In Vitro Cytotoxicity Evaluation of the Magnéli Phase Titanium Suboxides (TixO2x−1) on A549 Human Lung Cells
Abstract
1. Introduction
2. Results
2.1. Nanoparticle Characteristics
2.2. Cellular Protein Amount Assay
2.3. Metabolic Activity Assay
2.4. Lysosomal Integrity Assay
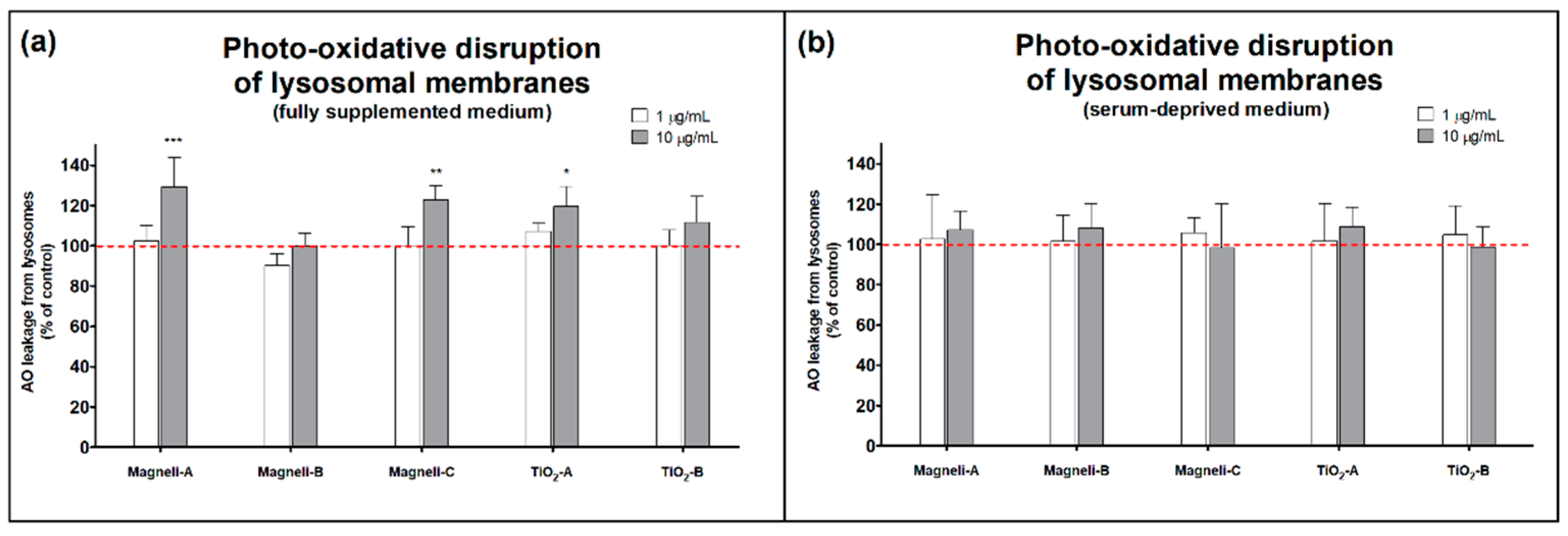
2.5. Photo-Oxidative Disruption of Lysosomal Membranes
2.6. Intracellular Ca2+ Level
2.7. Intracellular Reactive Oxygen Species Levels
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation and Characterization of Nanoparticle Suspensions
4.3. Cell Culture
4.4. Coomassie Blue Assay
4.5. Resazurin Assay
4.6. Neutral Red Uptake Assay
4.7. Acridine Orange Relocalization Assay
4.8. Reactive Oxygen Species Measurement
4.9. Calcium Imaging
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AO | Acridine Orange |
| ATP | Adenosine triphosphate |
| CB | Coomassie Blue |
| DCF | 2′,7′-dichlorofluorescein |
| DCFH-DA | 2′,7′-dichlorofluorescein diacetate |
| DLS | Dynamic Light Scattering |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DPBS | Dulbecco’s phosphate-buffered saline |
| FBS | Fetal Bovine Serum |
| NPs | Nanoparticles |
| NRU | Neutral Red Uptake |
| ROS | Reactive Oxygen Species |
| TEM | Transmission Electron Microscopy |
References
- De Matteis, V. Exposure to inorganic nanoparticles: Routes of entry, immune response, biodistribution and in vitro/in vivo toxicity evaluation. Toxics 2017, 5, 29. [Google Scholar] [CrossRef]
- Irache, J.M.; Martín-Arbella, N.; Ojer, P.; Azqueta, A.; de Cerain, A.L. Toxicological Aspects of Polymer Nanoparticles. In Polymer Nanoparticles for Nanomedicines; Springer: New York, NY, USA, 2016; pp. 521–550. [Google Scholar]
- Berger, E.; Breznan, D.; Stals, S.; Jasinghe, V.J.; Gonçalves, D.; Girard, D.; Faucher, S.; Vincent, R.; Thierry, A.R.; Lavigne, C. Cytotoxicity assessment, inflammatory properties, and cellular uptake of Neutraplex lipid-based nanoparticles in THP-1 monocyte-derived macrophages. Nanobiomedicine 2017, 4. [Google Scholar] [CrossRef]
- He, X.; Kiratipaiboon, C.; Porter, D.W.; Rojanasakul, L.W.; Dinu, C.Z.; Wang, K.; Yang, Y.; Rojanasakul, Y. Predicting Nanotube Fibrogenicity through Stem Cell-Mediated Fibroblast Focus and Spheroid Formation. Nano Lett. 2018, 18, 6500–6508. [Google Scholar] [CrossRef]
- Wang, K.; He, X.; Linthicum, W.; Mezan, R.; Wang, L.; Rojanasakul, Y.; Wen, Q.; Yang, Y. Carbon nanotubes induced fibrogenesis on nanostructured substrates. Environ. Sci. Nano 2017, 4, 689–699. [Google Scholar] [CrossRef]
- Pajnič, M.; Drašler, B.; Šuštar, V.; Krek, J.L.; Štukelj, R.; Šimundić, M.; Kononenko, V.; Makovec, D.; Hägerstrand, H.; Drobne, D.; et al. Effect of carbon black nanomaterial on biological membranes revealed by shape of human erythrocytes, platelets and phospholipid vesicles. J. Nanobiotechnol. 2015, 13, 28. [Google Scholar] [CrossRef]
- Martin, A.; Sarkar, A. Overview on biological implications of metal oxide nanoparticle exposure to human alveolar A549 cell line. Nanotoxicology 2017, 11, 713–724. [Google Scholar] [CrossRef]
- Kononenko, V.; Repar, N.; Marušič, N.; Drašler, B.; Romih, T.; Hočevar, S.; Drobne, D. Comparative in vitro genotoxicity study of ZnO nanoparticles, ZnO macroparticles and ZnCl2 to MDCK kidney cells: Size matters. Toxicol. In Vitro 2017, 40, 256–263. [Google Scholar] [CrossRef]
- Kononenko, V.; Erman, A.; Petan, T.; Križaj, I.; Kralj, S.; Makovec, D.; Drobne, D. Harmful at non-cytotoxic concentrations: SiO2-SPIONs affect surfactant metabolism and lamellar body biogenesis in A549 human alveolar epithelial cells. Nanotoxicology 2017, 11, 419–429. [Google Scholar] [CrossRef]
- Fu, P.P.; Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Ioroi, T.; Senoh, H.; Yamazaki, S.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Stability of corrosion-resistant Magnéli-phase Ti4O7-supported PEMFC catalysts at high potentials. J. Electrochem. Soc. 2008, 155, B321–B326. [Google Scholar] [CrossRef]
- Arif, A.F.; Balgis, R.; Ogi, T.; Iskandar, F.; Kinoshita, A.; Nakamura, K.; Okuyama, K. Highly conductive nano-sized Magnéli phases titanium oxide (TiOx). Sci. Rep. 2017, 7, 3646. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wills, R.G.A. The continuing development of Magnéli phase titanium sub-oxides and Ebonex®electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Geng, P.; Su, J.; Miles, C.; Comninellis, C.; Chen, G. Highly-ordered magnéli Ti4O7 nanotube arrays as effective anodic material for electro-oxidation. Electrochim. Acta 2015, 153, 316–324. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium dioxide nanomaterials for sensor applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced reactivity of titanium dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Awad, N.K.; Edwards, S.L.; Morsi, Y.S. A review of TiO2NTs on Ti metal: Electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C 2017, 76, 1401–1412. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Kononenko, V.; Imani, R.; Repar, N.; Benčina, M.; Lorenzetti, M.; Ermank, A.; Drobne, D.; Iglič, A. Phototoxicity of mesoporous TiO2+Gd microbeads with theranostic potential. In Advances in Biomembranes and Lipid Self-Assembly; Iglič, A., Rappolt, M., García-Sáez, A., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 26, pp. 153–171. ISBN 978-0-12-812079-8. [Google Scholar]
- Imani, R.; Dillert, R.; Bahnemann, D.W.; Pazoki, M.; Apih, T.; Kononenko, V.; Repar, N.; Kralj-Iglič, V.; Boschloo, G.; Drobne, D.; et al. Multifunctional Gadolinium-Doped Mesoporous TiO2 Nanobeads: Photoluminescence, Enhanced Spin Relaxation, and Reactive Oxygen Species Photogeneration, Beneficial for Cancer Diagnosis and Treatment. Small 2017, 13, 1700349. [Google Scholar] [CrossRef]
- Regonini, D.; Adamaki, V.; Bowen, C.R.; Pennock, S.R.; Taylor, J.; Dent, A.C.E. AC electrical properties of TiO2 and Magnéli phases, TinO2n−1. Solid State Ionics 2012, 229, 38–44. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, B.; Hower, J.; Schindler, M.; Winkler, C.; Brandt, J.; Di Giulio, R.; Ge, J.; Liu, M.; Fu, Y.; et al. Discovery and ramifications of incidental Magnéli phase generation and release from industrial coal-burning. Nat. Commun. 2017, 8, 194. [Google Scholar] [CrossRef]
- Jemec Kokalj, A.; Novak, S.; Talaber, I.; Kononenko, V.; Bizjak Mali, L.; Vodovnik, M.; Žegura, B.; Eleršek, T.; Kalčikova, G.; Žgajnar Gotvajn, A.; et al. Jemec Kokalj manuscript. Environ. Sci. Nano 2019. submitted for publication. [Google Scholar]
- Djurišić, A.B.; Leung, Y.H.; Ng, A.M.C.; Xu, X.Y.; Lee, P.K.H.; Degger, N.; Wu, R.S.S. Toxicity of metal oxide nanoparticles: Mechanisms, characterization, and avoiding experimental artefacts. Small 2015, 11, 26–44. [Google Scholar] [CrossRef]
- Huang, Y.W.; Cambre, M.; Lee, H.J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Stern, S.T.; Adiseshaiah, P.P.; Crist, R.M. Autophagy and lysosomal dysfunction as emerging mechanisms of nanomaterial toxicity. Part. Fibre Toxicol. 2012, 9, 20. [Google Scholar] [CrossRef]
- Meindl, C.; Kueznik, T.; Bösch, M.; Roblegg, E.; Fröhlich, E. Intracellular calcium levels as screening tool for nanoparticle toxicity. J. Appl. Toxicol. 2015, 35, 1150–1159. [Google Scholar] [CrossRef]
- Huang, C.C.; Aronstam, R.S.; Chen, D.R.; Huang, Y.W. Oxidative stress, calcium homeostasis, and altered gene expression in human lung epithelial cells exposed to ZnO nanoparticles. Toxicol. In Vitro 2010, 24, 45–55. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Brunk, U.T.; Dalen, H.; Roberg, K.; Hellquist, H.B. Photo-oxidative disruption of lysosomal membranes causes apoptosis of cultured human fibroblasts. Free Radic. Biol. Med. 1997, 23, 616–626. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Q.; Gao, S.; Song, Q.; Huang, R.; Wang, L.; Liu, L.; Dai, J.; Tang, M.; Cheng, G. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Albanese, A.; Chan, W.C.W. Effect of gold nanoparticle aggregation on cell uptake and toxicity. ACS Nano 2011, 5, 5478–5489. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef]
- Ji, Z.; Jin, X.; George, S.; Xia, T.; Meng, H.; Wang, X.; Suarez, E.; Zhang, H.; Hoek, E.M.V.; Godwin, H. Dispersion and stability optimization of TiO2 nanoparticles in cell culture media. Environ. Sci. Technol. 2010, 44, 7309–7314. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Decan, N.; Wu, D.; Williams, A.; Bernatchez, S.; Johnston, M.; Hill, M.; Halappanavar, S. Characterization of in vitro genotoxic, cytotoxic and transcriptomic responses following exposures to amorphous silica of different sizes. Mutat. Res. Toxicol. Environ. Mutagen. 2016, 796, 8–22. [Google Scholar] [CrossRef]
- Thai, S.; Wallace, K.A.; Jones, C.P.; Ren, H.; Grulke, E.; Castellon, B.T.; Crooks, J.; Kitchin, K.T. Differential genomic effects of six different TiO2 nanomaterials on human liver HepG2 cells. J. Biochem. Mol. Toxicol. 2016, 30, 331–341. [Google Scholar] [CrossRef]
- Tedja, R.; Lim, M.; Amal, R.; Marquis, C. Effects of serum adsorption on cellular uptake profile and consequent impact of titanium dioxide nanoparticles on human lung cell lines. ACS Nano 2012, 6, 4083–4093. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Patel, P.C.; Millstone, J.E.; Rosi, N.L.; Mirkin, C.A. Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles. Nano Lett. 2007, 7, 3818–3821. [Google Scholar] [CrossRef]
- Vippola, M.; Falck, G.C.M.; Lindberg, H.K.; Suhonen, S.; Vanhala, E.; Norppa, H.; Savolainen, K.; Tossavainen, A.; Tuomi, T. Preparation of nanoparticle dispersions for in-vitro toxicity testing. Hum. Exp. Toxicol. 2009, 28, 377–385. [Google Scholar] [CrossRef]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef]
- Lüllmann-Rauch, R. History and morphology of the lysosome. In Lysosomes; Springer: New York, NY, USA, 2005; pp. 1–16. [Google Scholar]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Intracellular calcium homeostasis and signaling. In Metallomics and the Cell; Springer: New York, NY, USA, 2013; pp. 119–168. [Google Scholar]
- Kong, H.; Xia, K.; Pan, L.; Zhang, J.; Luo, Y.; Zhang, Y.; Cui, Z.; El-Sayed, N.N.; Aldalbahi, A.; Chen, N.; et al. Autophagy and lysosomal dysfunction: A new insight into mechanism of synergistic pulmonary toxicity of carbon black-metal ions co-exposure. Carbon 2017, 111, 322–333. [Google Scholar] [CrossRef]
- Platt, F.M.; Boland, B.; van der Spoel, A.C. Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 2012, 199, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Thomassen, L.C.J.; Ferecatu, I.; Borot, M.C.; Andreau, K.; Martens, J.A.; Fleury, J.; Baeza-Squiban, A.; Marano, F.; Boland, S. Carbon black and titanium dioxide nanoparticles elicit distinct apoptotic pathways in bronchial epithelial cells. Part. Fibre Toxicol. 2010, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Zhu, B.; Wang, X.; Lu, Q. Cytotoxicity of Titanium Dioxide Nanoparticles in Mouse Fibroblast Cells Cytotoxicity of Titanium Dioxide Nanoparticles in Mouse Fibroblast. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef]
- Stone, V.; Tuinman, M.; Vamvakopoulos, J.E.; Shaw, J.; Brown, D.; Petterson, S.; Faux, S.P.; Borm, P.; MacNee, W.; Michaelangeli, F.; et al. Increased calcium influx in a monocytic cell line on exposure to ultrafine carbon black. Eur. Respir. J. 2000, 15, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Ong, C.; Bay, B.; Baeg, G. Nanotoxicity: An Interplay of Oxidative Stress, Inflammation and Cell Death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Oberdörster, G. Safety assessment for nanotechnology and nanomedicine: Concepts of nanotoxicology. J. Intern. Med. 2010, 267, 89–105. [Google Scholar] [CrossRef]
- Drasler, B.; Sayre, P.; Steinhaeuser, K.G.; Petri-Fink, A.; Rothen-Rutishauser, B. In vitro approaches to assess the hazard of nanomaterials. NanoImpact 2017, 8, 99–116. [Google Scholar] [CrossRef]
- Heinrich, U.; Fuhst, R.; Rittinghausen, S.; Creutzenberg, O.; Bellmann, B.; Koch, W.; Levsen, K. Chronic inhalation exposure of Wistar rats and two different strains of mice to diesel engine exhaust, carbon black, and titanium dioxide. Inhal. Toxicol. 1995, 7, 533–556. [Google Scholar] [CrossRef]
- Bondarenko, O.M.; Heinlaan, M.; Sihtmäe, M.; Ivask, A.; Kurvet, I.; Joonas, E.; Jemec, A.; Mannerström, M.; Heinonen, T.; Rekulapelly, R. Multilaboratory evaluation of 15 bioassays for (eco) toxicity screening and hazard ranking of engineered nanomaterials: FP7 project NANOVALID. Nanotoxicology 2016, 10, 1229–1242. [Google Scholar] [CrossRef]
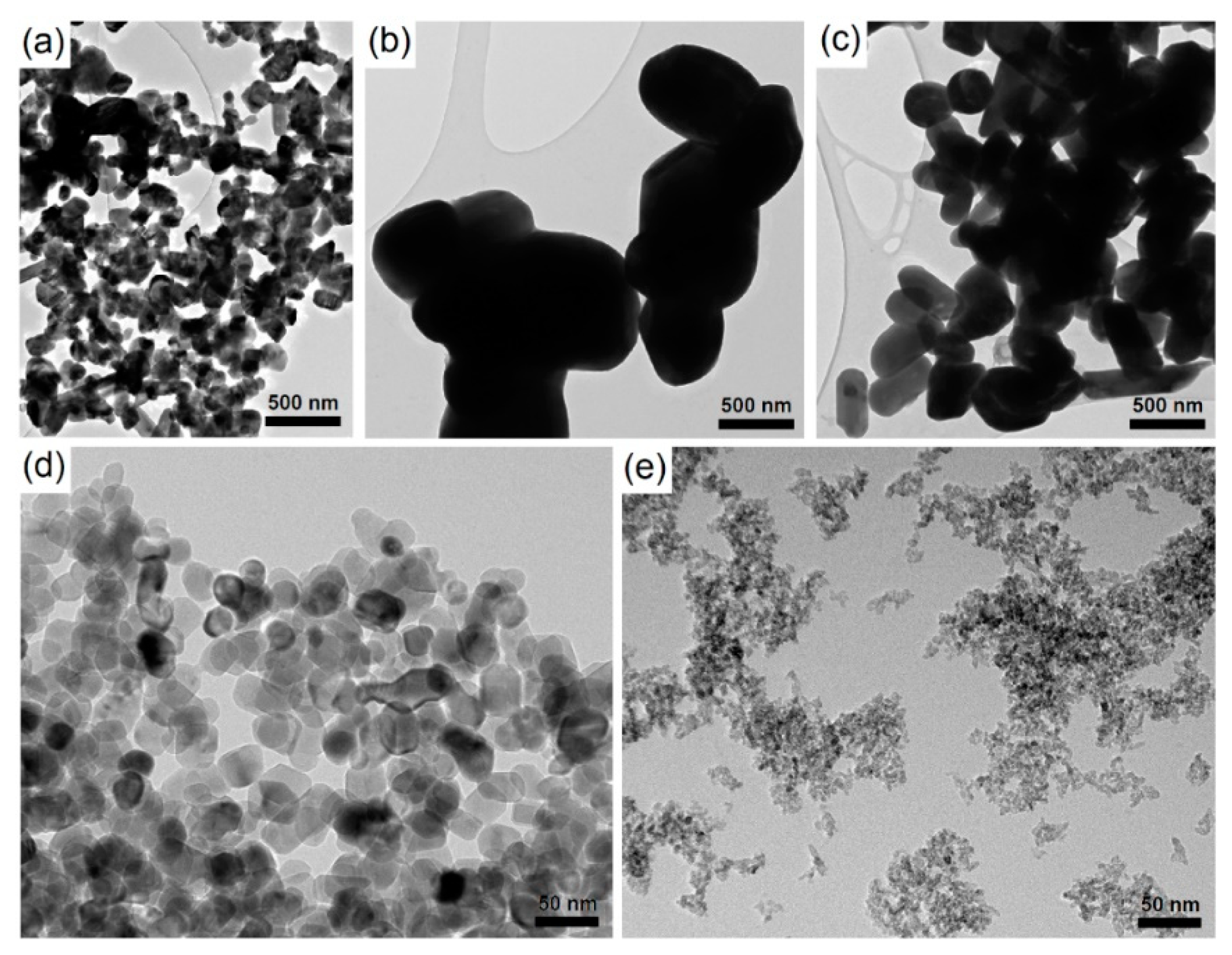



| Particles | Crystal Phase 1 | Average Diameter (nm) | Maximum Absorbance (nm) | Test Medium | Average Hydrodynamic Diameter (nm) | Zeta Potential (mV) |
|---|---|---|---|---|---|---|
| Magnéli-A | Magnéli phase | 192 ± 148 | 350 | Water | 163 ± 67 and 472 ± 194 | −32.9 ± 1.6 |
| Full cell medium | 90 ± 42 and 946 ± 442 | −20.8 ± 4.2 | ||||
| Serum-free medium | 991 ± 513 | −4.4 ± 11.6 | ||||
| Magnéli-B | Magnéli phase | 795 ± 298 | 545 | Water | 789 ± 280 | −38.1 ± 1.4 |
| Full cell medium | 124 ± 65 and 1087 ± 566 | 2.2 ± 5.9 | ||||
| Serum-free medium | 903 ± 506 | −22.2 ± 4.3 | ||||
| Magnéli-C | Magnéli phase | 507 ± 222 | 490 | Water | 543 ± 138 | −40.6 ± 1.2 |
| Full cell medium | 49 ± 8 and 1248 ± 216 | −23.1 ± 4.6 | ||||
| Serum-free medium | 991 ± 681 | −9.1 ± 6.7 | ||||
| TiO2-A | Anatase | 30 ± 7 | 350 | Water | 684 ± 318 | −6.9 ± 0.8 |
| Full cell medium | 136 ± 65 and 824 ± 393 | −0.5 ± 7.5 | ||||
| Serum-free medium | 2174 ± 1368 | −10.0 ± 9.1 | ||||
| TiO2-B | Anatase | 5.1 ± 1.2 | 250 | Water | 78 ± 35 and 473 ± 215 | 22.7 ± 1.3 |
| Full cell medium | 68 ± 64 | −7.5 ± 7.3 | ||||
| Serum-free medium | 1308 ± 451 | −8.6 ± 3.3 |
| Exposure Medium | Particles | Decreased Cell Number | Decreased Metabolic Activity | Affected Lysosomal Integrity | Photo-Oxidative Disruptions of Lysosomal Membranes | Increased ROS Levels | Increased Ca2+ Level |
|---|---|---|---|---|---|---|---|
| Fully supplemented cell medium | Magnéli-A | + | - | - | +++ | - | + |
| Magnéli-B | - | - | - | - | - | - | |
| Magnéli-C | + | - | - | ++ | - | ++ | |
| TiO2-A | - | - | - | + | +++ | - | |
| TiO2-B | - | - | - | - | - | - | |
| Serum-deprived cell medium | Magnéli-A | +++ | + | - | - | - | +++ |
| Magnéli-B | - | - | - | - | - | - | |
| Magnéli-C | + | - | - | - | - | +++ | |
| TiO2-A | + | +++ | +++ | - | +++ | + | |
| TiO2-B | - | +++ | +++ | - | +++ | +++ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kononenko, V.; Drobne, D. In Vitro Cytotoxicity Evaluation of the Magnéli Phase Titanium Suboxides (TixO2x−1) on A549 Human Lung Cells. Int. J. Mol. Sci. 2019, 20, 196. https://doi.org/10.3390/ijms20010196
Kononenko V, Drobne D. In Vitro Cytotoxicity Evaluation of the Magnéli Phase Titanium Suboxides (TixO2x−1) on A549 Human Lung Cells. International Journal of Molecular Sciences. 2019; 20(1):196. https://doi.org/10.3390/ijms20010196
Chicago/Turabian StyleKononenko, Veno, and Damjana Drobne. 2019. "In Vitro Cytotoxicity Evaluation of the Magnéli Phase Titanium Suboxides (TixO2x−1) on A549 Human Lung Cells" International Journal of Molecular Sciences 20, no. 1: 196. https://doi.org/10.3390/ijms20010196
APA StyleKononenko, V., & Drobne, D. (2019). In Vitro Cytotoxicity Evaluation of the Magnéli Phase Titanium Suboxides (TixO2x−1) on A549 Human Lung Cells. International Journal of Molecular Sciences, 20(1), 196. https://doi.org/10.3390/ijms20010196






