D.; Carson, P.L.; Fowlkes, J.B. Delivery of Water-Soluble Drugs Using Acoustically Triggered Perfluorocarbon Double Emulsions. Pharm. Res. 2010, 27, 2753–2765. [Google Scholar] [CrossRef] [Green Version]
- Fabiilli, M.L.; Haworth, K.J.; Sebastian, I.E.; Kripfgans, O.D.; Carson, P.L.; Fowlkes, J.B. Delivery of Chlorambucil Using an Acoustically-Triggered Perfluoropentane Emulsion. Ultrasound Med. Biol. 2010, 36, 1364–1375. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-H.; Kang, S.-T.; Lee, Y.-H.; Luo, Y.-L.; Huang, Y.-F.; Yeh, C.-K. Aptamer-conjugated and drug-loaded acoustic droplets for ultrasound theranosis. Biomaterials 2012, 33, 1939–1947. [Google Scholar] [CrossRef]
- Fabiilli, M.L.; Wilson, C.G.; Padilla, F.; Martín-Saavedra, F.M.; Fowlkes, J.B.; Franceschi, R.T. Acoustic droplet–hydrogel composites for spatial and temporal control of growth factor delivery and scaffold stiffness. Acta Biomater. 2013, 9, 7399–7409. [Google Scholar] [CrossRef] [PubMed]
- Moncion, A.; Arlotta, K.J.; O’Neill, E.G.; Lin, M.; Mohr, L.A.; Franceschi, R.T.; Kripfgans, O.D.; Putnam, A.J.; Fabiilli, M.L. In vitro and in vivo assessment of controlled release and degradation of acoustically responsive scaffolds. Acta Biomater. 2016, 46, 221–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncion, A.; Lin, M.; O’Neill, E.G.; Franceschi, R.T.; Kripfgans, O.D.; Putnam, A.J.; Fabiilli, M.L. Controlled release of basic fibroblast growth factor for angiogenesis using acoustically-responsive scaffolds. Biomaterials 2017, 140, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343. [Google Scholar] [CrossRef] [PubMed]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimcevski, G.; Kotopoulis, S.; Bjånes, T.; Hoem, D.; Schjøtt, J.; Gjertsen, B.T.; Biermann, M.; Molven, A.; Sorbye, H.; McCormack, E.; et al. A human clinical trial using ultrasound and microbubbles to enhance gemcitabine treatment of inoperable pancreatic cancer. J. Control. Release 2016, 243, 172–181. [Google Scholar] [CrossRef] [Green Version]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorick, C.M.; Chappell, J.C.; Price, R.J. Applications of Ultrasound to Stimulate Therapeutic Revascularization. Int. J. Mol. Sci. 2019, 20, 3081. https://doi.org/10.3390/ijms20123081
Gorick CM, Chappell JC, Price RJ. Applications of Ultrasound to Stimulate Therapeutic Revascularization. International Journal of Molecular Sciences. 2019; 20(12):3081. https://doi.org/10.3390/ijms20123081
Chicago/Turabian StyleGorick, Catherine M., John C. Chappell, and Richard J. Price. 2019. "Applications of Ultrasound to Stimulate Therapeutic Revascularization" International Journal of Molecular Sciences 20, no. 12: 3081. https://doi.org/10.3390/ijms20123081