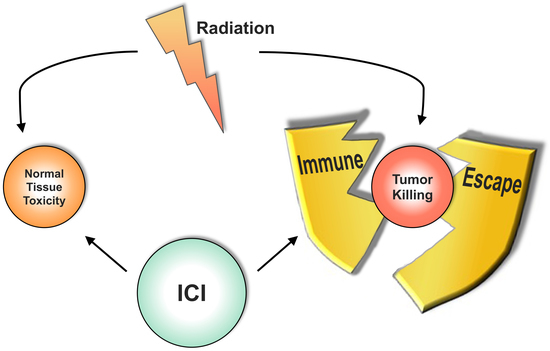
Combining Radiotherapy and Immunotherapy in Lung Cancer: Can We Expect Limitations Due to Altered Normal Tissue Toxicity?
Abstract
:1. Introduction
2. Radiotherapy in the Thoracic Region—Tumor Control Versus Immune Escape
3. Radiation-Induced Normal Tissue Toxicity in the Lung
4. Immunotherapy in Lung Cancer
5. Combining Radiotherapy and Immunotherapy in Lung Cancer
6. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| CAR | Chimeric Antigen Receptor |
| CEACAM-1 | Carcinoembryonic antigen-related cell adhesion molecule 1 |
| cGAS | cGMP–AMP synthase |
| CTLA-4 | Cytotoxic T-lymphocyte-associated Protein 4 |
| DAMPs | Damage-associated molecular patterns |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| HMGB1 | High-Mobility-Group-Protein B1 |
| ICI | Immune Checkpoint Inhibition |
| IRAEs | Immune-related adverse effects |
| MHCI | Major histocompatibility complex I |
| NSCLC | Non-small cell lung cancer |
| NTCP | Normal tissue complication probability |
| PD1 | Programmed cell death protein 1 |
| PD-L1 | Programmed cell death 1 ligand 1 |
| PRR | Pathogen recognition receptor |
| ROS | Reactive oxygen species |
| RT | Radiotherapy |
| SBRT | Stereotactic body radiotherapy |
| SCLC | small cell lung cancer |
| SIRS | systemic inflammatory response syndrome |
| STING | stimulator of interferon genes |
| TGF-β | transforming growth factor beta |
| TNFR | tumor necrosis factor receptor |
| TLR | TOLL-like receptor |
| TREX1 | three prime repair exonuclease 1 |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Auperin, A.; Le Pechoux, C.; Rolland, E.; Curran, W.J.; Furuse, K.; Fournel, P.; Belderbos, J.; Clamon, G.; Ulutin, H.C.; Paulus, R.; et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 2181–2190. [Google Scholar] [CrossRef]
- Crabtree, T.D.; Denlinger, C.E.; Meyers, B.F.; El Naqa, I.; Zoole, J.; Krupnick, A.S.; Kreisel, D.; Patterson, G.A.; Bradley, J.D. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2010, 140, 377–386. [Google Scholar] [CrossRef]
- Grills, I.S.; Mangona, V.S.; Welsh, R.; Chmielewski, G.; McInerney, E.; Martin, S.; Wloch, J.; Ye, H.; Kestin, L.L. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J. Clin. Oncol. 2010, 28, 928–935. [Google Scholar] [CrossRef]
- Onishi, H.; Shirato, H.; Nagata, Y.; Hiraoka, M.; Fujino, M.; Gomi, K.; Karasawa, K.; Hayakawa, K.; Niibe, Y.; Takai, Y.; et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: Can SBRT be comparable to surgery? Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 1352–1358. [Google Scholar] [CrossRef]
- Ohri, N. Radiotherapy Dosing for Locally Advanced Non-Small Cell Lung Carcinoma: “MTD” or “ALARA”? Front. Oncol. 2017, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Niyazi, M.; Maihoefer, C.; Krause, M.; Rodel, C.; Budach, W.; Belka, C. Radiotherapy and “new” drugs-new side effects? Radiat. Oncol. 2011, 6, 177. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, S.M.; Jiang, J.; Gomez, D.R.; Chang, J.Y.; Buchholz, T.A.; Smith, B.D. Intensity modulated radiotherapy for stage III non-small cell lung cancer in the United States: Predictors of use and association with toxicities. Lung Cancer 2013, 82, 252–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chun, S.G.; Hu, C.; Choy, H.; Komaki, R.U.; Timmerman, R.D.; Schild, S.E.; Bogart, J.A.; Dobelbower, M.C.; Bosch, W.; Galvin, J.M.; et al. Impact of Intensity-Modulated Radiation Therapy Technique for Locally Advanced Non-Small-Cell Lung Cancer: A Secondary Analysis of the NRG Oncology RTOG 0617 Randomized Clinical Trial. J. Clin. Oncol. 2017, 35, 56–62. [Google Scholar] [CrossRef]
- Higgins, K.A.; O’Connell, K.; Liu, Y.; Gillespie, T.W.; McDonald, M.W.; Pillai, R.N.; Patel, K.R.; Patel, P.R.; Robinson, C.G.; Simone, C.B., 2nd; et al. National Cancer Database Analysis of Proton Versus Photon Radiation Therapy in Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 128–137. [Google Scholar] [CrossRef]
- Bradley, J.D.; Paulus, R.; Komaki, R.; Masters, G.; Blumenschein, G.; Schild, S.; Bogart, J.; Hu, C.; Forster, K.; Magliocco, A.; et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015, 16, 187–199. [Google Scholar] [CrossRef]
- Farr, K.P.; Khalil, A.A.; Knap, M.M.; Moller, D.S.; Grau, C. Development of radiation pneumopathy and generalised radiological changes after radiotherapy are independent negative prognostic factors for survival in non-small cell lung cancer patients. Radiother. Oncol. 2013, 107, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Boon, T.; Coulie, P.G.; Van den Eynde, B. Tumor antigens recognized by T cells. Immunol. Today 1997, 18, 267–268. [Google Scholar] [CrossRef]
- Karanikas, V.; Colau, D.; Baurain, J.F.; Chiari, R.; Thonnard, J.; Gutierrez-Roelens, I.; Goffinet, C.; Van Schaftingen, E.V.; Weynants, P.; Boon, T.; et al. High frequency of cytolytic T lymphocytes directed against a tumor-specific mutated antigen detectable with HLA tetramers in the blood of a lung carcinoma patient with long survival. Cancer Res. 2001, 61, 3718–3724. [Google Scholar] [PubMed]
- Galon, J.; Fridman, W.H.; Pages, F. The adaptive immunologic microenvironment in colorectal cancer: A novel perspective. Cancer Res. 2007, 67, 1883–1886. [Google Scholar] [CrossRef]
- Gajewski, T.F. The Next Hurdle in Cancer Immunotherapy: Overcoming the Non-T-Cell-Inflamed Tumor Microenvironment. Semin. Oncol. 2015, 42, 663–671. [Google Scholar] [CrossRef]
- Zamanakou, M.; Germenis, A.E.; Karanikas, V. Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunol. Lett. 2007, 111, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Beatty, G.L.; Gladney, W.L. Immune escape mechanisms as a guide for cancer immunotherapy. Clin. Cancer Res. 2015, 21, 687–692. [Google Scholar] [CrossRef]
- Facciabene, A.; Motz, G.T.; Coukos, G. T-regulatory cells: Key players in tumor immune escape and angiogenesis. Cancer Res. 2012, 72, 2162–2171. [Google Scholar] [CrossRef]
- Paulsen, E.E.; Kilvaer, T.K.; Rakaee, M.; Richardsen, E.; Hald, S.M.; Andersen, S.; Busund, L.T.; Bremnes, R.M.; Donnem, T. CTLA-4 expression in the non-small cell lung cancer patient tumor microenvironment: Diverging prognostic impact in primary tumors and lymph node metastases. Cancer Immunol. Immunother. 2017, 66, 1449–1461. [Google Scholar] [CrossRef]
- Karanikas, V.; Zamanakou, M.; Kerenidi, T.; Dahabreh, J.; Hevas, A.; Nakou, M.; Gourgoulianis, K.I.; Germenis, A.E. Indoleamine 2,3-dioxygenase (IDO) expression in lung cancer. Cancer Biol. Ther. 2007, 6, 1258–1262. [Google Scholar] [CrossRef]
- Holmgaard, R.B.; Zamarin, D.; Munn, D.H.; Wolchok, J.D.; Allison, J.P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 2013, 210, 1389–1402. [Google Scholar] [CrossRef] [Green Version]
- Gubin, M.M.; Zhang, X.; Schuster, H.; Caron, E.; Ward, J.P.; Noguchi, T.; Ivanova, Y.; Hundal, J.; Arthur, C.D.; Krebber, W.J.; et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 2014, 515, 577–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef]
- Judd, J.; Zibelman, M.; Handorf, E.; O’Neill, J.; Ramamurthy, C.; Bentota, S.; Doyle, J.; Uzzo, R.G.; Bauman, J.; Borghaei, H.; et al. Immune-Related Adverse Events as a Biomarker in Non-Melanoma Patients Treated with Programmed Cell Death 1 Inhibitors. Oncologist 2017, 22, 1232–1237. [Google Scholar] [CrossRef]
- Brix, N.; Tiefenthaller, A.; Anders, H.; Belka, C.; Lauber, K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol. Rev. 2017, 280, 249–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derer, A.; Frey, B.; Fietkau, R.; Gaipl, U.S. Immune-modulating properties of ionizing radiation: Rationale for the treatment of cancer by combination radiotherapy and immune checkpoint inhibitors. Cancer Immunol. Immunother. 2016, 65, 779–786. [Google Scholar] [CrossRef]
- Abuodeh, Y.; Venkat, P.; Kim, S. Systematic review of case reports on the abscopal effect. Curr. Probl. Cancer 2016, 40, 25–37. [Google Scholar] [CrossRef]
- Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Pilones, K.A.; Garcia-Martinez, E.; Rudqvist, N.P.; Formenti, S.C.; Demaria, S. Barriers to Radiation-Induced In Situ Tumor Vaccination. Front. Immunol. 2017, 8, 229. [Google Scholar] [CrossRef]
- Ngwa, W.; Ouyang, Z. Following the Preclinical Data: Leveraging the Abscopal Effect More Efficaciously. Front. Oncol. 2017, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Shaverdian, N.; Lisberg, A.E.; Bornazyan, K.; Veruttipong, D.; Goldman, J.W.; Formenti, S.C.; Garon, E.B.; Lee, P. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017, 18, 895–903. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018. [Google Scholar] [CrossRef]
- Graves, P.R.; Siddiqui, F.; Anscher, M.S.; Movsas, B. Radiation pulmonary toxicity: From mechanisms to management. Semin. Radiat. Oncol. 2010, 20, 201–207. [Google Scholar] [CrossRef]
- Shibaki, R.; Akamatsu, H.; Fujimoto, M.; Koh, Y.; Yamamoto, N. Nivolumab induced radiation recall pneumonitis after two years of radiotherapy. Ann. Oncol. 2017, 28, 1404–1405. [Google Scholar] [CrossRef]
- Ko, E.C.; Raben, D.; Formenti, S.C. The Integration of Radiotherapy with Immunotherapy for the Treatment of Non-Small Cell Lung Cancer. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef]
- Bhalla, N.; Brooker, R.; Brada, M. Combining immunotherapy and radiotherapy in lung cancer. J. Thorac. Dis. 2018, 10, S1447–S1460. [Google Scholar] [CrossRef]
- Hwang, W.L.; Pike, L.R.G.; Royce, T.J.; Mahal, B.A.; Loeffler, J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 2018, 15, 477–494. [Google Scholar] [CrossRef]
- Bockel, S.; Durand, B.; Deutsch, E. Combining radiation therapy and cancer immune therapies: From preclinical findings to clinical applications. Cancer Radiother. 2018, 22, 567–580. [Google Scholar] [CrossRef]
- Ko, E.C.; Formenti, S.C. Radiotherapy and checkpoint inhibitors: A winning new combination? Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Durm, G.; Hanna, N.; Einhorn, L.H.; Kong, F.S. Can radiotherapy potentiate the effectiveness of immune checkpoint inhibitors in lung cancer? Future Oncol. 2017, 13, 2503–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badiyan, S.N.; Roach, M.C.; Chuong, M.D.; Rice, S.R.; Onyeuku, N.E.; Remick, J.; Chilukuri, S.; Glass, E.; Mohindra, P.; Simone, C.B., 2nd. Combining immunotherapy with radiation therapy in thoracic oncology. J. Thorac. Dis. 2018, 10, S2492–S2507. [Google Scholar] [CrossRef] [PubMed]
- Kalbasi, A.; Rengan, R. Clinical experiences of combining immunotherapy and radiation therapy in non-small cell lung cancer: Lessons from melanoma. Transl. Lung Cancer Res. 2017, 6, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.H.; Rimner, A.; Cohen, R.B. Combining immunotherapy and radiation therapy for small cell lung cancer and thymic tumors. Transl. Lung Cancer Res. 2017, 6, 186–195. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Stanic, S.; Paulus, R.; Timmerman, R.D.; Michalski, J.M.; Barriger, R.B.; Bezjak, A.; Videtic, G.M.; Bradley, J. No clinically significant changes in pulmonary function following stereotactic body radiation therapy for early- stage peripheral non-small cell lung cancer: An analysis of RTOG 0236. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1092–1099. [Google Scholar] [CrossRef]
- Timmerman, R.; Paulus, R.; Galvin, J.; Michalski, J.; Straube, W.; Bradley, J.; Fakiris, A.; Bezjak, A.; Videtic, G.; Johnstone, D.; et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010, 303, 1070–1076. [Google Scholar] [CrossRef]
- O’Rourke, N.; Roque, I.F.M.; Farre Bernado, N.; Macbeth, F. Concurrent chemoradiotherapy in non-small cell lung cancer. Cochrane Database Syst. Rev. 2010, CD002140. [Google Scholar] [CrossRef]
- Maciejczyk, A.; Skrzypczynska, I.; Janiszewska, M. Lung cancer. Radiotherapy in lung cancer: Actual methods and future trends. Rep. Pract. Oncol. Radiother. 2014, 19, 353–360. [Google Scholar] [CrossRef]
- Baker, S.; Dahele, M.; Lagerwaard, F.J.; Senan, S. A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiat. Oncol. 2016, 11, 115. [Google Scholar] [CrossRef]
- Loganadane, G.; Martinetti, F.; Mercier, O.; Krhili, S.; Riet, F.G.; Mbagui, R.; To, H.; Le Pechoux, C.; Levy, A. Stereotactic ablative radiotherapy for early stage non-small cell lung cancer: A critical literature review of predictive factors of relapse. Cancer Treat. Rev. 2016, 50, 240–246. [Google Scholar] [CrossRef]
- Walls, G.M.; Hanna, G.G.; Qi, F.; Zhao, S.; Xia, J.; Ansari, M.T.; Landau, D. Predicting Outcomes from Radical Radiotherapy for Non-small Cell Lung Cancer: A Systematic Review of the Existing Literature. Front. Oncol. 2018, 8, 433. [Google Scholar] [CrossRef] [PubMed]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, H.; Pitt, J.M.; Kroemer, G.; Zitvogel, L. Therapy-induced microenvironmental changes in cancer. J. Mol. Med. 2016, 94, 497–508. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Eichten, A.; Coussens, L.M. Paradoxical roles of the immune system during cancer development. Nat. Rev. Cancer 2006, 6, 24–37. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, K.J.; Hudson, A.L.; Back, M.; Eade, T.; Diakos, C.I. Radiation, inflammation and the immune response in cancer. Mamm. Genome 2018. [Google Scholar] [CrossRef] [PubMed]
- Frey, B.; Ruckert, M.; Deloch, L.; Ruhle, P.F.; Derer, A.; Fietkau, R.; Gaipl, U.S. Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol. Rev. 2017, 280, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Bok, S.; Hong, B.J.; Choi, H.S.; Ahn, G.O. Radiation-induced immune responses: Mechanisms and therapeutic perspectives. Blood Res. 2016, 51, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Klein, D. The Tumor Vascular Endothelium as Decision Maker in Cancer Therapy. Front. Oncol. 2018, 8, 367. [Google Scholar] [CrossRef]
- Herrera, F.G.; Bourhis, J.; Coukos, G. Radiotherapy combination opportunities leveraging immunity for the next oncology practice. CA Cancer J. Clin. 2017, 67, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S.C. The convergence of radiation and immunogenic cell death signaling pathways. Front. Oncol. 2012, 2, 88. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Huebener, P.; Schwabe, R.F. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene 2016, 35, 5931–5941. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Probst, H.C.; Vuong, V.; Landshammer, A.; Muth, S.; Yagita, H.; Schwendener, R.; Pruschy, M.; Knuth, A.; van den Broek, M. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J. Immunol. 2012, 189, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.D.; Mauceri, H.; Beckett, M.; Darga, T.; et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsou, P.; Katayama, H.; Ostrin, E.J.; Hanash, S.M. The Emerging Role of B Cells in Tumor Immunity. Cancer Res. 2016, 76, 5597–5601. [Google Scholar] [CrossRef]
- Diamond, J.M.; Vanpouille-Box, C.; Spada, S.; Rudqvist, N.P.; Chapman, J.R.; Ueberheide, B.M.; Pilones, K.A.; Sarfraz, Y.; Formenti, S.C.; Demaria, S. Exosomes Shuttle TREX1-Sensitive IFN-Stimulatory dsDNA from Irradiated Cancer Cells to DCs. Cancer Immunol. Res. 2018, 6, 910–920. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Formenti, S.C.; Demaria, S. TREX1 dictates the immune fate of irradiated cancer cells. Oncoimmunology 2017, 6, e1339857. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Diamond, J.M.; Pilones, K.A.; Zavadil, J.; Babb, J.S.; Formenti, S.C.; Barcellos-Hoff, M.H.; Demaria, S. TGFbeta Is a Master Regulator of Radiation Therapy-Induced Antitumor Immunity. Cancer Res. 2015, 75, 2232–2242. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Coussens, L.M. The inflammatory tumor microenvironment and its impact on cancer development. Contrib. Microbiol. 2006, 13, 118–137. [Google Scholar] [CrossRef] [PubMed]
- De Visser, K.E.; Coussens, L.M. The interplay between innate and adaptive immunity regulates cancer development. Cancer Immunol. Immunother. 2005, 54, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Barbera-Guillem, E.; May, K.F., Jr.; Nyhus, J.K.; Nelson, M.B. Promotion of tumor invasion by cooperation of granulocytes and macrophages activated by anti-tumor antibodies. Neoplasia 1999, 1, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Yuen, G.J.; Demissie, E.; Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer 2016, 2, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Hagerling, C.; Casbon, A.J.; Werb, Z. Balancing the innate immune system in tumor development. Trends Cell Biol. 2015, 25, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, G.O.; Brown, J.M. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: Role of bone marrow-derived myelomonocytic cells. Cancer Cell 2008, 13, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.O.; Tseng, D.; Liao, C.H.; Dorie, M.J.; Czechowicz, A.; Brown, J.M. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl. Acad. Sci. USA 2010, 107, 8363–8368. [Google Scholar] [CrossRef] [Green Version]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.W.; Dong, K.; Zhang, H.Z. The roles of CD73 in cancer. BioMed Res. Int. 2014, 2014, 460654. [Google Scholar] [CrossRef]
- Qin, A.; Coffey, D.G.; Warren, E.H.; Ramnath, N. Mechanisms of immune evasion and current status of checkpoint inhibitors in non-small cell lung cancer. Cancer Med. 2016, 5, 2567–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nouvion, A.L.; Beauchemin, N. [CEACAM1 as a central modulator of metabolism, tumor progression, angiogenesis and immunity]. Med. Sci. 2009, 25, 247–252. [Google Scholar] [CrossRef]
- Rudqvist, N.P.; Pilones, K.A.; Lhuillier, C.; Wennerberg, E.; Sidhom, J.W.; Emerson, R.O.; Robins, H.S.; Schneck, J.; Formenti, S.C.; Demaria, S. Radiotherapy and CTLA-4 Blockade Shape the TCR Repertoire of Tumor-Infiltrating T Cells. Cancer Immunol. Res. 2018, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Lhuillier, C.; Vanpouille-Box, C.; Galluzzi, L.; Formenti, S.C.; Demaria, S. Emerging biomarkers for the combination of radiotherapy and immune checkpoint blockers. Semin. Cancer Biol. 2018, 52, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Chow, C.W.; Downey, G.P. Role of innate immune cells and their products in lung immunopathology. Int. J. Biochem. Cell Biol. 2008, 40, 1348–1361. [Google Scholar] [CrossRef] [PubMed]
- Ratikan, J.A.; Micewicz, E.D.; Xie, M.W.; Schaue, D. Radiation takes its Toll. Cancer Lett. 2015, 368, 238–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaue, D.; Micewicz, E.D.; Ratikan, J.A.; Xie, M.W.; Cheng, G.; McBride, W.H. Radiation and inflammation. Semin. Radiat. Oncol. 2015, 25, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Formenti, S.C.; Demaria, S. Systemic effects of local radiotherapy. Lancet Oncol. 2009, 10, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Lumniczky, K.; Safrany, G. The impact of radiation therapy on the antitumor immunity: Local effects and systemic consequences. Cancer Lett. 2015, 356, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, I.V.; Laskaratou, D.A.; Frey, B.; Candeias, S.M.; Gaipl, U.S.; Lumniczky, K.; Georgakilas, A.G. Key mechanisms involved in ionizing radiation-induced systemic effects. A current review. Toxicol. Res. 2016, 5, 12–33. [Google Scholar] [CrossRef] [Green Version]
- Jelonek, K.; Pietrowska, M.; Widlak, P. Systemic effects of ionizing radiation at the proteome and metabolome levels in the blood of cancer patients treated with radiotherapy: The influence of inflammation and radiation toxicity. Int. J. Radiat. Biol. 2017, 93, 683–696. [Google Scholar] [CrossRef] [PubMed]
- Wirsdorfer, F.; Jendrossek, V. Modeling DNA damage-induced pneumopathy in mice: Insight from danger signaling cascades. Radiat. Oncol. 2017, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Demaria, S.; Formenti, S.C.; Galluzzi, L. Cytosolic DNA Sensing in Organismal Tumor Control. Cancer Cell 2018, 34, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Hekim, N.; Cetin, Z.; Nikitaki, Z.; Cort, A.; Saygili, E.I. Radiation triggering immune response and inflammation. Cancer Lett. 2015, 368, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Kunitoh, H.; Sekine, I.; Sumi, M.; Tokuuye, K.; Saijo, N. Radiation pneumonitis in lung cancer patients: A retrospective study of risk factors and the long-term prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 649–655. [Google Scholar] [CrossRef]
- McDonald, S.; Rubin, P.; Phillips, T.L.; Marks, L.B. Injury to the lung from cancer therapy: Clinical syndromes, measurable endpoints, and potential scoring systems. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1187–1203. [Google Scholar] [CrossRef]
- Provatopoulou, X.; Athanasiou, E.; Gounaris, A. Predictive markers of radiation pneumonitis. Anticancer Res. 2008, 28, 2421–2432. [Google Scholar]
- Giridhar, P.; Mallick, S.; Rath, G.K.; Julka, P.K. Radiation induced lung injury: Prediction, assessment and management. Asian Pac. J. Cancer Prev. 2015, 16, 2613–2617. [Google Scholar] [CrossRef]
- Yarnold, J.; Brotons, M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother. Oncol. 2010, 97, 149–161. [Google Scholar] [CrossRef]
- Citrin, D.; Cotrim, A.P.; Hyodo, F.; Baum, B.J.; Krishna, M.C.; Mitchell, J.B. Radioprotectors and mitigators of radiation-induced normal tissue injury. Oncologist 2010, 15, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014, 32, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Barnett, G.C.; West, C.M.; Dunning, A.M.; Elliott, R.M.; Coles, C.E.; Pharoah, P.D.; Burnet, N.G. Normal tissue reactions to radiotherapy: Towards tailoring treatment dose by genotype. Nat. Rev. Cancer 2009, 9, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.B.; Coleman, C.N.; Anscher, M.S.; McBride, W.H. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003, 4, 529–536. [Google Scholar] [CrossRef]
- Ruhle, A.; Huber, P.E. Normal tissue: Radiosensitivity, toxicity, consequences for planning. Radiologe 2018. [Google Scholar] [CrossRef]
- Wirsdorfer, F.; Jendrossek, V. The Role of Lymphocytes in Radiotherapy-Induced Adverse Late Effects in the Lung. Front. Immunol. 2016, 7, 591. [Google Scholar] [CrossRef]
- Simone, C.B., 2nd. Thoracic Radiation Normal Tissue Injury. Semin. Radiat. Oncol. 2017, 27, 370–377. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef]
- Tsoutsou, P.G.; Koukourakis, M.I. Radiation pneumonitis and fibrosis: Mechanisms underlying its pathogenesis and implications for future research. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1281–1293. [Google Scholar] [CrossRef]
- Wirsdorfer, F.; de Leve, S.; Cappuccini, F.; Eldh, T.; Meyer, A.V.; Gau, E.; Thompson, L.F.; Chen, N.Y.; Karmouty-Quintana, H.; Fischer, U.; et al. Extracellular Adenosine Production by ecto-5′-Nucleotidase (CD73) Enhances Radiation-Induced Lung Fibrosis. Cancer Res. 2016, 76, 3045–3056. [Google Scholar] [CrossRef]
- De Leve, S.; Wirsdorfer, F.; Cappuccini, F.; Schutze, A.; Meyer, A.V.; Rock, K.; Thompson, L.F.; Fischer, J.W.; Stuschke, M.; Jendrossek, V. Loss of CD73 prevents accumulation of alternatively activated macrophages and the formation of prefibrotic macrophage clusters in irradiated lungs. FASEB J. 2017, 31, 2869–2880. [Google Scholar] [CrossRef] [PubMed]
- Lanitis, E.; Dangaj, D.; Irving, M.; Coukos, G. Mechanisms regulating T-cell infiltration and activity in solid tumors. Ann. Oncol. 2017, 28, xii18–xii32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dutta, P.; Liu, J.; Sabri, N.; Song, Y.; Li, W.X.; Li, J. Tumour cell-intrinsic CTLA4 regulates PD-L1 expression in non-small cell lung cancer. J. Cell. Mol. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hamerlik, P.; Lathia, J.D.; Rasmussen, R.; Wu, Q.; Bartkova, J.; Lee, M.; Moudry, P.; Bartek, J., Jr.; Fischer, W.; Lukas, J.; et al. Autocrine VEGF-VEGFR2-Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J. Exp. Med. 2012, 209, 507–520. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.; Lewis, C.E.; Harris, A.L.; McGee, J.O. Secretion of epidermal growth factor by macrophages associated with breast carcinoma. Lancet 1993, 342, 148–149. [Google Scholar] [CrossRef]
- Barbera-Guillem, E.; Nyhus, J.K.; Wolford, C.C.; Friece, C.R.; Sampsel, J.W. Vascular endothelial growth factor secretion by tumor-infiltrating macrophages essentially supports tumor angiogenesis, and IgG immune complexes potentiate the process. Cancer Res. 2002, 62, 7042–7049. [Google Scholar]
- Mulligan, J.K.; Rosenzweig, S.A.; Young, M.R. Tumor secretion of VEGF induces endothelial cells to suppress T cell functions through the production of PGE2. J. Immunother. 2010, 33, 126–135. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Chen, H.L.; Girgis, K.R.; Cunningham, H.T.; Meny, G.M.; Nadaf, S.; Kavanaugh, D.; Carbone, D.P. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 1996, 2, 1096–1103. [Google Scholar] [CrossRef]
- Su, J.L.; Yen, C.J.; Chen, P.S.; Chuang, S.E.; Hong, C.C.; Kuo, I.H.; Chen, H.Y.; Hung, M.C.; Kuo, M.L. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br. J. Cancer 2007, 96, 541–545. [Google Scholar] [CrossRef]
- Wada, J.; Suzuki, H.; Fuchino, R.; Yamasaki, A.; Nagai, S.; Yanai, K.; Koga, K.; Nakamura, M.; Tanaka, M.; Morisaki, T.; et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 2009, 29, 881–888. [Google Scholar]
- Li, Y.L.; Zhao, H.; Ren, X.B. Relationship of VEGF/VEGFR with immune and cancer cells: Staggering or forward? Cancer Biol. Med. 2016, 13, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, J.; Liu, B. Targeting VEGF/VEGFR to Modulate Antitumor Immunity. Front. Immunol. 2018, 9, 978. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Cantley, L.C. The role of the ErbB family members in non-small cell lung cancers sensitive to epidermal growth factor receptor kinase inhibitors. Clin. Cancer Res. 2006, 12, 4372s–4376s. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Wislez, M.; Zhang, J.; Iwanaga, K.; Dackor, J.; Hanna, A.E.; Kalyankrishna, S.; Cody, D.D.; Price, R.E.; Sato, M.; et al. High expression of ErbB family members and their ligands in lung adenocarcinomas that are sensitive to inhibition of epidermal growth factor receptor. Cancer Res. 2005, 65, 11478–11485. [Google Scholar] [CrossRef] [PubMed]
- Minder, P.; Zajac, E.; Quigley, J.P.; Deryugina, E.I. EGFR regulates the development and microarchitecture of intratumoral angiogenic vasculature capable of sustaining cancer cell intravasation. Neoplasia 2015, 17, 634–649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yuan, Q. Current mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and updated therapy strategies in human nonsmall cell lung cancer. J. Cancer Res. Ther. 2016, 12, C131–C137. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.C.; Lin, C.C.; Lee, J.H.; Yang, J.C. Optimal management of EGFR-mutant non-small cell lung cancer with disease progression on first-line tyrosine kinase inhibitor therapy. Lung Cancer 2017, 110, 7–13. [Google Scholar] [CrossRef]
- Sun, J.M.; Park, K. Can we define the optimal sequence of epidermal growth factor receptor tyrosine kinase inhibitors for the treatment of epidermal growth factor receptor-mutant nonsmall cell lung cancer? Curr. Opin. Oncol. 2017, 29, 89–96. [Google Scholar] [CrossRef]
- Moya-Horno, I.; Viteri, S.; Karachaliou, N.; Rosell, R. Combination of immunotherapy with targeted therapies in advanced non-small cell lung cancer (NSCLC). Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucktrout, S.L.; Bluestone, J.A.; Ramsdell, F. Recent advances in immunotherapies: From infection and autoimmunity, to cancer, and back again. Genome Med. 2018, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Malapelle, U.; Rocco, D.; Perrotta, F.; Mazzarella, G. Targeting immune checkpoints in non small cell lung cancer. Curr. Opin. Pharmacol. 2018, 40, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Donini, C.; D’Ambrosio, L.; Grignani, G.; Aglietta, M.; Sangiolo, D. Next generation immune-checkpoints for cancer therapy. J. Thorac. Dis. 2018, 10, S1581–S1601. [Google Scholar] [CrossRef]
- De Sousa Linhares, A.; Leitner, J.; Grabmeier-Pfistershammer, K.; Steinberger, P. Not All Immune Checkpoints Are Created Equal. Front. Immunol. 2018, 9, 1909. [Google Scholar] [CrossRef]
- Bustamante Alvarez, J.G.; Gonzalez-Cao, M.; Karachaliou, N.; Santarpia, M.; Viteri, S.; Teixido, C.; Rosell, R. Advances in immunotherapy for treatment of lung cancer. Cancer Biol. Med. 2015, 12, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ramirez, R.A. The Role of Checkpoint Inhibition in Non-Small Cell Lung Cancer. Ochsner J. 2017, 17, 379–387. [Google Scholar] [PubMed]
- Malhotra, J.; Jabbour, S.K.; Aisner, J. Current state of immunotherapy for non-small cell lung cancer. Transl. Lung Cancer Res. 2017, 6, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. Epidermal Growth Factor Receptor Mutation (EGFR) Testing for Prediction of Response to EGFR-Targeting Tyrosine Kinase Inhibitor (TKI) Drugs in Patients with Advanced Non-Small-Cell Lung Cancer: An Evidence-Based Analysis. Ont. Health Technol. Assess. Ser. 2010, 10, 1–48. [Google Scholar] [PubMed]
- Villanueva, N.; Bazhenova, L. New strategies in immunotherapy for lung cancer: Beyond PD-1/PD-L1. Ther. Adv. Respir. Dis. 2018, 12. [Google Scholar] [CrossRef] [PubMed]
- Zeltsman, M.; Dozier, J.; McGee, E.; Ngai, D.; Adusumilli, P.S. CAR T-cell therapy for lung cancer and malignant pleural mesothelioma. Transl. Res. 2017, 187, 1–10. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Dillman, R.O.; Schwarzenberger, P.O.; Senzer, N.; Cunningham, C.; Cutler, J.; Tong, A.; Kumar, P.; Pappen, B.; Hamilton, C.; et al. Phase II study of belagenpumatucel-L, a transforming growth factor beta-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 4721–4730. [Google Scholar] [CrossRef]
- Gough, M.J.; Crittenden, M.R.; Sarff, M.; Pang, P.; Seung, S.K.; Vetto, J.T.; Hu, H.M.; Redmond, W.L.; Holland, J.; Weinberg, A.D. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J. Immunother. 2010, 33, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.; Zielinski, M.; Linder, A.; Dahabreh, J.; Gonzalez, E.E.; Malinowski, W.; Lopez-Brea, M.; Vanakesa, T.; Jassem, J.; Kalofonos, H.; et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: Phase II randomized study results. J. Clin. Oncol. 2013, 31, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Li, J.F.; Niu, Y.Y.; Xing, Y.L.; Liu, F. A novel bispecific c-MET/CTLA-4 antibody targeting lung cancer stem cell-like cells with therapeutic potential in human non-small cell lung cancer. Biosci. Rep. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamurthy, A.; Jimeno, A. Bispecific antibodies for cancer therapy: A review. Pharmacol. Ther. 2018, 185, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Conkling, P.; Richards, D.A.; Nemunaitis, J.J.; Boyd, T.E.; Mita, A.C.; de La Bourdonnaye, G.; Wages, D.; Bexon, A.S. Antitumor activity and safety of combination therapy with the Toll-like receptor 9 agonist IMO-2055, erlotinib, and bevacizumab in advanced or metastatic non-small cell lung cancer patients who have progressed following chemotherapy. Cancer Immunol. Immunother. 2014, 63, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Iribarren, K.; Bloy, N.; Buque, A.; Cremer, I.; Eggermont, A.; Fridman, W.H.; Fucikova, J.; Galon, J.; Spisek, R.; Zitvogel, L.; et al. Trial Watch: Immunostimulation with Toll-like receptor agonists in cancer therapy. Oncoimmunology 2016, 5, e1088631. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Lin, L.; Shu, R.; Dong, B.; Cao, D.; Li, Q.; Wang, Z. A targeted transforming growth factor-beta (TGF-beta) blocker, TTB, inhibits tumor growth and metastasis. Oncotarget 2018, 9, 23102–23113. [Google Scholar] [CrossRef]
- Russo, A.E.; Priolo, D.; Antonelli, G.; Libra, M.; McCubrey, J.A.; Ferrau, F. Bevacizumab in the treatment of NSCLC: Patient selection and perspectives. Lung Cancer 2017, 8, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.K.; Wozniak, A.J. Spotlight on necitumumab in the treatment of non-small-cell lung carcinoma. Lung Cancer 2017, 8, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Ciuleanu, T.E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.; Prelaj, A.; Califano, R. Immune checkpoint blockade for advanced non-small cell lung cancer: Challenging clinical scenarios. J. Thorac. Dis. 2018, 10, S1494–S1502. [Google Scholar] [CrossRef] [PubMed]
- Ellis, P.M.; Vella, E.T.; Ung, Y.C. Immune Checkpoint Inhibitors for Patients With Advanced Non-Small-Cell Lung Cancer: A Systematic Review. Clin. Lung Cancer 2017, 18, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.E.; Bryan, P.M.; Banerji, S.; Morris, D.G. Targeting the PD-1/PD-L1 axis for the treatment of non-small-cell lung cancer. Curr. Oncol. 2018, 25, e324–e334. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Kim, S.; Arrington, J.; Naghavi, A.O.; Dilling, T.J.; Creelan, B.C.; Antonia, S.J.; Caudell, J.J.; Harrison, L.B.; Sahebjam, S.; et al. Outcomes targeting the PD-1/PD-L1 axis in conjunction with stereotactic radiation for patients with non-small cell lung cancer brain metastases. J. Neurooncol. 2017, 133, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Collins, D.; Dolly, S.; McDonald, F.; O’Brien, M.E.R.; Yap, T.A. Targeting the PD-1/PD-L1 axis in non-small cell lung cancer. Curr. Probl. Cancer 2017, 41, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, M.; Caron, P.; Sibaud, V.; Godillot, C.; Collot, S.; Milia, J.; Prevot, G.; Mazieres, J. Toxicity of immune checkpoints inhibitors. Rev. Mal. Respir. 2018. [Google Scholar] [CrossRef]
- Varricchi, G.; Marone, G.; Mercurio, V.; Galdiero, M.R.; Bonaduce, D.; Tocchetti, C.G. Immune Checkpoint Inhibitors and Cardiac Toxicity: An Emerging Issue. Curr. Med. Chem. 2018, 25, 1327–1339. [Google Scholar] [CrossRef]
- Byun, D.J.; Wolchok, J.D.; Rosenberg, L.M.; Girotra, M. Cancer immunotherapy—Immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol. 2017, 13, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Johnston, R.L.; Lutzky, J.; Chodhry, A.; Barkin, J.S. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig. Dis. Sci. 2009, 54, 2538–2540. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Ramaiya, N.H.; Krajewski, K.M.; Jagannathan, J.P.; Tirumani, S.H.; Srivastava, A.; Ibrahim, N. Ipilimumab associated hepatitis: Imaging and clinicopathologic findings. Investig. New Drugs 2013, 31, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarevic, I.Z.; Qadir, N.; Suri, A.; Santamauro, J.T.; Stover, D. Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 2013, 143, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Seferian, A.; Parent, F.; Humbert, M. Immune checkpoint inhibitor-associated interstitial lung diseases: Some progress but still many issues. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wu, Q.; Zhou, Y.L.; Guo, X.; Ge, J.; Fu, J. Immune-related adverse events from combination immunotherapy in cancer patients: A comprehensive meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2018, 63, 292–298. [Google Scholar] [CrossRef]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Sandigursky, S.; Mor, A. Immune-Related Adverse Events in Cancer Patients Treated With Immune Checkpoint Inhibitors. Curr. Rheumatol. Rep. 2018, 20, 65. [Google Scholar] [CrossRef]
- Simmons, D.; Lang, E. The Most Recent Oncologic Emergency: What Emergency Physicians Need to Know About the Potential Complications of Immune Checkpoint Inhibitors. Cureus 2017, 9, e1774. [Google Scholar] [CrossRef]
- Winer, A.; Bodor, J.N.; Borghaei, H. Identifying and managing the adverse effects of immune checkpoint blockade. J. Thorac. Dis. 2018, 10, S480–S489. [Google Scholar] [CrossRef] [PubMed]
- King, G.T.; Sharma, P.; Davis, S.L.; Jimeno, A. Immune and autoimmune-related adverse events associated with immune checkpoint inhibitors in cancer therapy. Drugs Today 2018, 54, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef]
- Sharma, N.; Atluri, P.; Stroud, C.R.G.; Walker, P.R.; Cherukuri, S.D.; Cherry, C.R.; Gibbs, P.; Parent, T.; Hardin, J. Immune related adverse events (irAEs): A unique profile based on tumor type. J. Clin. Oncol. 2017, 35, e14606. [Google Scholar] [CrossRef]
- Pillai, R.N.; Behera, M.; Owonikoko, T.K.; Kamphorst, A.O.; Pakkala, S.; Belani, C.P.; Khuri, F.R.; Ahmed, R.; Ramalingam, S.S. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer 2018, 124, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Khunger, M.; Rakshit, S.; Pasupuleti, V.; Hernandez, A.V.; Mazzone, P.; Stevenson, J.; Pennell, N.A.; Velcheti, V. Incidence of Pneumonitis With Use of Programmed Death 1 and Programmed Death-Ligand 1 Inhibitors in Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Trials. Chest 2017, 152, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Fromm, A.; Ahmed, K.A.; Grass, G.D.; Yang, G.Q.; Oliver, D.E.; Dilling, T.J.; Antonia, S.J.; Perez, B.A. Radiotherapy Rescue of a Nivolumab-Refractory Immune Response in a Patient with PD-L1-Negative Metastatic Squamous Cell Carcinoma of the Lung. J. Thorac. Oncol. 2017, 12, e135–e136. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, T.; Nakamura, K.; Kawase, A. Abscopal Effect of Nivolumab in a Patient with Primary Lung Cancer. J. Thorac. Oncol. 2017, 12, e143–e144. [Google Scholar] [CrossRef]
- Louvel, G.; Bahleda, R.; Ammari, S.; Le Pechoux, C.; Levy, A.; Massard, C.; Le Pavec, J.; Champiat, S.; Deutsch, E. Immunotherapy and pulmonary toxicities: Can concomitant immune-checkpoint inhibitors with radiotherapy increase the risk of radiation pneumonitis? Eur. Respir. J. 2018, 51. [Google Scholar] [CrossRef]
- Wirsdorfer, F.; Cappuccini, F.; Niazman, M.; de Leve, S.; Westendorf, A.M.; Ludemann, L.; Stuschke, M.; Jendrossek, V. Thorax irradiation triggers a local and systemic accumulation of immunosuppressive CD4+ FoxP3+ regulatory T cells. Radiat. Oncol. 2014, 9, 98. [Google Scholar] [CrossRef] [Green Version]
- Kainthola, A.; Haritwal, T.; Tiwari, M.; Gupta, N.; Parvez, S.; Tiwari, M.; Prakash, H.; Agrawala, P.K. Immunological Aspect of Radiation-Induced Pneumonitis, Current Treatment Strategies, and Future Prospects. Front. Immunol. 2017, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Domagala-Kulawik, J.; Raniszewska, A. How to evaluate the immune status of lung cancer patients before immunotherapy. Breathe 2017, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Kakimi, K.; Karasaki, T.; Matsushita, H.; Sugie, T. Advances in personalized cancer immunotherapy. Breast Cancer 2017, 24, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kiyotani, K.; Chan, H.T.; Nakamura, Y. Immunopharmacogenomics towards personalized cancer immunotherapy targeting neoantigens. Cancer Sci. 2018, 109, 542–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zappasodi, R.; Wolchok, J.D.; Merghoub, T. Strategies for Predicting Response to Checkpoint Inhibitors. Curr. Hematol. Malig. Rep. 2018, 13, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Bentzen, S.M.; Deasy, J.O.; Kong, F.M.; Bradley, J.D.; Vogelius, I.S.; El Naqa, I.; Hubbs, J.L.; Lebesque, J.V.; Timmerman, R.D.; et al. Radiation dose-volume effects in the lung. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S70–S76. [Google Scholar] [CrossRef] [PubMed]
- Shahzadeh, S.; Gholami, S.; Aghamiri, S.M.R.; Mahani, H.; Nabavi, M.; Kalantari, F. Evaluation of normal lung tissue complication probability in gated and conventional radiotherapy using the 4D XCAT digital phantom. Comput. Biol. Med. 2018, 97, 21–29. [Google Scholar] [CrossRef]
- Tekatli, H.; Duijm, M.; Oomen-de Hoop, E.; Verbakel, W.; Schillemans, W.; Slotman, B.J.; Nuyttens, J.J.; Senan, S. Normal Tissue Complication Probability Modeling of Pulmonary Toxicity After Stereotactic and Hypofractionated Radiation Therapy for Central Lung Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 738–747. [Google Scholar] [CrossRef]
- Ghita, M.; Dunne, V.L.; McMahon, S.J.; Osman, S.O.; Small, D.M.; Weldon, S.; Taggart, C.C.; McGarry, C.K.; Hounsell, A.R.; Graves, E.E.; et al. Preclinical Evaluation of Dose-Volume Effects and Lung Toxicity Occurring in- and out-of-field. Int. J. Radiat. Oncol. Biol. Phys. 2018. [Google Scholar] [CrossRef]
- Krafft, S.P.; Rao, A.; Stingo, F.; Briere, T.M.; Court, L.E.; Liao, Z.; Martel, M.K. The utility of quantitative CT radiomics features for improved prediction of radiation pneumonitis. Med. Phys. 2018, 45, 5317–5324. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, G.; Chen, J.; Yang, Y.; Meng, X.; Wang, X.; Wu, Q.; Huo, Z.; Yu, Q.; Yu, J.; et al. Comparison of predictive powers of functional and anatomic dosimetric parameters for radiation-induced lung toxicity in locally advanced non-small cell lung cancer. Radiother. Oncol. 2018, 129, 242–248. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wirsdörfer, F.; De Leve, S.; Jendrossek, V. Combining Radiotherapy and Immunotherapy in Lung Cancer: Can We Expect Limitations Due to Altered Normal Tissue Toxicity? Int. J. Mol. Sci. 2019, 20, 24. https://doi.org/10.3390/ijms20010024
Wirsdörfer F, De Leve S, Jendrossek V. Combining Radiotherapy and Immunotherapy in Lung Cancer: Can We Expect Limitations Due to Altered Normal Tissue Toxicity? International Journal of Molecular Sciences. 2019; 20(1):24. https://doi.org/10.3390/ijms20010024
Chicago/Turabian StyleWirsdörfer, Florian, Simone De Leve, and Verena Jendrossek. 2019. "Combining Radiotherapy and Immunotherapy in Lung Cancer: Can We Expect Limitations Due to Altered Normal Tissue Toxicity?" International Journal of Molecular Sciences 20, no. 1: 24. https://doi.org/10.3390/ijms20010024