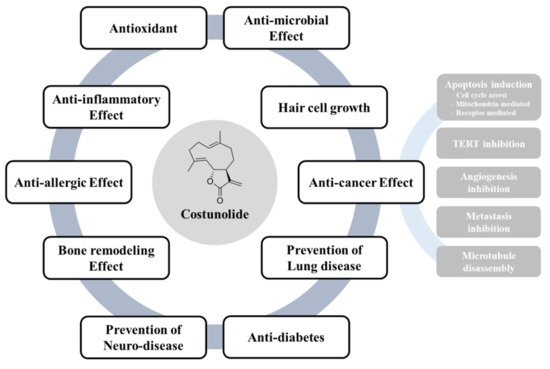
Costunolide—A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential
Abstract
:1. Introduction
2. Therapeutic Potential of Costunolide
2.1. Antioxidant and Anti-Inflammatory Effects of Costunolide
2.2. Anti-Allergic Effects of Costunolide
2.3. Costunolide in Bone Remodeling
2.4. Costunolide as a Neuroprotective Agent
2.5. Antimicrobial Properties of Costunolide
2.6. Costunolide in the Treatment of Alopecia
2.7. Costunolide as an Anticancer Agent
2.7.1. Inhibition of Cell Proliferation
2.7.2. Induction of Apoptosis
Mitochondria-Mediated Apoptosis
Endoplasmic Reticulum (ER) Stress-Mediated Apoptosis
Death Receptor-Mediated Apoptosis
2.7.3. Telomerase Reverse Transcriptase (TERT) Inhibition
2.7.4. Inhibition of Angiogenesis
2.7.5. Inhibition of Tumor Metastasis
2.8. Antidiabetic Effects of Costunolide
3. Pharmacokinetics and Toxicity Profile
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Lahlou, M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013, 4, 17–31. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.; Bidigare, R.R. Terpenoids as therapeutic drugs and pharmaceutical agents. In Natural Products; Springer: Berlin, Germany, 2005; pp. 197–227. [Google Scholar]
- De Kraker, J.-W.; Franssen, M.C.; Dalm, M.C.; de Groot, A.; Bouwmeester, H.J. Biosynthesis of germacrene A carboxylic acid in chicory roots. Demonstration of a cytochrome P450 (+)-germacrene A hydroxylase and NADP+-dependent sesquiterpenoid dehydrogenase (s) involved in sesquiterpene lactone biosynthesis. Plant Physiol. 2001, 125, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-J.; Ge, W.-Z.; Li, Q.-Y.; Lu, Y.; Gong, J.-M.; Kuang, B.-J.; Xi, X.; Wu, H.; Zhang, Q.; Chen, Y. Syntheses and biological evaluation of costunolide, parthenolide, and their fluorinated analogues. J. Med. Chem. 2015, 58, 7007–7020. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Parveen, S.; Ma, T. Costunolide: A novel anti Costunolide: A novel anti-cancer sesquiterpene lactone cancer sesquiterpene lactone cancer sesquiterpene lactone. Bangladesh J. Pharmacol. 2012, 7, 6–13. [Google Scholar] [CrossRef]
- Rao, A.S.; Kelkar, G.; Bhattacharyya, S. Terpenoids—XXI: The structure of costunolide, a new sesquiterpene lactone from costus root oil. Tetrahedron 1960, 9, 275–283. [Google Scholar] [CrossRef]
- Rice-Evans, C. Flavonoids and isoflavones: Absorption, metabolism, and bioactivity. Free Radic. Biol. Med. 2004, 7, 827–828. [Google Scholar] [CrossRef] [PubMed]
- Eliza, J.; Daisy, P.; Ignacimuthu, S. Antioxidant activity of costunolide and eremanthin isolated from Costus speciosus (Koen ex. Retz) Sm. Chem. Biol. Interact. 2010, 188, 467–472. [Google Scholar] [CrossRef]
- Wu, G.; Fang, Y.-Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Rajalakshmi, M.; Anita, R. In vitro and in silico evaluation of antioxidant activity of a sesquiterpene lactone, costunolide, isolated from costus specious rhizome on MCF-7 and MDA-MB-231 human breast cancer cell lines. World J. Pharm. Pharm. Sci. 2014, 3, 1334–1347. [Google Scholar]
- Chen, Y.; Zheng, H.; Zhang, J.; Wang, L.; Jin, Z.; Gao, W. Intestinal mucositis repaired activity of costunolide and dehydrocostus in 5-fluorouracil-induced mice model. RSC. Adv. 2016, 6, 5249–5258. [Google Scholar] [CrossRef]
- Cheong, C.-U.; Yeh, C.-S.; Hsieh, Y.-W.; Lee, Y.-R.; Lin, M.-Y.; Chen, C.-Y.; Lee, C.-H. Protective effects of Costunolide against hydrogen peroxide-induced injury in PC12 cells. Molecules 2016, 21, 898. [Google Scholar] [CrossRef] [PubMed]
- Kassuya, C.A.L.; Cremoneze, A.; Barros, L.F.L.; Simas, A.S.; da Rocha Lapa, F.; Mello-Silva, R.; Stefanello, M.É.A.; Zampronio, A.R. Antipyretic and anti-inflammatory properties of the ethanolic extract, dichloromethane fraction and costunolide from Magnolia ovata (Magnoliaceae). J. Ethnopharmacol. 2009, 124, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.H.; Lee, J.-H.; Park, Y.J.; Hong, Y.-S.; Kim, H.S.; Kim, K.-W.; Lee, J.J. A sesquiterpene lactone, costunolide, from Magnolia grandiflora inhibits NF-κB by targeting IκB phosphorylation. Planta Med. 2001, 67, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Scarponi, C.; Butturini, E.; Sestito, R.; Madonna, S.; Cavani, A.; Mariotto, S.; Albanesi, C. Inhibition of inflammatory and proliferative responses of human keratinocytes exposed to the sesquiterpene lactones dehydrocostuslactone and costunolide. PLoS ONE 2014, 9, e107904. [Google Scholar] [CrossRef] [PubMed]
- Butturini, E.; Cavalieri, E.; de Prati, A.C.; Darra, E.; Rigo, A.; Shoji, K.; Murayama, N.; Yamazaki, H.; Watanabe, Y.; Suzuki, H. Two naturally occurring terpenes, dehydrocostuslactone and costunolide, decrease intracellular GSH content and inhibit STAT3 activation. PLoS ONE 2011, 6, e20174. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Chen, Y.; Zhang, J.; Wang, L.; Jin, Z.; Huang, H.; Man, S.; Gao, W. Evaluation of protective effects of costunolide and dehydrocostuslactone on ethanol-induced gastric ulcer in mice based on multi-pathway regulation. Chem. Biol. Interact. 2016, 250, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Yoon, Y.D.; Lee, K.H.; Park, S.-K.; Kim, H.M. Costunolide inhibits interleukin-1β expression by down-regulation of AP-1 and MAPK activity in LPS-stimulated RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2004, 313, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Butturini, E.; Di Paola, R.; Suzuki, H.; Paterniti, I.; Ahmad, A.; Mariotto, S.; Cuzzocrea, S. Costunolide and Dehydrocostuslactone, two natural sesquiterpene lactones, ameliorate the inflammatory process associated to experimental pleurisy in mice. Eur. J. Pharmacol. 2014, 730, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Prawan, A.; Kundu, J.K.; Surh, Y.J. Molecular basis of heme oxygenase-1 induction: Implications for chemoprevention and chemoprotection. Antioxid. Redox Signal. 2005, 7, 1688–1703. [Google Scholar] [CrossRef]
- Pae, H.-O.; Jeong, G.-S.; Kim, H.-S.; Woo, W.; Rhew, H.; Kim, H.; Sohn, D.; Kim, Y.-C.; Chung, H.-T. Costunolide inhibits production of tumor necrosis factor-α and interleukin-6 by inducing heme oxygenase-1 in RAW264. 7 macrophages. Inflamm. Res. 2007, 56, 520–526. [Google Scholar] [CrossRef]
- Rahimi, K.; Ahmadi, A.; Hassanzadeh, K.; Soleimani, Z.; Sathyapalan, T.; Mohammadi, A.; Sahebkar, A. Targeting the balance of T helper cell responses by curcumin in inflammatory and autoimmune states. Autoimmun. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Song, J.H.; Kim, M.S.; Park, S.-H.; Kim, T.S. Costunolide, a sesquiterpene lactone, inhibits the differentiation of pro-inflammatory CD4+ T cells through the modulation of mitogen-activated protein kinases. Int. Immunopharmacol. 2016, 40, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, J.S.; Cho, D.H.; Park, H.J. Molecular Mechanisms of Cutaneous Inflammatory Disorder: Atopic Dermatitis. Int. J. Mol. Sci. 2016, 17, 1234. [Google Scholar] [CrossRef] [PubMed]
- Seo, C.S.; Lim, H.S.; Jeong, S.J.; Shin, H.K. Anti-allergic effects of sesquiterpene lactones from the root of Aucklandia lappa Decne. Mol. Med. Rep. 2015, 12, 7789–7795. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-K.; Park, S.-J.; Nam, S.-Y.; Kang, S.; Hwang, J.; Lee, S.-J.; Im, D.-S. Anti-allergic effects of sesquiterpene lactones from Saussurea costus (Falc.) Lipsch. determined using in vivo and in vitro experiments. J. Ethnopharmacol. 2018, 213, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Nam, K.W.; Kim, B.; Lee, S.J.; Oh, K.B.; Kim, K.H.; Mar, W.; Shin, J. Inhibitory Effects of Costunolide Isolated from Laurus nobilis on IgE-induced Degranulation of Mast Cell-like RBL-2H3 Cells and the Growth of Y16 pro-B Cells. Phytother. Res. 2011, 25, 1392–1397. [Google Scholar] [CrossRef]
- Khosla, S. Increasing options for the treatment of osteoporosis. N. Engl. J. Med. 2009, 361, 818–820. [Google Scholar] [CrossRef]
- Lee, Y.S.; Choi, E.M. Costunolide stimulates the function of osteoblastic MC3T3-E1 cells. Int. Immunopharmacol. 2011, 11, 712–718. [Google Scholar] [CrossRef]
- Jeon, W.-J.; Kim, K.-M.; Kim, E.-J.; Jang, W.-G. Costunolide increases osteoblast differentiation via ATF4-dependent HO-1 expression in C3H10T1/2 cells. Life Sci. 2017, 178, 94–99. [Google Scholar] [CrossRef]
- Cheon, Y.H.; Song, M.J.; Kim, J.Y.; Kwak, S.C.; Park, J.H.; Lee, C.H.; Kim, J.J.; Kim, J.Y.; Choi, M.K.; Oh, J. Costunolide Inhibits Osteoclast Differentiation by Suppressing c-Fos Transcriptional Activity. Phytother. Res. 2014, 28, 586–592. [Google Scholar] [CrossRef]
- Hermanson, E.; Joseph, B.; Castro, D.; Lindqvist, E.; Aarnisalo, P.; Wallen, A.; Benoit, G.; Hengerer, B.; Olson, L.; Perlmann, T. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells. Exp. Cell Res. 2003, 288, 324–334. [Google Scholar] [CrossRef]
- Ham, A.; Lee, S.-J.; Shin, J.; Kim, K.-H.; Mar, W. Regulatory effects of costunolide on dopamine metabolism-associated genes inhibit dopamine-induced apoptosis in human dopaminergic SH-SY5Y cells. Neurosci. Lett. 2012, 507, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Rayan, N.; Baby, N.; Pitchai, D.; Indraswari, F.; Ling, E.; Lu, J.; Dheen, T. Costunolide inhibits proinflammatory cytokines and iNOS in activated murine BV2 microglia. Front. Biosci. (Elite Ed.) 2011, 3, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Luna-Herrera, J.; Costa, M.; Gonzalez, H.; Rodrigues, A.; Castilho, P. Synergistic antimycobacterial activities of sesquiterpene lactones from Laurus spp. J. Antimicrob. Chemother. 2007, 59, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Fischer, N.H.; Lu, T.; Cantrell, C.L.; Castañeda-Acosta, J.; Quijano, L.; Franzblau, S.G. Antimycobacterial evaluation of germacranolides in honour of professor GH Neil Towers 75th birthday. Phytochemistry 1998, 49, 559–564. [Google Scholar] [CrossRef]
- Alaagib, R.M.O.; Ayoub, S.M.H. On the chemical composition and antibacterial activity of Saussurea lappa (Asteraceae). Pharma Innov. 2015, 4 Pt C, 73–76. [Google Scholar]
- Park, J.-B.; Lee, C.-K.; Park, H.J. Anti-Helicobacter pylori effect of costunolide isolated from the stem bark ofMagnolia sieboldii. Arch. Pharmacal Res. 1997, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Duraipandiyan, V.; Al-Harbi, N.A.; Ignacimuthu, S.; Muthukumar, C. Antimicrobial activity of sesquiterpene lactones isolated from traditional medicinal plant, Costus speciosus (Koen ex. Retz.) Sm. BMC Complement. Altern. Med. 2012, 12, 13. [Google Scholar] [CrossRef]
- Wedge, D.; Galindo, J.; Macıas, F. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry 2000, 53, 747–757. [Google Scholar] [CrossRef]
- Barrero, A.F.; Oltra, J.E.; Álvarez, M.R.; Raslan, D.S.; Saúde, D.A.; Akssira, M. New sources and antifungal activity of sesquiterpene lactones. Fitoterapia 2000, 71, 60–64. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chou, C.-K.; Lee, S.-D.; Wang, J.-C.; Yeh, S.-F. Active compounds from Saussurea lappa Clarks that suppress hepatitis B virus surface antigen gene expression in human hepatoma cells. Antivir. Res. 1995, 27, 99–109. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, D.; Li, M.; Wang, B. Costunolide ameliorates lipoteichoic acid-induced acute lung injury via attenuating MAPK signaling pathway. Int. Immunopharmacol. 2018, 61, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y. Hair-Growth Potential of Ginseng and Its Major Metabolites: A Review on Its Molecular Mechanisms. Int. J. Mol. Sci 2018, 19, 2703. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of Hair Follicle Dermal Papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Choi, H.C.; Nam, G.; Choi, B.Y. Costunolide promotes the proliferation of human hair follicle dermal papilla cells and induces hair growth in C57 BL/6 mice. J. Cosmet. Dermatol. 2018, 18, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Bocca, C.; Gabriel, L.; Bozzo, F.; Miglietta, A. A sesquiterpene lactone, costunolide, interacts with microtubule protein and inhibits the growth of MCF-7 cells. Chem. Biol. Interact. 2004, 147, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; He, X.; Yang, C. Costunolide promotes imatinib-induced apoptosis in chronic myeloid leukemia cells via the Bcr/Abl-Stat5 pathway. Phytother. Res. 2018, 32, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.Z.; Shim, A.R.; Hyeon, J.S.; Lee, H.J.; Ryu, J.H. Inhibition of Wnt/β-Catenin Pathway by Dehydrocostus Lactone and Costunolide in Colon Cancer Cells. Phytother. Res. 2015, 29, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.-L.; Pan, S.-L.; Ho, Y.-F.; Hwang, T.-L.; Kung, F.-L.; Guh, J.-H. Costunolide induces apoptosis through nuclear calcium2+ overload and DNA damage response in human prostate cancer. J. Urol. 2011, 185, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Chang, H.-S.; Chen, I.-S.; Chen, C.-J.; Hsu, M.-L.; Fu, S.-L.; Chen, Y.-J. Costunolide causes mitotic arrest and enhances radiosensitivity in human hepatocellular carcinoma cells. Radiat. Oncol. 2011, 6, 56. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Liu, L.; Yao, W. Activation of p53 by costunolide blocks glutaminolysis and inhibits proliferation in human colorectal cancer cells. Gene 2018, 678, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.; Zhang, G.; Zhang, Y.; Sun, M.; Cui, R.; Li, X.; Li, B.; Zhang, X. Costunolide induces G1/S phase arrest and activates mitochondrial-mediated apoptotic pathways in SK-MES 1 human lung squamous carcinoma cells. Oncol. Lett. 2016, 11, 2780–2786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasul, A.; Yu, B.; Yang, L.-F.; Arshad, M.; Khan, M.; Ma, T.; Yang, H. Costunolide, a sesquiterpene lactone induces G2/M phase arrest and mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-7901 cells. J. Med. Plants Res. 2012, 6, 1191–1200. [Google Scholar]
- Peng, Z.; Wang, Y.; Fan, J.; Lin, X.; Liu, C.; Xu, Y.; Ji, W.; Yan, C.; Su, C. Costunolide and dehydrocostuslactone combination treatment inhibit breast cancer by inducing cell cycle arrest and apoptosis through c-Myc/p53 and AKT/14-3-3 pathway. Sci. Rep. 2017, 7, 41254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, Y.K.; Seo, H.S.; Choi, H.S.; Choi, H.S.; Kim, S.R.; Shin, Y.C.; Ko, S.-G. Induction of Fas-mediated extrinsic apoptosis, p21WAF1-related G2/M cell cycle arrest and ROS generation by costunolide in estrogen receptor-negative breast cancer cells, MDA-MB-231. Mol. Cell. Biochem. 2012, 363, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Manikkam, R. Cytotoxic Impact of Costunolide Isolated from Costus speciosus on Breast Cancer via Differential Regulation of Cell Cycle—An In-vitro and In-silico Approach. Phytother. Res. 2015, 29, 1532–1539. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, L.; Jiang, J.; Zhao, C.; Yang, C. Costunolide enhances sensitivity of K562/ADR chronic myeloid leukemia cells to doxorubicin through PI3K/Akt pathway. Phytother. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.; Sun, M.; Zhang, G.; Zhang, Y.; Song, G.; Liu, Z.; Li, X.; Zhang, X.; Li, B. Costunolide Induces Apoptosis through Generation of ROS and Activation of P53 in Human Esophageal Cancer Eca-109 Cells. J. Biochem. Mol. Toxicol. 2016, 30, 462–469. [Google Scholar] [CrossRef]
- Chen, J.; Chen, B.; Zou, Z.; Li, W.; Zhang, Y.; Xie, J.; Liu, C. Costunolide enhances doxorubicin-induced apoptosis in prostate cancer cells via activated mitogen-activated protein kinases and generation of reactive oxygen species. Oncotarget 2017, 8, 107701–107705. [Google Scholar] [CrossRef]
- Rasul, A.; Bao, R.; Malhi, M.; Zhao, B.; Tsuji, I.; Li, J.; Li, X. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules 2013, 18, 1418–1433. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, K.-T. Costunolide-induced apoptosis in human leukemia cells: Involvement of c-jun N-terminal kinase activation. Biol. Pharm. Bull. 2009, 32, 1803–1808. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-I.; Kim, J.-H.; Lee, K.-T.; Choi, J.-H. Costunolide induces apoptosis in platinum-resistant human ovarian cancer cells by generating reactive oxygen species. Gynecol. Oncol. 2011, 123, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Yang, Y.-I.; Lee, K.-T.; Park, H.-J.; Choi, J.-H. Costunolide induces apoptosis in human endometriotic cells through inhibition of the prosurvival Akt and nuclear factor kappa B signaling pathway. Biol. Pharm. Bull. 2011, 34, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, J.; Wu, Y.; Gui, J.; Shen, Y. Costunolide Inhibits the Growth of OAW42-A Multidrug-Resistant Human Ovarian Cancer Cells by Activating Apoptotic and Autophagic Pathways, Production of Reactive Oxygen Species (ROS), Cleaved Caspase-3 and Cleaved Caspase-9. Med. Sci. Monit. 2019, 25, 3231–3237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, X.; Gong, X. Costunolide induces lung adenocarcinoma cell line A549 cells apoptosis through ROS (reactive oxygen species)—Mediated endoplasmic reticulum stress. Cell Biol. Int. 2016, 40, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, T.; Wang, G.-D.; Ma, C.; Zhou, Y.-F. Costunolide, an active sesquiterpene lactone, induced apoptosis via ROS-mediated ER stress and JNK pathway in human U2OS cells. Biomed. Pharmacother. 2016, 80, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Zhuge, W.; Chen, R.; Vladimir, K.; Dong, X.; Zia, K.; Sun, X.; Dai, X.; Bao, M.; Shen, X.; Liang, G. Costunolide specifically binds and inhibits thioredoxin reductase 1 to induce apoptosis in colon cancer. Cancer Lett. 2018, 412, 46–58. [Google Scholar] [CrossRef]
- Kanno, S.-I.; Kitajima, Y.; Kakuta, M.; Osanai, Y.; Kurauchi, K.; Ujibe, M.; Ishikawa, M. Costunolide-induced apoptosis is caused by receptor-mediated pathway and inhibition of telomerase activity in NALM-6 cells. Biol. Pharm. Bull. 2008, 31, 1024–1028. [Google Scholar] [CrossRef]
- Choi, S.-H.; Im, E.; Kang, H.K.; Lee, J.-H.; Kwak, H.-S.; Bae, Y.-T.; Park, H.-J.; Kim, N.D. Inhibitory effects of costunolide on the telomerase activity in human breast carcinoma cells. Cancer Lett. 2005, 227, 153–162. [Google Scholar] [CrossRef]
- Ahmad, F.; Dixit, D.; Sharma, V.; Kumar, A.; Joshi, S.; Sarkar, C.; Sen, E. Nrf2-driven TERT regulates pentose phosphate pathway in glioblastoma. Cell Death Dis. 2017, 7, e2213. [Google Scholar] [CrossRef]
- Tahtouh, R.; Azzi, A.-S.; Alaaeddine, N.; Chamat, S.; Bouharoun-Tayoun, H.; Wardi, L.; Raad, I.; Sarkis, R.; Antoun, N.A.; Hilal, G. Telomerase inhibition decreases alpha-fetoprotein expression and secretion by hepatocellular carcinoma cell lines: In vitro and in vivo study. PLoS ONE 2015, 10, e0119512. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Yano, H.; Nishida, T.; Kamura, T.; Kojiro, M. Angiogenesis in cancer. Vasc. Health Risk Manag. 2006, 2, 213–219. [Google Scholar] [CrossRef]
- Saraswati, S.; Alhaider, A.A.; Abdelgadir, A.M. Costunolide suppresses an inflammatory angiogenic response in a subcutaneous murine sponge model. Apmis 2018, 126, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.-J.; Itokawa, T.; Shibuya, M.; Kuwano, M.; Ono, M.; Higuchi, R.; Miyamoto, T. Costunolide, a sesquiterpene lactone from Saussurea lappa, inhibits the VEGFR KDR/Flk-1 signaling pathway. Cancer Lett. 2002, 187, 129–133. [Google Scholar] [CrossRef]
- Mahfouz, N.; Tahtouh, R.; Alaaeddine, N.; El Hajj, J.; Sarkis, R.; Hachem, R.; Raad, I.; Hilal, G. Gastrointestinal cancer cells treatment with bevacizumab activates a VEGF autoregulatory mechanism involving telomerase catalytic subunit hTERT via PI3K-AKT, HIF-1α and VEGF receptors. PLoS ONE 2017, 12, e0179202. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Cho, S.-G.; Woo, S.-M.; Yun, Y.J.; Jo, J.; Kim, W.; Shin, Y.C.; Ko, S.-G. Saussurea lappa Clarke-derived costunolide prevents TNFα-induced breast cancer cell migration and invasion by inhibiting NF-κB activity. Evid. Based Complement. Altern. Med. 2013, 2013, 936257. [Google Scholar] [CrossRef] [PubMed]
- Tabata, K.; Nishimura, Y.; Takeda, T.; Kurita, M.; Uchiyama, T.; Suzuki, T. Sesquiterpene lactones derived from Saussurea lappa induce apoptosis and inhibit invasion and migration in neuroblastoma cells. J. Pharmacol. Sci. 2015, 127, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Lohberger, B.; Rinner, B.; Stuendl, N.; Kaltenegger, H.; Steinecker-Frohnwieser, B.; Bernhart, E.; Rad, E.B.; Weinberg, A.M.; Leithner, A.; Bauer, R. Sesquiterpene lactones downregulate G2/M cell cycle regulator proteins and affect the invasive potential of human soft tissue sarcoma cells. PLoS ONE 2013, 8, e66300. [Google Scholar] [CrossRef]
- Jeong, D.; Watari, K.; Shirouzu, T.; Ono, M.; Koizumi, K.; Saiki, I.; Kim, Y.-C.; Tanaka, C.; Higuchi, R.; Miyamoto, T. Studies on lymphangiogenesis inhibitors from Korean and Japanese crude drugs. Biol. Pharm. Bull. 2013, 36, 152–157. [Google Scholar] [CrossRef]
- Whipple, R.A.; Vitolo, M.I.; Boggs, A.E.; Charpentier, M.S.; Thompson, K.; Martin, S.S. Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from NF-κB inhibition. Breast Cancer Res. 2013, 15, R83. [Google Scholar] [CrossRef]
- Perera, H.K.; Premadasa, W.K.; Poongunran, J. alpha-glucosidase and glycation inhibitory effects of costus speciosus leaves. BMC Complement. Altern Med. 2016, 16, 2. [Google Scholar]
- Eliza, J.; Daisy, P.; Ignacimuthu, S.; Duraipandiyan, V. Normo-glycemic and hypolipidemic effect of costunolide isolated from Costus speciosus (Koen ex. Retz.) Sm. in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2009, 179, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Feng, S.; Wu, Y.; Bi, Y.; Wang, C.; Li, W. Quantitative analysis of costunolide and dehydrocostuslactone in rat plasma by ultraperformance liquid chromatography–electrospray ionization–mass spectrometry. Biomed. Chromatogr. 2011, 25, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, X.; Gao, W.; Qu, Z.; Guo, H.; Liu, Z.; Liu, C. Pharmacokinetic study on costunolide and dehydrocostuslactone after oral administration of traditional medicine Aucklandia lappa Decne. by LC/MS/MS. J. Ethnopharmacol. 2014, 151, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Ma, L.-Y.; Liu, Y.-T.; Yu, M.; Jia, H.-M.; Zhang, H.-W.; Yu, C.-Y.; Zou, Z.-M. Pharmacokinetics of costunolide and dehydrocostuslactone after oral administration of Radix aucklandiae extract in normal and gastric ulcer rats. J. Asian Nat. Prod. Res. 2018, 11, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, Y.; Gu, X.; Guo, X.; Yan, C. Study on the pharmacokinetics and metabolism of costunolide and dehydrocostus lactone in rats by HPLC-UV and UPLC-Q-TOF/MS. Biomed. Chromatogr. 2014, 28, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Singireesu, S.; Misra, S.; Mondal, S.K.; Yerramsetty, S.; Sahu, N.; Katragadda, S.B. Costunolide induces micronuclei formation, chromosomal aberrations, cytostasis, and mitochondrial-mediated apoptosis in Chinese hamster ovary cells. Cell Biol. Toxicol. 2018, 34, 125–142. [Google Scholar] [CrossRef]
- Muschietti, L.V.; Ulloa, J.L. Natural Sesquiterpene Lactones as Potential Trypanocidal Therapeutic Agents: A Review. Nat. Prod. Commun. 2016, 11, 1569–1578. [Google Scholar] [CrossRef]




| Effect | Tested Organisms | Concentration | Reference | |
|---|---|---|---|---|
| Antibacterial activity | M. tuberculosis | MIC (mg/L) | 12.5 | [35] |
| S. aureus E. coli P. aeruginosa | MIDZ (mm) | 18 19 14 | [37] | |
| M. avium M. tuberculosis | MIC (μg/mL) | 128 32 | [36] | |
| H. pylori | MIC (μg/mL) | 100–200 | [38] | |
| Antifungal activity | Trichophyton mentagrophytes T. simum T. rubrum Epidermophyton floccosum Scopulariopsis sp. Aspergillus niger Curvulari lunata Magnaporthe grisea | MIC (µg/mL) | 62.5 62 31 or 62 125 250 125 250 | [39] |
| Colletotrichum acutatum Colletotrichum fragariae | MDIZ (mm) | 4 6 | [40] | |
| C. echinulata | EC50 (μg/mL) | 6 | [41] | |
| Antiviral activity | Hepatitis B virus (HBV) | IC50 (μM) | 1 | [42] |
| Type | Experimental Model | Dose/Concentration | Mechanism of Action | Ref. |
|---|---|---|---|---|
| Antioxidant effect | STZ-induced diabetic rats | 20 mg/kg day | Decreased in TBARS level; increased in GSH content | [8] |
| MCF-7, MDA-MB-231 | 20, 40 μM | Decreased in TBARS level; increased in SOD, catalase, GPx activity | [10] | |
| 5-FU-induced IM | 5, 20 mg/kg | Increased in SOD level | [11] | |
| H2O2-stimulated PC12 cells | 50, 100 μM | Decreased intracellular ROS | [12] | |
| Anti-inflammatory effect | Cg-induced edema; LPS-induced fever | 0.015, 0.15, 0.3 mg/kg | Inhibited edema formation; Reduced the fever index | [13] |
| LPS-stimulated RAW264.7 cells | 0.5, 1.5, 3 μg/ml | Inhibited NF-κB activity, phosphorylation of IκBα and NO production; suppressed iNOS mRNA expression | [14] | |
| 5-FU-induced IM | 5, 20 mg/kg | Decreased the expression of iNOS, COX-2, TNF-α and NO | [11] | |
| IL-22 or IFN-γ-stimulated keratinocytes | 12.5 μM | Inhibited STAT1/3 phosphorylation | [15] | |
| IL-6-stimulated THP-1 cells | 6, 12, 25 ng/ml | Inhibited STAT3 and JAK1/2 phosphorylation | [16] | |
| Ethanol-induced gastric ulcer | 5, 20 mg/kg | Suppressed the activation of NF-κB, TNF-α, COX-2, NO and iNOS | [17] | |
| LPS-stimulated RAW264.7 cells | 0.1, 0.3, 1, 3 μM | Suppressed the protein and mRNA expression of IL-1β; inhibited the activity of AP-1 and the phosphorylation of MAPKs | [18] | |
| Carrageenan-induced pleurisy | 5, 10, 15 mg/kg | Reduced accumulation of PMNs and expression of T TNF-α, ICAM-1, P-selectin and nitrotyrosine | [19] | |
| LPS-stimulated RAW264.7 cells | 0.1, 0.5, 1 μM | Induced HO-1 expression and Nrf2 nuclear accumulation; inhibited production of TNF-α and IL-6 | [21] | |
| CD3/CD28-stimulated CD4+ T cells | 0.5, 1, 2 μM | Inhibited the expression of T-bet, GATA3 and RORγt; suppressed the proliferation of CD4+ T cells and expression of CD69; decreased the phosphorylation of ERK and p38 | [23] | |
| Antiallergic effect | TNF-α/IFN-γ-stimulated HaCaT cells | 2.5, 5, 10 μM | Inhibited the expression of TARC, MDC, RANTES and IL-8 | [25] |
| IgE-sensitized RBL-2H3 | 10 μM | Inhibited the expression of β-hexosaminidase | [26] | |
| OVA-induced mouse asthma model | 10 mg/kg | Reduced eosinophil filtration, inflammation score and mucin secretion; decreased the expression of IL-4 and IL-13 | ||
| Ketotifen-stimulated RBL-2H3 | 0.32, 1.6, 8, 40 μM | Inhibited the release of β-hexosaminidase | [27] | |
| IL-5-stimulated Y16 cells | 0.16, 0.8, 4, 20, 40 μM | Inhibited the proliferation Y16 cells | ||
| Bone remodeling | MC3T3-E1 cells differentiation | 10 μM | Increased ALP activity, collagen deposition and mineralization | [29] |
| C3H10T1/2 cells differentiation | 1, 10, 102, 103, 104 ng/ml | Increased the expression of Dlx5, Runx2, ALP, and OC; reduced the activity of ATF4 and expression of HO-1 | [30] | |
| RANKL-induced osteoclast differentiation | 5 μM | Suppressed NFATc1 expression and c-Fos activity | [31] | |
| Neuroprotective agent | DA-stimulated SH-SY5Y | 0.8, 4, 2 μM | Decreased the expression of ASYN; increased the expression of Nurr1, VMAT2 and DAT | [33] |
| LPS-stimulated BV2 microglial cells | 1 μM | Attenuated the expression of TNF-α, IL-1,6, iNOS, MCP-1 and COX-2; inhibited the activation of NF-κB | [34] | |
| Treatment of alopecia | Testosterone-stimulated hHFDPCs | 3 μM | Promotes the growth of hHFDPCs; inhibits the 5α-reductase activity | [46] |
| Hair growth in mice | 3 μM/L | Improved the hair growth | ||
| Inhibition of proliferation | MCF-7 breast cancer cells | 10, 100 nM | Inhibited the cell growth; stimulated tubulin assembly | [47] |
| K562 leukemia cells | 15 μM | Induced cell cycle arrest; induced apoptosis | [48] | |
| S480 colon cancer cells | 5 μM | Suppressed cyclin D1, survivin, β-catenin, and galectin-3; inhibited proliferation and survival of cells | [49] | |
| LNCaP, PC-3, DU-145 prostate cancer cells | 1.3 μM | Inhibited cell proliferation; induced cell cycle arrest at the G1phase | [50] | |
| HA22T/VGH hepatocellular carcinoma cells | 5 μM | Caused G2/M arrest; up-regulated phosphorylation of Chk2, Cdc25c, Cdk1, and cyclin B1 | [51] | |
| HCT-116 colorectal cancer cells | 10, 20, 40 μM | Inhibited proliferation; suppressed mTOR phosphorylation and GLS1 activity | [52] | |
| SK-MES-1 lung squamous carcinoma cells | 40, 80 μM | Inhibited growth of cells; induced cell cycle arrest at G1/S phase; upregulated expression of p53 and Bax; downregulated Bcl-2 expression; activated caspase-3 | [53] | |
| SGC-7901 gastric adenocarcinoma cells | 20, 40 μM | Arrested cell cycle at G2/M phase; activated caspase-3 | [54] | |
| MCF-7, MDA-MB-231 breast cancer cells | 0.9, 1.3, 2.2 μg/mL | Arrested cell cycle at G2/M phase; induced p53 and 14-3-3 expression; inhibited c-Myc, p-Akt and p-BID expression | [55] | |
| MDA-MB-231 breast cancer cells | 15 μM | Induced G2/M cell cycle arrest; upregulated p21WAF1 expression; inhibited cdc2 and cyclin B1 expression | [56] | |
| MCF-7, MDA-MB-231 breast cancer cells | 40 μM | Arrested cell cycle arrest at G2/M phase; inhibited the expression of cyclin D1, D3, CDK-4, CDK-6, p18 INK4c, p21 CIP1/Waf-1 and p27 KIP1 | [57] | |
| K562/ADR chronic myeloid leukemia cells | 0.1, 1, 10, 100 μM | Sensitized K562 cells to doxorubicin; inhibited PI3K/Akt activity | [58] | |
| Eca-109 human esophageal cancer cells | 40, 80 μM | Induced cell cycle arrest in G1/S phase; upregulated the expression of p53, p21, Bax and caspase-3; downregulated Bcl-2 | [59] | |
| Mitochondria-mediated apoptosis | PC-3, DU-145 prostate cancer cells | 20 μM | Enhanced doxorubicin to change of MMP; increased Bax expression and cytochrome c release | [60] |
| T24 human bladder cancer cells | 25, 50 μM | Increased expression of Bax, downregulated Bcl-2 and surviving; activated caspase-3 and PARP | [48] | |
| U937 human promonocytic leukemia cells | 5, 10 | Increased the activation of JNK; inhibited the expression of Bcl-2; induced DNA fragmentation | [61] | |
| SKOV3, A2780, MPSC1 ovarian cancer cells | 10, 20, 30 μM | Triggered the activation of caspase-3, -8, and -9; down-regulated Bcl-2 expression, | [62] | |
| 11Z human epithelial endometriotic cells | IC50 14.21 μM | Induced the activation of caspase-3, -8, and -9; inhibited the activation of Akt and NF-κB | [63] | |
| ovarian cancer cell line, OAW42-A | 12.5, 25, 50 μM | Reduced the mitochondrial membrane potential; increased protein expression of LC3 II and beclin 1 | [64] | |
| ER stress-mediated apoptosis | A549 lung adenocarcinoma cells | 10, 20, 30 μM | Activated UPR signaling pathways; upregulated GRP78 and IRE1α expression; induced ASK1 and JNK activation | [65] |
| U2OS human osteosarcoma cells, A549 human alveolar adenocarcinoma cells, Hela cells | 10, 20, 30 μM | Increased expressions of Bip and IREa; increased expressions of p-ASK1, p-JNK and p-ERK; induced generation of Ca2+ | [66] | |
| HCT-116, HT-29, SW620 colon cancer cells | 10, 20, 30 μM | Inhibited the activity of TrxR1; induced the expression of p-eIF2a, ATF4 and CHOP | [67] | |
| Death receptor mediated apoptosis | NALM-6 human B cell leukemia cell | 10 μM | Increased the phosphorylation of FADD; activated caspase-8 | [68] |
| TERT inhibition | NALM-6 human B cell leukemia cell | 10 μM | Suppressed telomerase activity; inhibited the expression of hTERT mRNA and protein | |
| MCF-7, MDA-MB-231 breast cancer cells | 10, 50, 80, 100 μM | Inhibited the cell growth, telomerase activity and hTERT mRNA expression; inhibited bindings of hTERT promoters; inhibited the expression of c-Myc and Sp1 | [69] | |
| A172, U87MG, T98G glioma cells | 10, 20, 30, 40 μM | Decreases Nrf2 levels; Suppressed telomerase activity; decreased expression of G6PD and TKT | [70] | |
| A172, U87MG glioma cells | 30 μM | Inhibited hTERT expression | [71] | |
| HepG2/C3A, PLC/PRF/5 HCC cells | 5, 10, 50 μM | Inhibited AFP secretion and mRNA expression; decreased cell migration | [72] | |
| Inhibition of angiogenesis | subcutaneous murine sponge model | 5, 10, 20 mg/kg | Reduced hemoglobin concentration and VEGF levels | [74] |
| VEGF-stimulated HUVECs | IC50 5.7 μM | Inhibited VEGF-induced proliferation and migration; inhibited the VEGF-induced autophosphorylation of KDR/Flk-1 | [75] | |
| AGS, Caco-2, HepG2/C3A cancer cells | 10 μM | Decreased VEGF secretion and mRNA levels | [76] | |
| Inhibition of tumor metastasis | MDA-MB-231 breast cancer cells | 20 μM | Inhibited TNF𝛼-induced cells migration and invasion; reduced phosphorylation of IKK and I𝜅B𝛼; inhibited p65 NF-𝜅B subunit | [77] |
| IMR-32, LA-N-1, SK-N-SH neuroblastoma cell | 0.1, 1, 10 μM | Inhibited migration and invasion; suppressed MMP2 expression | [78] | |
| SW-872, SW-982, TE-671 soft tissue sarcomas | 3, 10, 20 μg/mL | Inhibited the invasion potential; changed the expression of MMPs | [79] | |
| TR-LE (temperature-sensitive rat lymphatic endothelial) cells | IC50 1.37 μM | Suppressed cell proliferation; inhibited capillary-like tube formation | [80] | |
| MDA-MB-157, MDA-MB-436, Bt-549 breast cancer cells | 10, 25 μM | Reduced detyrosinated tubulin; decreased microtentacle (McTN) frequency; reduced tumor cell attachment | [81] | |
| Antidiabetic effect | α-Amylase, α-Glucosidase, fructosamine formation, glycation | IC50 5.88 or 67.5 μM | Inhibited the activity of α-Amylase, α-Glucosidase; inhibited fructosamine formation; | [82] |
| streptozotocin-induced diabetic rats | 5, 10, 20 mg/kg | Reduced glucose levels and HbA1c; increased insulin levels; reduced cholesterol, TG, LDL; increased HDL | [8] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Choi, B.Y. Costunolide—A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential. Int. J. Mol. Sci. 2019, 20, 2926. https://doi.org/10.3390/ijms20122926
Kim DY, Choi BY. Costunolide—A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential. International Journal of Molecular Sciences. 2019; 20(12):2926. https://doi.org/10.3390/ijms20122926
Chicago/Turabian StyleKim, Dae Yong, and Bu Young Choi. 2019. "Costunolide—A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential" International Journal of Molecular Sciences 20, no. 12: 2926. https://doi.org/10.3390/ijms20122926