Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary
Abstract
:1. Introduction
2. Results
2.1. Gene Expression Profiles of the Serotonin Transporter SERT and Enzymes of Serotonin Synthesis DDC, TPH1 and TPH2, during Postnatal Development of Mouse Ovary
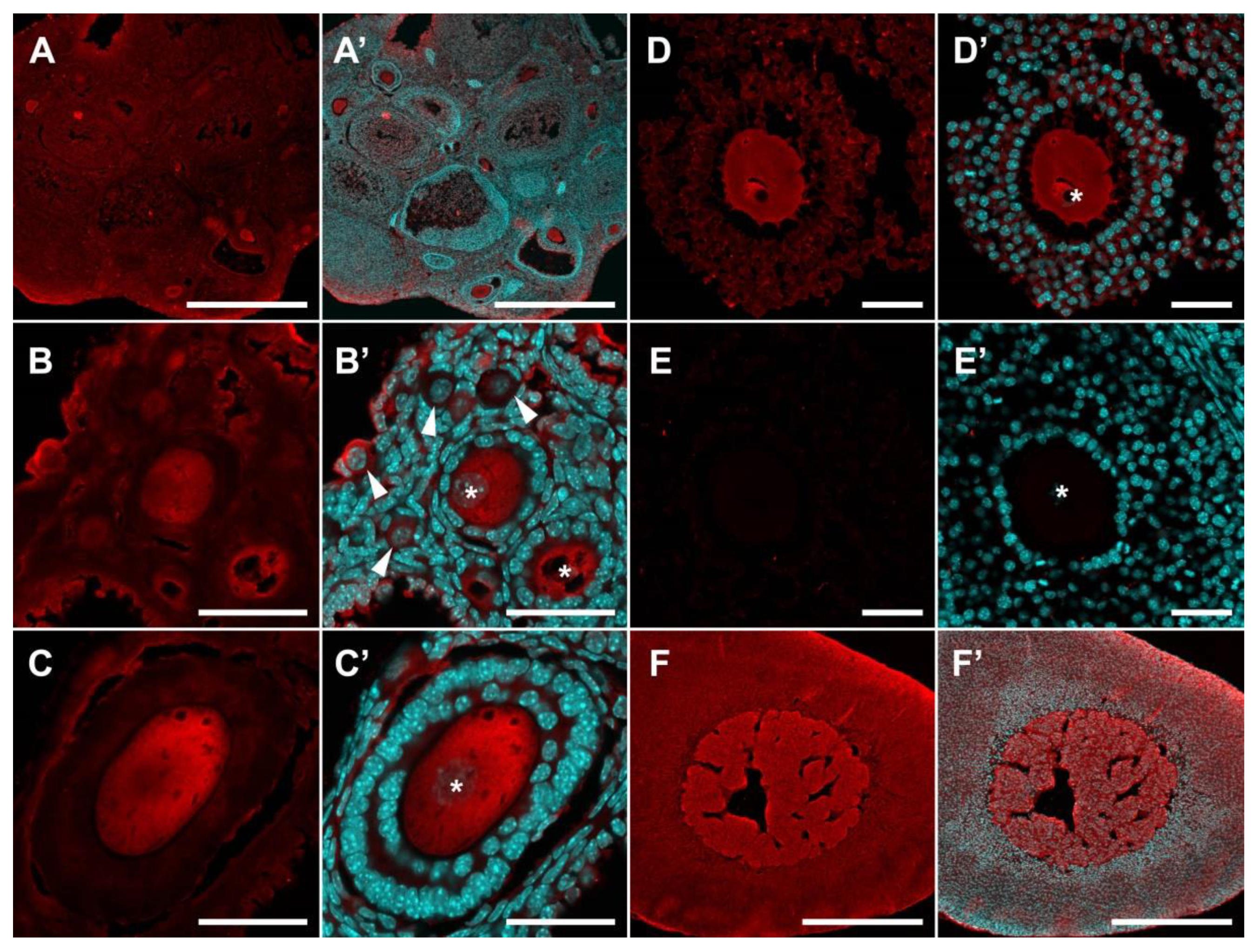
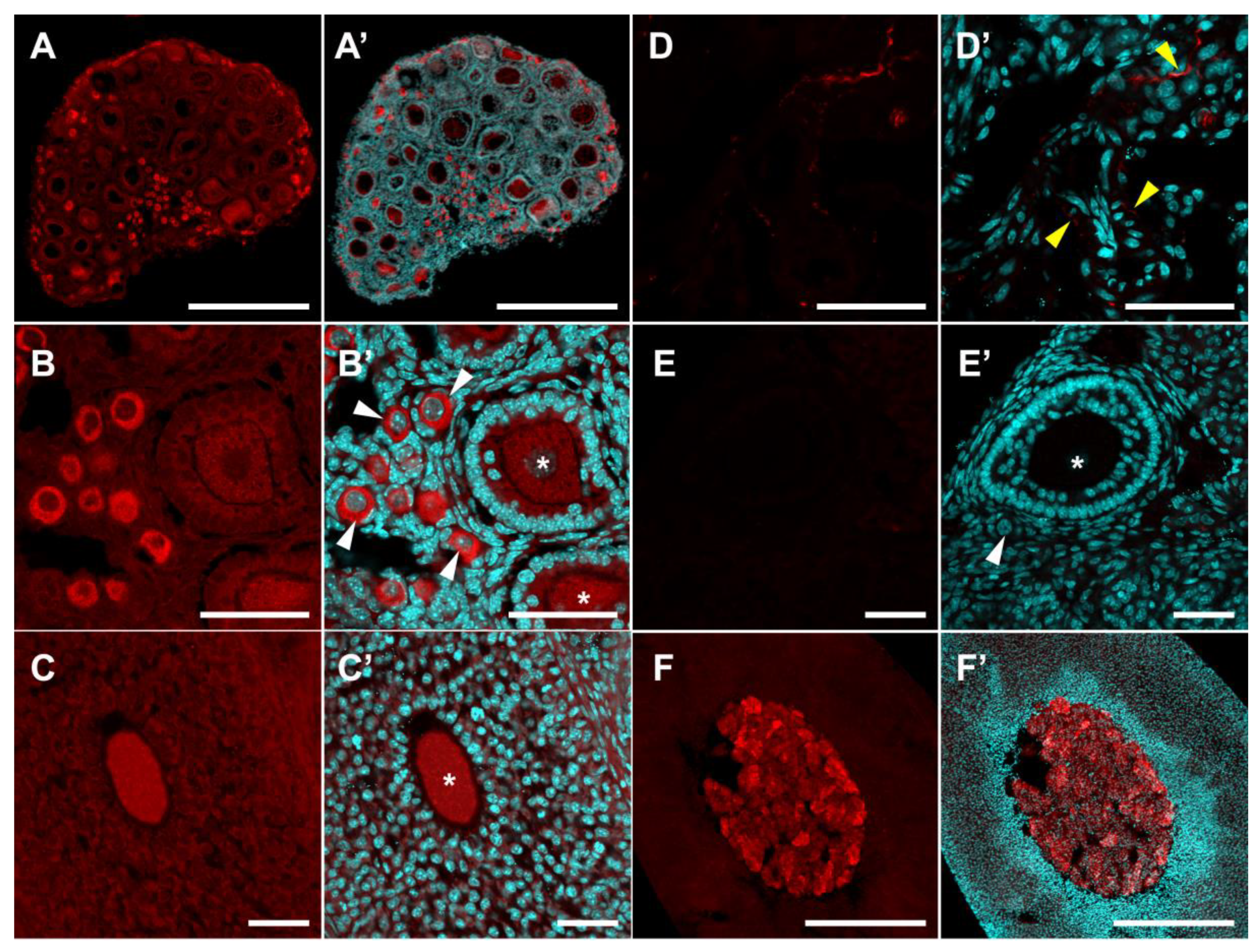
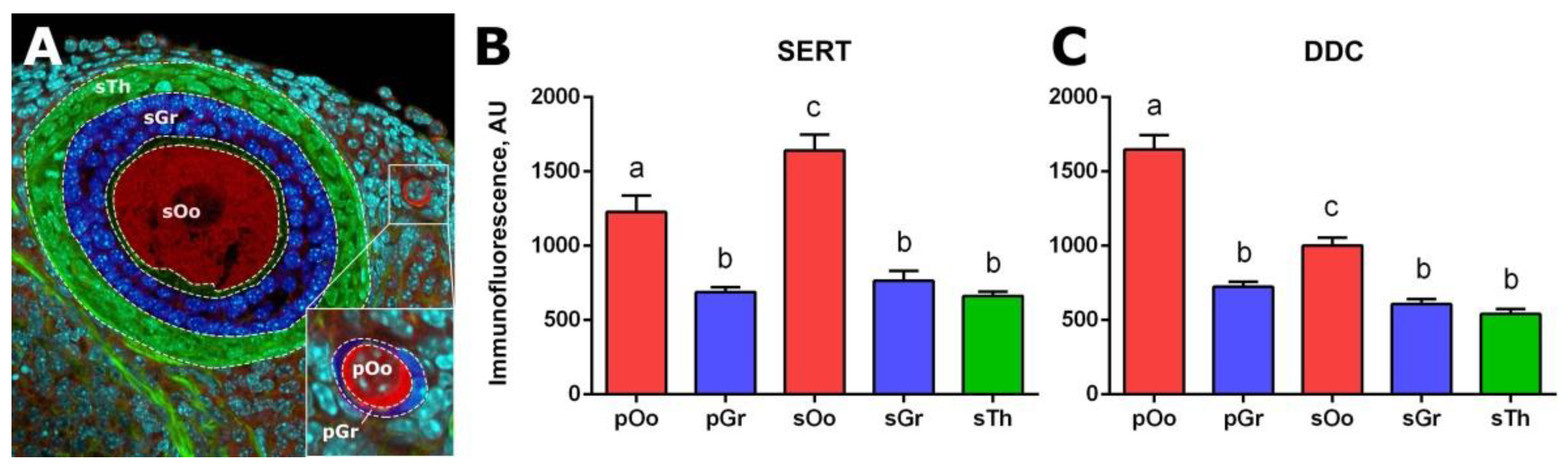
2.2. Localization of SERT and DDC Immunoreactivity in Mouse Ovary
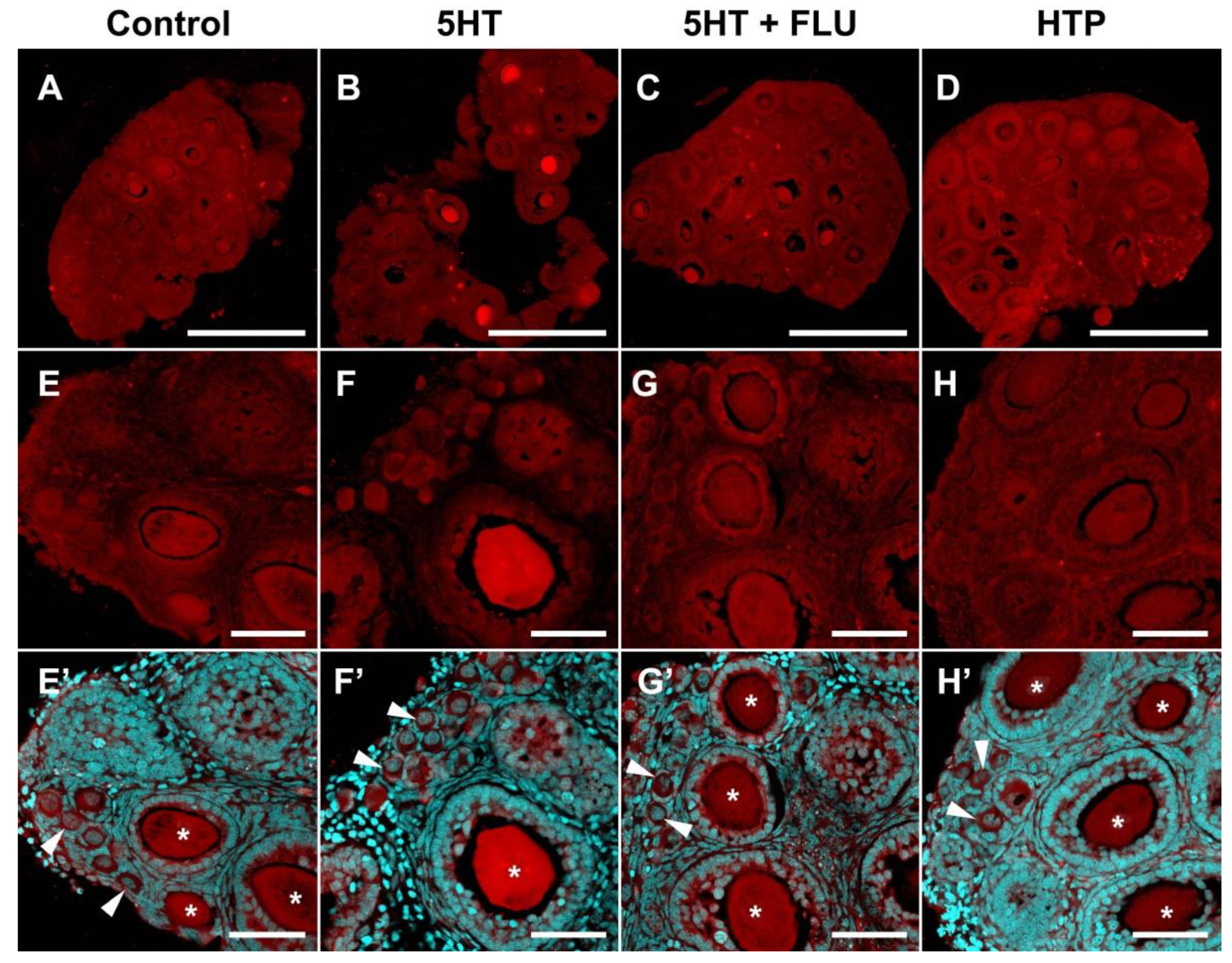
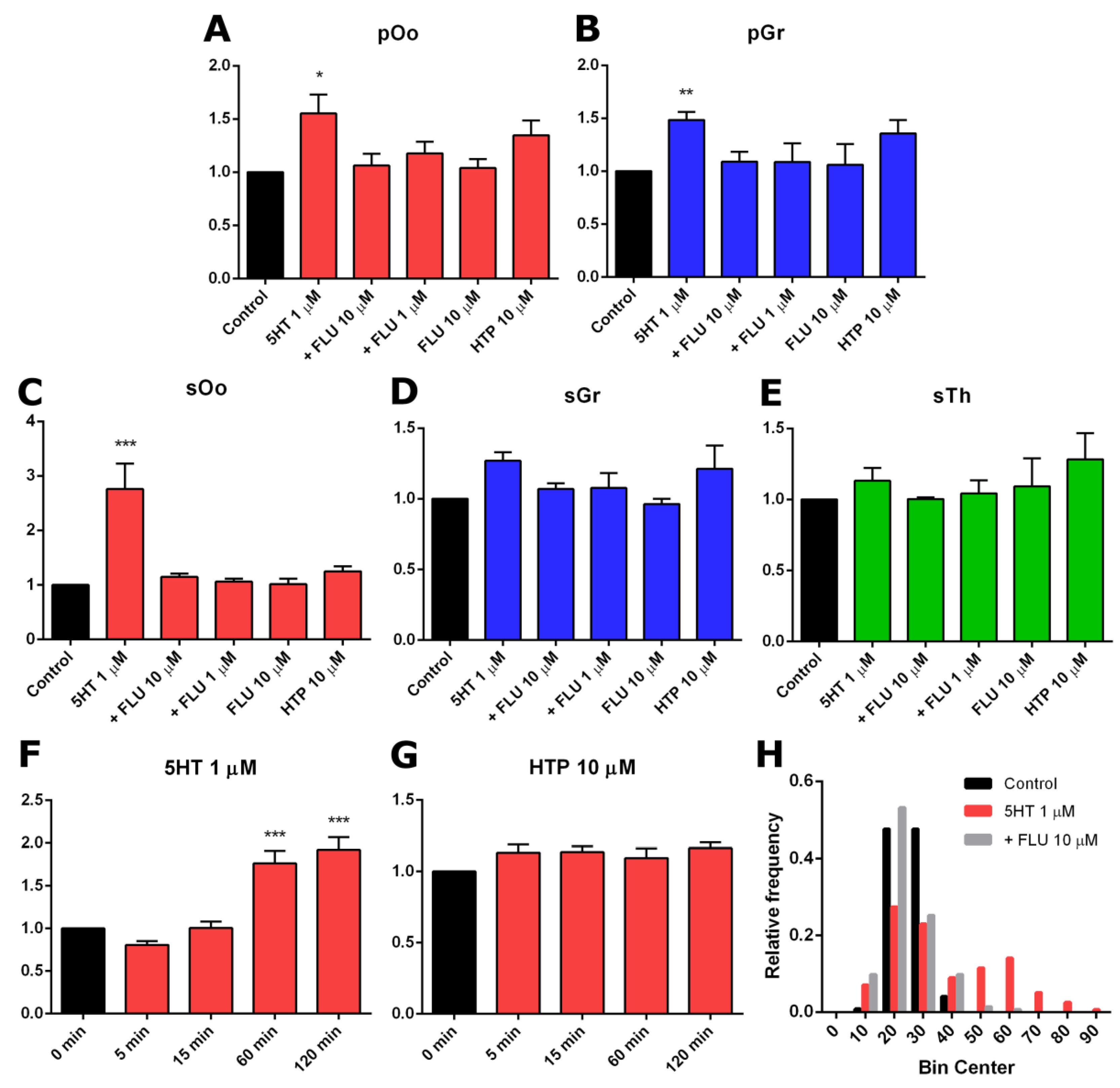
2.3. Functional Activity of Serotonin Synthesis and Uptake in Mouse Ovary
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Quantitative Polymerase Chain Reaction (qPCR)
4.3. In Vitro Ovary Incubation Experiments
4.4. Immunohistochemistry
4.5. Estimation of Serotonin Immunoreactivity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AU | arbitrary units |
| BSA | bovine serum albumin |
| dpp | day postpartum |
| 5HT | 5-hydroxytryptamine, serotonin |
| HTP | 5-hydroxy-L-tryptophan |
| PBS | phosphate buffered saline |
| PCR | polymerase chain reaction |
| PFA | paraformaldehyde |
| qPCR | quantitative real-time PCR |
| ROI | region of interest |
| SSRI | selective serotonin reuptake inhibitor |
References
- Clausell, D.E.; Soliman, K.F. Ovarian serotonin content in relation to ovulation. Experientia 1978, 34, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Bòdis, J.; Bognàr, Z.; Hartmann, G.; Török, A.; Csaba, I.F. Measurement of noradrenaline, dopamine and serotonin contents in follicular fluid of human graafian follicles after superovulation treatment. Gynecol. Obstet. Investig. 1992, 33, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Amireault, P.; Dubé, F. Serotonin and its antidepressant-sensitive transport in mouse cumulus-oocyte complexes and early embryos. Biol. Reprod. 2005, 73, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Buznikov, G.A.; Nikitina, L.A.; Galanov, A.Y.; Malchenko, L.A.; Trubnikova, O.B. The control of oocyte maturation in the starfish and amphibians by serotonin and its antagonists. Int. J. Dev. Biol. 1993, 37, 363–364. [Google Scholar] [PubMed]
- Terranova, P.F.; Uilenbroek, J.T.; Saville, L.; Horst, D.; Nakamura, Y. Serotonin enhances oestradiol production by hamster preovulatory follicles in vitro: effects of experimentally induced atresia. J. Endocrinol. 1990, 125, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, E.; Baba, N.; Toshida, K.; Suzuki, K. Serotonin stimulates steroidogenesis in rat preovulatory follicles: involvement of 5-HT2 receptor. Life Sci. 1993, 53, 563–570. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, L.; Liu, X.S.J.S.; Montplaisir, V.; Tiberi, M.; Baltz, J.M.; Liu, X.J. A serotonin receptor antagonist induces oocyte maturation in both frogs and mice: evidence that the same G protein ptor is responsible for maintaining meiosis arrest in both species. J. Cell Physiol. 2005, 202, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Bódis, J.; Hartmann, G.; Tinneberg, H.R.; Török, A.; Hanf, V.; Papenfuss, F.; Schwarz, H. Relationship between the monoamine, progesterone and estradiol content in follicular fluid of preovulatory graafian follicles after superovulation treatment. Gynecol. Obstet. Invest. 1993, 35, 232–235. [Google Scholar] [CrossRef]
- Koppan, M.; Bodis, J.; Verzar, Z.; Tinneberg, H.-R.; Torok, A. Serotonin may alter the pattern of gonadotropin-induced progesterone release of human granulosa cells in superfusion system. Endocrine 2004, 24, 155–159. [Google Scholar] [CrossRef]
- Graveleau, C.; Paust, H.J.; Schmidt-Grimminger, D.; Mukhopadhyay, A.K. Presence of a 5-HT7 receptor positively coupled to adenylate cyclase activation in human granulosa-lutein cells. J. Clin Endocrinol. Metab. 2000, 85, 1277–1286. [Google Scholar] [CrossRef]
- Basu, B.; Desai, R.; Balaji, J.; Chaerkady, R.; Sriram, V.; Maiti, S.; Panicker, M.M. Serotonin in pre-implantation mouse embryos is localized to the mitochondria and can modulate mitochondrial potential. Reproduction 2008, 135, 657–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amenta, F.; Vega, J.A.; Ricci, A.; Collier, W.L. Localization of 5-hydroxytryptamine-like immunoreactive cells and nerve fibers in the rat female reproductive system. Anat Rec. 1992, 233, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Jequier, E.; Robinson, D.S.; Lovenberg, W.; Sjoerdsma, A. Further studies on tryptophan hydroxylase in rat brainstem and beef pineal. Biochem. Pharmacol. 1969, 18, 1071–1081. [Google Scholar] [CrossRef]
- Dubé, F.; Amireault, P. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci. 2007, 81, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Nikishin, D.A.; Khramova, Y.V.; Bagayeva, T.S.; Semenova, M.L.; Shmukler, Y.B. Expression of Components of the Serotonergic System in Folliculogenesis and Preimplantation Development in Mice. Russ. J. Dev. Biol. 2018, 49, 184–192. [Google Scholar] [CrossRef]
- Comai, S.; Bertazzo, A.; Carretti, N.; Podfigurna-Stopa, A.; Luisi, S.; Costa, C.V.L. Serum levels of tryptophan, 5-hydroxytryptophan and serotonin in patients affected with different forms of amenorrhea. Int. J. Tryptophan Res. 2010, 3, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Wang, Y.; Wang, Z.; Xin, Q.; Wang, Y.; Feng, L.; Zhao, L.; Wen, J.; Zhang, H.; Wang, C.; Xia, G. JNK signaling regulates E-cadherin junctions in germline cysts and determines primordial follicle formation in mice. Development 2016, 143, 1778–1787. [Google Scholar] [CrossRef] [Green Version]
- François, C.M.; Petit, F.; Giton, F.; Gougeon, A.; Ravel, C.; Magre, S.; Cohen-Tannoudji, J.; Guigon, C.J. A novel action of follicle-stimulating hormone in the ovary promotes estradiol production without inducing excessive follicular growth before puberty. Sci. Rep. 2017, 7, 46222. [Google Scholar] [CrossRef]
- Morohaku, K.; Tanimoto, R.; Sasaki, K.; Kawahara-Miki, R.; Kono, T.; Hayashi, K.; Hirao, Y.; Obata, Y. Complete in vitro generation of fertile oocytes from mouse primordial germ cells. Proc. Natl. Acad. Sci. USA 2016, 113, 9021–9026. [Google Scholar] [CrossRef] [Green Version]
- Daws, L.C. Unfaithful neurotransmitter transporters: focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol. Ther. 2009, 121, 89–99. [Google Scholar] [CrossRef]
- Kim, C.-W.; Choe, C.; Kim, E.-J.; Lee, J.I.; Yoon, S.Y.; Cho, Y.W.; Han, S.; Tak, H.M.; Han, J.; Kang, D. Dual effects of fluoxetine on mouse early embryonic development. Toxicol. Appl. Pharmacol. 2012, 265, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Wenthur, C.J.; Bennett, M.R.; Lindsley, C.W. Classics in chemical neuroscience: Fluoxetine (Prozac). ACS Chem. Neurosci. 2014, 5, 14–23. [Google Scholar] [CrossRef]
- Greiner, M.; Paredes, A.; Rey-Ares, V.V.; Saller, S.; Mayerhofer, A.; Lara, H.E.H.E. Catecholamine uptake, storage, and regulated release by ovarian granulosa cells. Endocrinology 2008, 149, 4988–4996. [Google Scholar] [CrossRef] [PubMed]
- Boxberger, K.H.; Hagenbuch, B.; Lampe, J.N. Common drugs inhibit human organic cation transporter 1 (oct1)-mediated neurotransmitter uptake. Drug Metab. Dispos. 2014, 42, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Zha, W.; Ho, H.T.B.; Hu, T.; Hebert, M.F.; Wang, J. Serotonin transporter deficiency drives estrogen-dependent obesity and glucose intolerance. Sci. Rep. 2017, 7, 1137. [Google Scholar] [CrossRef]
- Lister, A.; Regan, C.; Van Zwol, J.; Van Der Kraak, G. Inhibition of egg production in zebrafish by fluoxetine and municipal effluents: A mechanistic evaluation. Aquat. Toxicol. 2009, 95, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A Review of Melatonin, Its Receptors and Drugs. Eurasian J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef]
- Sakaguchi, K.; Itoh, M.T.; Takahashi, N.; Tarumi, W.; Ishizuka, B. The rat oocyte synthesises melatonin. Reprod. Fertil. Dev. 2013, 25, 674–682. [Google Scholar] [CrossRef]
- Ivashkin, E.; Khabarova, M.Y.; Melnikova, V.; Nezlin, L.P.; Kharchenko, O.; Voronezhskaya, E.E.; Adameyko, I. Serotonin Mediates Maternal Effects and Directs Developmental and Behavioral Changes in the Progeny of Snails. Cell Rep. 2015, 12, 1144–1158. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, T.; Nakada, K.; Shirakawa, H.; Miyazaki, S. Development of inositol trisphosphate-induced calcium release mechanism during maturation of hamster oocytes. Dev. Biol. 1993, 156, 69–79. [Google Scholar] [CrossRef]
- Vitale, S.G.; Rossetti, P.; Corrado, F.; Rapisarda, A.M.; La Vignera, S.; Condorelli, R.A.; Valenti, G.; Sapia, F.; Laganà, A.S.; Buscema, M. How to Achieve High-Quality Oocytes? The Key Role of Myo-Inositol and Melatonin. Int. J. Endocrinol. 2016, 2016, 4987436. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Garzon, S.; Casarin, J.; Franchi, M.; Ghezzi, F. Inositol in Polycystic Ovary Syndrome: Restoring Fertility through a Pathophysiology-Based Approach. Trends Endocrinol. Metab. 2018, 29, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Nikishin, D.A.; Milošević, I.; Gojković, M.; Rakić, L.; Bezuglov, V.V.; Shmukler, Y.B. Expression and functional activity of neurotransmitter system components in sea urchins’ early development. Zygote 2016, 24, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.M.Y.; Kaplan, B.J. Perinatal Depression: Prevalence, Risks, and the Nutrition Link—A Review of the Literature. J. Am. Diet Assoc. 2009, 109, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; Mitchell, A.A.; Louik, C.; Werler, M.M.; Chambers, C.D.; Hernández-Díaz, S. Antidepressant use during pregnancy and the risk of preterm delivery and fetal growth restriction. J. Clin. Psychopharmacol. 2009, 29, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Reefhuis, J.; Devine, O.; Friedman, J.M.; Louik, C.; Honein, M.A. Specific SSRIs and birth defects: Bayesian analysis to interpret new data in the context of previous reports. BMJ 2015, 351, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaihola, H.; Yaldir, F.G.; Hreinsson, J.; Hörnaeus, K.; Bergquist, J.; Olivier, J.D.; Åkerud, H.; Sundström-Poromaa, I. Effects of Fluoxetine on Human Embryo Development. Front. Cell. Neurosci. 2016, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 1–11. [Google Scholar] [CrossRef]
- Rooney, K.L.; Domar, A.D. The relationship between stress and infertility. Dialogues Clin. Neurosci. 2018, 20, 41–47. [Google Scholar]
- Sylvester, C.; Menke, M.; Gopalan, P. Selective Serotonin Reuptake Inhibitors and Fertility: Considerations for Couples Trying to Conceive. Harv. Rev. Psychiatry 2019, 27, 108–118. [Google Scholar]
- Nikishin, D.A.; Filatov, M.A.; Kiseleva, M.V.; Bagaeva, T.S.; Konduktorova, V.V.; Khramova, Y.V.; Malinova, I.V.; Komarova, E.V.; Semenova, M.L. Selection of stable expressed reference genes in native and vitrified/thawed human ovarian tissue for analysis by qRT-PCR and Western blot. J. Assist Reprod. Genet. 2018, 35, 1851–1860. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | NCBI Gene ID | Forward Primer | Reverse Primer | PCR Product Length, bp | PCR Product Tm, °C |
|---|---|---|---|---|---|
| SERT (SLC6A4) | 15567 | GGGAGACCTGGGGCAAGAAG | CAGGGCGAGCTCCATGTAGAAGA | 182 | 87.1 |
| DDC | 13195 | TCCCCACGGCTAGCTCATACCC | TTCCCCAGCCAGTCCATCATCA | 133 | 86.5 |
| TPH1 | 21990 | TGCGACATCAGCCGAGAACAGT | GGCGCAGAAGTCCAGGTCAGA | 162 | 86.4 |
| TPH2 | 216343 | CATGGCTCCGACCCCCTCTACA | ATACGCCCGCAGTTGACCCTCTT | 219 | 86.8 |
| RPS18 | 20084 | AAGAAAATTCGAGCCCATAGAGG | TAACAGCAAAGGCCCAGAGACT | 138 | 86.1 |
| Antibody | Manufacturer | Catalogue Number | Dilution |
|---|---|---|---|
| Goat polyclonal Anti-Serotonin transporter (Sert) antibody | Abcam, UK | ab130130 | 1:500 |
| Rabbit polyclonal Anti-DOPA Decarboxylase (Ddc) antibody | Abcam, UK | ab3905 | 1:500 |
| Anti-Serotonin antibody produced in rabbitwhole antiserum | Sigma-Aldrich, Germany | S5545 | 1:1000 |
| Fluorescein (FITC) AffiniPure Goat Anti-Rabbit IgG (H + L) | Jackson Immuno Research, UK | 111-095-003 | 1:200 |
| Donkey F(ab’)2 Anti-Rabbit IgG H&L (Alexa Fluor® 568) preadsorbed | Abcam, UK | ab175694 | 1:200 |
| Donkey F(ab’)2 Anti-Goat IgG H&L (Alexa Fluor® 647) preadsorbed | Abcam, UK | ab150139 | 1:200 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikishin, D.A.; Alyoshina, N.M.; Semenova, M.L.; Shmukler, Y.B. Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary. Int. J. Mol. Sci. 2019, 20, 3070. https://doi.org/10.3390/ijms20123070
Nikishin DA, Alyoshina NM, Semenova ML, Shmukler YB. Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary. International Journal of Molecular Sciences. 2019; 20(12):3070. https://doi.org/10.3390/ijms20123070
Chicago/Turabian StyleNikishin, Denis A., Nina M. Alyoshina, Maria L. Semenova, and Yuri B. Shmukler. 2019. "Analysis of Expression and Functional Activity of Aromatic L-Amino Acid Decarboxylase (DDC) and Serotonin Transporter (SERT) as Potential Sources of Serotonin in Mouse Ovary" International Journal of Molecular Sciences 20, no. 12: 3070. https://doi.org/10.3390/ijms20123070





