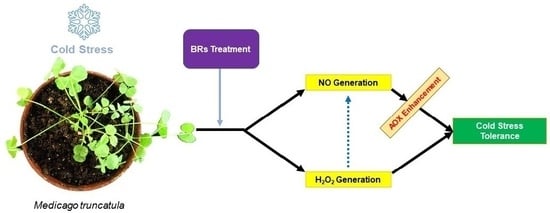
Hydrogen Peroxide and Nitric Oxide Crosstalk Mediates Brassinosteroids Induced Cold Stress Tolerance in Medicago truncatula
Abstract
:1. Introduction
2. Results
2.1. Brassinosteroids (BRs) Treatment Results in the Improvement of Cold Stress Tolerance
2.2. BRs Regulate Cold-Related Genes’ Expression
2.3. BRs-Treatment Induced Hydrogen Peroxide and Nitric Oxide Accumulation in Leaves
2.4. Relationship between BRs-Induced Hydrogen Peroxide and Nitric Oxide Accumulation
2.5. The Role of Hydrogen Peroxide and Nitric Oxide in BRs-Induced Alternative Respiratory Pathway
2.6. Hydrogen Peroxide and Nitric Oxide Involved in BRs-Induced Defense of Photosystem
3. Discussion
3.1. Involvement of BRs in Cold Stress Tolerance
3.2. BRs-Induced H2O2 and NO Generation in Medicago truncatula
3.3. Involvement of H2O2 and NO in BRs-Induced Alternative Pathway and PSII
4. Materials and Methods
4.1. Plant Material, Growth Condition, and Chemical Treatment
4.2. Quantification of Superoxide (O2−) Anion, Hydrogen Peroxide (H2O2), and Malondialdehyde (MDA)
4.3. Determination of Antioxidant Enzymes Activities
4.4. Measurement of Endogenous H2O2 and NO
4.5. Respiration Measurements
4.6. Chlorophyll Fluorescence Analysis
4.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BRs | Brassinosteroids |
| BL | Brassinolide |
| BRZ | Brassinazole |
| DMTU | Dimethylthiourea |
| PTIO | 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide |
References
- Zhang, S.; Cai, Z.; Wang, X. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 4543–4548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ning, J.; Li, X.; Hicks, L.M.; Xiong, L. A Raf-like MAPKKK gene DSM1 mediates drought resistance through reactive oxygen species scavenging in rice. Plant Physiol. 2010, 152, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.L. Cold acclimation and freezing stress tolerance: Role of protein metabolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1990, 41, 187–223. [Google Scholar] [CrossRef]
- Xin, Z.G.; Browse, J. Cold comfort farm: The acclimation of plants to freezing temperatures. Plant Cell Environ. 2000, 23, 893–902. [Google Scholar] [CrossRef]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Sasse, J.M. Physiological actions of brassinosteroids: An update. J. Plant Growth Regul. 2003, 22, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Kagale, S.; Divi, U.K.; Krochko, J.E.; Keller, W.A.; Krishna, P. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta 2007, 225, 353–364. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Cui, J.X.; Zhou, Y.H.; Ding, J.G.; Xia, X.J.; Shi, K.; Chen, S.C.; Asami, T.; Chen, Z.; Yu, J.Q. Role of nitric oxide in hydrogen peroxide dependent induction of abiotic stress tolerance by brassinosteroids in cucumber. Plant Cell Environ. 2011, 34, 347–358. [Google Scholar] [CrossRef]
- Xia, X.J.; Huang, L.F.; Zhou, Y.H.; Mao, W.H.; Shi, K.; Wu, J.X.; Asami, T.; Chen, Z.X.; Yu, J.Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 2009, 230, 1185–1196. [Google Scholar] [CrossRef]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Tao, Y.; Mao, W.H.; Shi, K.; Asami, T.; Chen, Z.X.; Yu, J.Q. Reactive oxygen species are involved in brassinosteroid induced stress tolerance in cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2013, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dat, J.; Vandenabeele, S.; Vranova, E.; van Montagu, M.; Inze, D.; van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Iwahashi, H.; Rackwal, R. Small GTPase (ROP) molecular switch for plant defense responses. FEBS Lett. 2003, 546, 173–180. [Google Scholar] [CrossRef]
- Gilroy, S.; Suzuki, N.; Miller, G.; Choi, W.G.; Toyota, M.; Devireddy, A.R.; Mittler, R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014, 19, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Hancock, J. Hydrogen peroxide signaling. Curr. Opin. Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, J.; Li, X.; Xia, X.J.; Zhou, Y.H.; Shi, K.; Chen, Z.; Yu, J.Q. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. J. Exp. Bot. 2014, 65, 4371–4383. [Google Scholar] [CrossRef] [PubMed]
- Neill, S.J.; Desikan, R.; Hancock, J. Nitric oxide signaling in plants. New Phytol. 2003, 159, 11–35. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Zhang, F.; Guo, J.K.; Yang, Y.L.; Li, B.B.; Zhang, L.X. Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol. 2004, 134, 849–857. [Google Scholar] [CrossRef]
- Wilson, I.D.; Neill, S.J.; Hancock, J.T. Nitric oxide synthesis and signaling in plants. Plant Cell Environ. 2008, 31, 622–631. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B.; Carreras, A.; Valderrama, R.; Palma, J.M.; Leon, A.M.; Sandalio, L.M.; Rio, L.A. Constitutive arginine-dependent nitric oxide synthase activity in different organs of pea seedlings during plant development. Planta 2006, 224, 246–254. [Google Scholar] [CrossRef]
- Shi, C.; Qi, C.; Ren, H.; Huang, A.; Hei, S.; She, X. Ethylene mediates brassinosteroid-induced stomatal closure via Ga protein-activated hydrogen peroxide and nitric oxide production in Arabidopsis. Plant J. 2015, 82, 280–301. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.L.; Albury, M.S.; Crichton, P.G.; Affourtit, C. Function of the alternative oxidase: Is it still a scavenger? Trends Plant Sci. 2002, 7, 478–481. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, D.W.; Zhu, F.; Tang, H.; Lv, X.; Cheng, J.; Xie, H.F.; Lin, H.H. A novel role for cyanide in the control of cucumber (Cucumis sativus L.) seedlings response to environmental stress. Plant Cell Environ. 2012, 35, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, J.; Liang, X.; Bi, Y. Involvement of hydrogen peroxide, calcium, and ethylene in the induction of the alternative pathway in chilling-stressed Arabidopsis callus. Planta 2012, 235, 53–67. [Google Scholar] [CrossRef]
- Panda, S.K.; Sahoo, L.; Katsuhara, M.; Matsumoto, H. Overexpression of alternative oxidase gene confers aluminum tolerance by altering the respiratory capacity and the response to oxidative stress in tobacco cells. Mol. Biotechnol. 2013, 54, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Watanabe, C.; Kato, Y.; Sakamoto, W.; Noguchi, K. Influence of chloroplastic photo-oxidative stress on mitochondrial alternative oxidase capacity and respiratory properties: A case study with Arabidopsis yellow variegated 2. Plant Cell Physiol. 2008, 49, 592–603. [Google Scholar] [CrossRef]
- Zhang, D.W.; Yuan, S.; Xu, F.; Zhu, F.; Yuan, M.; Ye, H.X.; Guo, H.Q.; Lv, X.; Yin, Y.H.; Lin, H.H. Light intensity affects chlorophyll synthesis during greening process by metabolite signal from mitochondrial alternative oxidase in Arabidopsis. Plant Cell Environ. 2016, 39, 12–25. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef]
- Barker, D.G.; Bianchi, S.; Blondon, F.; Dattee, Y.; Duc, G.; Essad, S.; Flament, P.; Gallusci, P.; Genier, G.; Guy, P.; et al. Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobium-legume symbiosis. Plant Mol. Biol. Rep. 1990, 8, 40–49. [Google Scholar] [CrossRef]
- Luo, S.S.; Sun, Y.N.; Zhou, X.; Zhu, T.; Zhu, L.S.; Arfan, M.; Zou, L.J.; Lin, H.H. Medicago truncatula genotypes Jemalong A17 and R108 show contrasting variations under drought stress. Plant Physiol. Biochem. 2016, 109, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.G.; Zhu, T.; Zhang, D.W.; Lin, H.H. The alternative respiratory pathway is involved in brassinosteroid-induced environmental stress tolerance in Nicotiana benthamiana. J. Exp. Bot. 2015, 66, 6219–6232. [Google Scholar] [CrossRef]
- Deng, X.G.; Zhu, T.; Zou, L.J.; Han, X.Y.; Zhou, X.; Xi, D.H.; Zhang, D.W.; Lin, H.H. Orchestration of hydrogen peroxide and nitric oxide in brassinosteroid-mediated systemic virus resistance in Nicotiana benthamiana. Plant J. 2016, 85, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Deng, X.G.; Tan, W.R.; Zhou, X.; Luo, S.S.; Han, X.Y.; Zhang, D.W.; Lin, H.H. Nitric oxide is involved in brassinosteroid-induced alternative respiratory pathway in Nicotiana benthamiana seedlings response to salt stress. Physiol. Plant. 2015, 156, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Liu, W.; Xia, X.; Wang, T.; Zhang, W.H. Cold acclimation-induced freezing tolerance of Medicago truncatula seedlings is negatively regulated by ethylene. Physiol. Plant. 2014, 152, 115–129. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, X.; Liu, W.; Min, X.; Jin, X.; Ndayambaza, B.; Wang, Y. Genome-wide identification and expression analysis of the fatty acid desaturase genes in Medicago truncatula. Biochem. Biophys. Res. Commun. 2018, 499, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Székely, G.; Ábrahám, E.; Cséplő, Á.; Rigó, G.; Zsigmond, L.; Csiszár, J.; Ayaydin, F.; Strizhov, N.; Jásik, J.; Schmelzer, E.; et al. Duplicated P5CS genes of Arabidopsis play distinct roles in stress regulation and developmental control of proline biosynthesis. Plant J. 2008, 53, 11–28. [Google Scholar] [CrossRef]
- Bowler, C.; Montagu, M.V.; Inzé, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Asghari, M.; Farmani, B.; Mohayeji, M.; Moradbeygi, H. Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci. Hort. 2012, 144, 116–120. [Google Scholar] [CrossRef]
- Hu, W.H.; Wu, Y.; Zeng, J.Z.; He, L.; Zeng, Q.M. Chill-induced inhibition of photosynthesis was alleviated by 24-epibrassinolide pretreatment in cucumber during chilling and subsequent recovery. Photosynthetica 2010, 48, 537–544. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Yusuf, M.; Chalkoo, S.; Hayat, S.; Ahmad, A. 28-Homobrassinolide improves growth and photosynthesis in Cucumis sativus L. through an enhanced antioxidant system in the presence of chilling stress. Photosynthetica 2011, 49, 55–64. [Google Scholar] [CrossRef]
- Ereminaa, M.; Unterholzner, S.J.; Rathnayake, A.I.; Castellanos, M.; Khan, M.; Kugler, K.G.; May, S.T.; Mayer, K.F.X.; Rozhon, W.; Poppenberger, B. Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. USA 2016, 113, 5982–5991. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Chae, H.S.; Kieber, J.J. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2009, 57, 606–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshioka, H.; Mase, K.; Yoshioka, M.; Kobayashi, M.; Asai, S. Regulatory mechanisms of nitric oxide and reactive oxygen species generation and their role in plant immunity. Nitric Oxide 2011, 25, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Li, C.; Fan, L.M. Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ. Exp. Bot. 2014, 100, 84–93. [Google Scholar] [CrossRef]
- Bright, J.; Desikan, R.; Hancock, J.T.; Weir, I.S.; Neill, S.J. ABA induced NO generation and stomatal closure in Arabidopsis is dependent on H2O2 synthesis. Plant J. 2006, 45, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Jiang, M.Y.; Zhang, J.H.; Ding, H.D.; Xu, S.C.; Hu, X.L.; Tan, M.P. Nitric oxide induced by hydrogen peroxide mediates abscisic acid-induced activation of the mitogen activated protein kinase cascade involved in antioxidant defense in maize leaves. New Phytol. 2007, 175, 36–50. [Google Scholar] [CrossRef]
- Mizuno, N.; Sugie, A.; Kobayashi, F.; Takumi, S. Mitochondrial alternative pathway is associated with development of freezing tolerance in common wheat. J. Plant Physiol. 2008, 165, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Matos, A.R.; Mendes, A.T.; Scotti-Campos, P.; Arrabaça, J.D. Study of the effects of salicylic acid on soybean mitochondrial lipids and respiratory properties using the alternative oxidase as a stress-reporter protein. Physiol. Plant. 2009, 137, 485–497. [Google Scholar] [CrossRef]
- Fung, R.W.M.; Wang, C.Y.; Smith, D.L.; Gross, K.C.; Tian, M.S. MeSA and MeJA increase steady-state transcript levels of alternative oxidase and resistance against chilling injury in sweet peppers (Capsicum annuum L.). Plant Sci. 2004, 166, 711–719. [Google Scholar] [CrossRef]
- Wang, H.; Liang, X.; Huang, J.; Zhang, D.; Lu, H.; Liu, Z.; Bi, Y. Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt treated Arabidopsis calluses. Plant Cell Physiol. 2010, 51, 1754–1765. [Google Scholar] [CrossRef]
- Giraud, E.; Van Aken, O.; Ho, L.H.; Whelan, J. The transcription factor ABI4 is a regulator of mitochondrial retrograde expression of ALTERNATIVE OXIDASE1a. Plant Physiol. 2009, 150, 1286–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.T.; Zhang, Z.S.; Gao, H.Y.; Xue, Z.C.; Yang, C.; Meng, X.L.; Meng, Q.W. Mitochondrial alternative oxidase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photo damaged PSII through preventing formation of reactive oxygen species in RumexK-1 leaves. Physiol. Plant. 2011, 143, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Tanir, H.M.; Sener, T.; Inal, M.; Akyuz, F.; Uzuner, K.; Sivri, E. Effect of quercetine and glutathione on the level of superoxide dismutase, catalase, malonyldialdehyde, blood pressure and neonatal outcome in a rat model of pre-eclampsia induced by NG-nitro-l-arginine-methyl ester. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 118, 190–195. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Wei, X.; Li, D.; Liu, G. Anti-oxidative responses of Elodea nuttallii (Planch.) H. St. John to short-term iron exposure. Plant Physiol. Bioch. 2010, 48, 873–878. [Google Scholar]
- García-Triana, A.; Zenteno-Savínb, T.; Peregrino-Uriartea, A.B.; Yepiz-Plascenciaa, G. Hypoxia, reoxygenation and cytosolic manganese superoxide dismutase (cMnSOD) silencing in Litopenaeus vannamei: Effects on cMnSOD transcripts, superox ide dismutase activity and superoxide anion production capacity. Dev. Comp. Immunol. 2010, 34, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, M.; Butler, W.L. Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim. Biophys. Acta 1975, 376, 105–115. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Jian, W.; Zhang, D.W.; Zhu, F.; Wang, S.X.; Pu, X.J.; Deng, X.G.; Luo, S.S.; Lin, H.H. Alternative oxidase pathway is involved in the exogenous SNP-elevated tolerance of Medicago truncatula to salt stress. J. Plant Physiol. 2016, 193, 79–87. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arfan, M.; Zhang, D.-W.; Zou, L.-J.; Luo, S.-S.; Tan, W.-R.; Zhu, T.; Lin, H.-H. Hydrogen Peroxide and Nitric Oxide Crosstalk Mediates Brassinosteroids Induced Cold Stress Tolerance in Medicago truncatula. Int. J. Mol. Sci. 2019, 20, 144. https://doi.org/10.3390/ijms20010144
Arfan M, Zhang D-W, Zou L-J, Luo S-S, Tan W-R, Zhu T, Lin H-H. Hydrogen Peroxide and Nitric Oxide Crosstalk Mediates Brassinosteroids Induced Cold Stress Tolerance in Medicago truncatula. International Journal of Molecular Sciences. 2019; 20(1):144. https://doi.org/10.3390/ijms20010144
Chicago/Turabian StyleArfan, Muhammad, Da-Wei Zhang, Li-Juan Zou, Shi-Shuai Luo, Wen-Rong Tan, Tong Zhu, and Hong-Hui Lin. 2019. "Hydrogen Peroxide and Nitric Oxide Crosstalk Mediates Brassinosteroids Induced Cold Stress Tolerance in Medicago truncatula" International Journal of Molecular Sciences 20, no. 1: 144. https://doi.org/10.3390/ijms20010144