Altered Glucosinolate Profiles and Expression of Glucosinolate Biosynthesis Genes in Ringspot-Resistant and Susceptible Cabbage Lines
Abstract
:1. Introduction
2. Results
2.1. Resistance of the Cabbage Lines to M. brassicicola
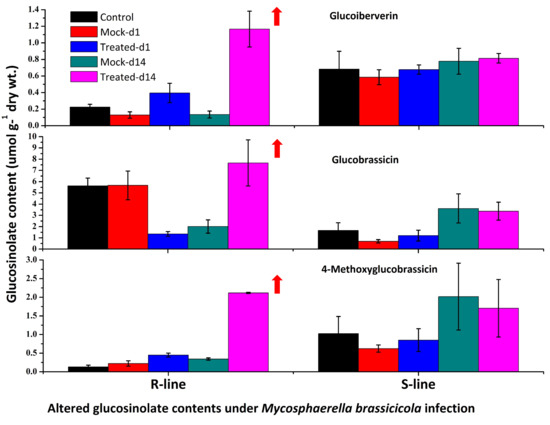
2.2. Overview of the Profile of Individual GSLs in the R and S Lines of Cabbage after M. Brassicicola Inoculation
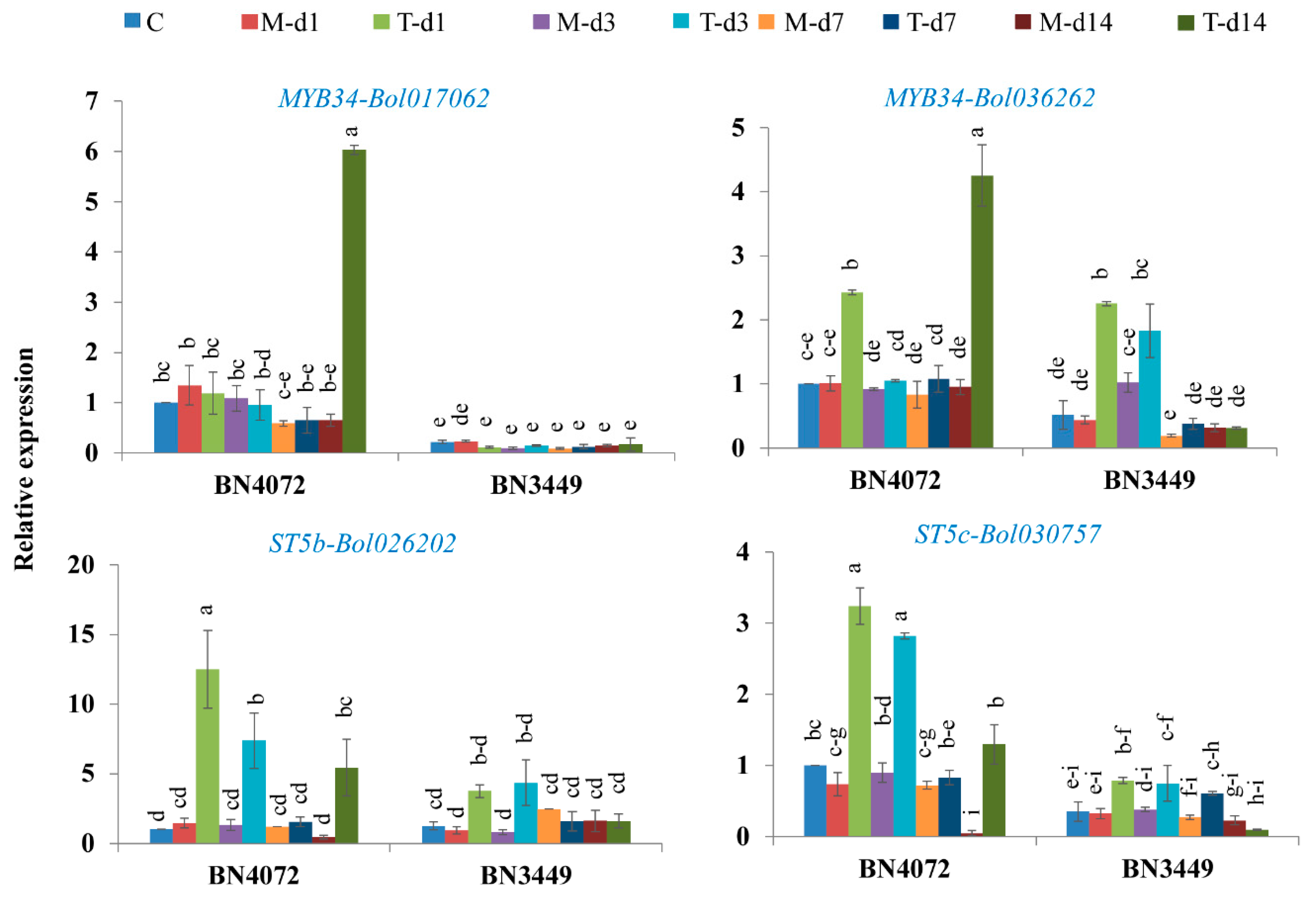
2.3. Upregulation of MYB34-Bol017062, MYB34-Bol036262, ST5b-Bol026202, and ST5c-Bol030757 in the R Line after Inoculation
2.4. Upregulation of Transcription Factor-Related Genes and GSL Biosynthesis Genes in the S Line after Inoculation
2.5. Upregulation of Transcription Factor-Related Genes and GSL Biosynthesis Genes in the R and S Lines
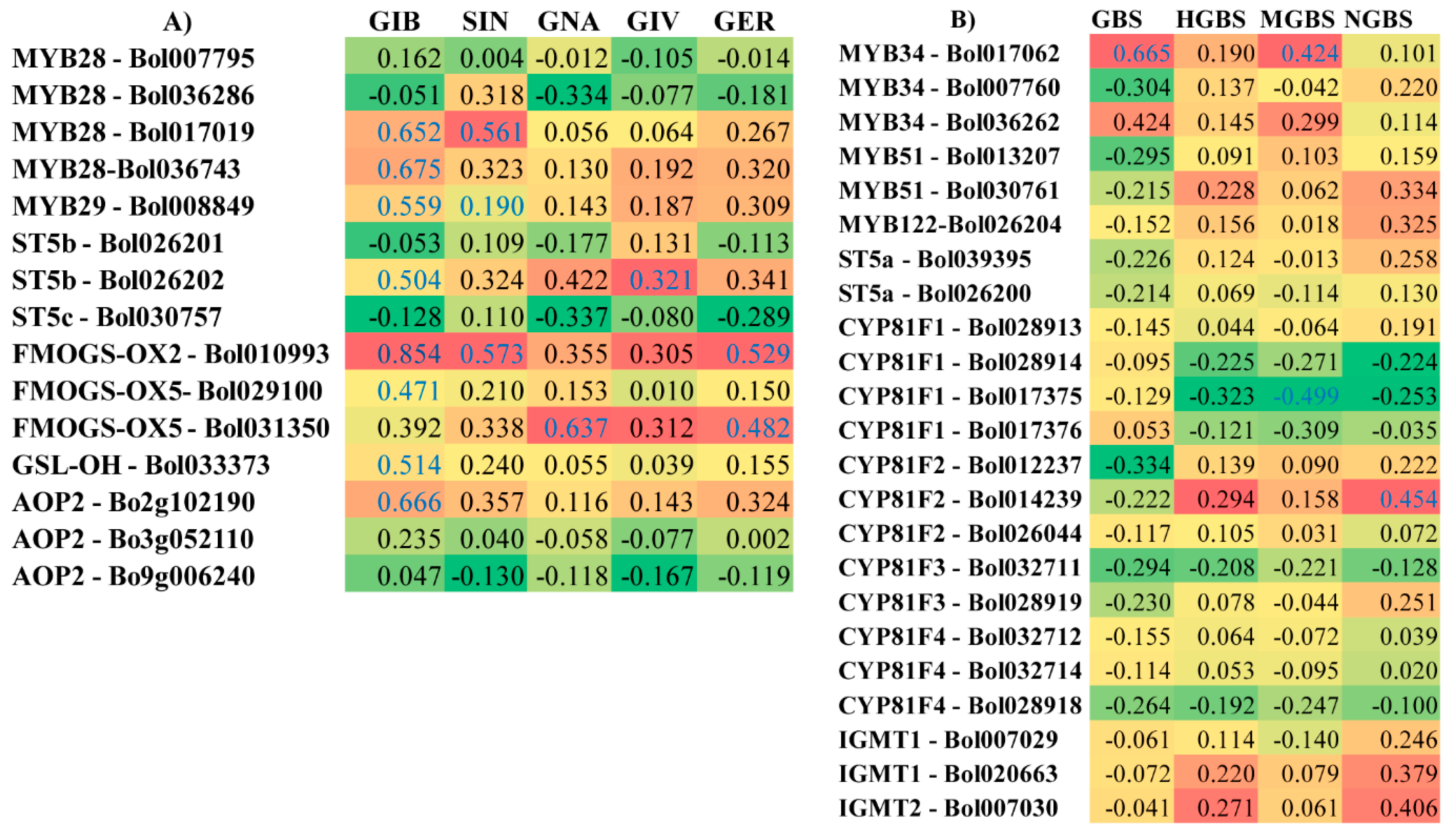
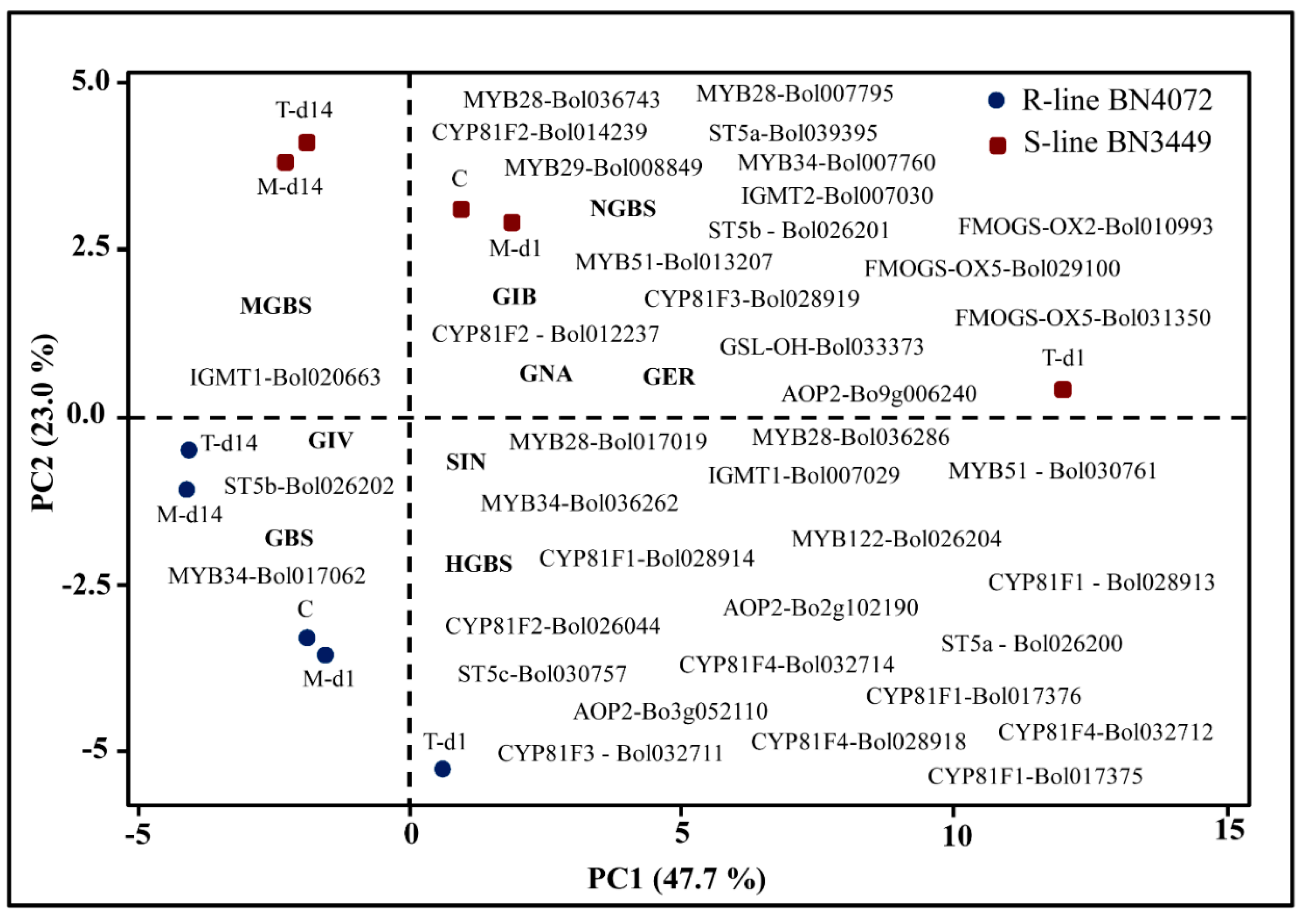
2.6. Correlation between the Levels of Individual GSLs and the Expression Level of GSL Biosynthesis Pathway Genes Induced by M. brassicicola in the R and S Lines
3. Discussion
3.1. Levels of Total GSLs, GIV, and GBS Are Related to Ringspot Resistance
3.2. Increased Expression of ST5b-Bol026202 Led to Increased Levels of GIV in the R Line
3.3. Increased Levels of Aliphatic GIV and Indolic GBS and MGBS Are Associated with Ringspot Resistance
3.4. Expression of MYB34 Likely Induces the Expression of GSL Biosynthesis Genes, Leading to Increases in GBS and MGBS in the R Line
3.5. Accumulation of Indolic GBS and MGBS in the R Line is Activated by Increased Expression of GSL Biosynthesis Genes
3.6. Accumulation of Aliphatic GIV with Expression of ST5b-Bol026202 and Indolic GBS with MYB34-Bol017062 May Play a Role in Resistance
3.7. Association of GSL Biosynthesis Genes and Accumulation of Individual GSLs in the S Line
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Inoculum Preparation
4.3. Inoculation Technique
4.4. Disease Assessment
4.5. Leaf Sampling and Preparation for High-Performance Liquid Chromatography and Gene Expression Analysis
4.6. Assessment of GSL Content
4.7. Primer Design for Expression Analysis of GSL Biosynthesis Genes
4.8. cDNA Synthesis and RT-qPCR Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Messiaen, C.-M.; Blancard, D.; Rouxel, F. Les Maladies des Plantes Maraîchères, 3rd ed.; Inra: Paris, France, 1991. [Google Scholar]
- Jouan, B.; Lemaire, J.; Hervé, Y. Etude des Maladies du Chou-Fleur (Brassica Oleracea l. Var. Botrytis d. C.) i. Les Parasites des Porte-Graines de Chou-Fleur d’hiver; Annales de Phytopathologie: Paris, France, 1972. [Google Scholar]
- Götz, M.; Zornbach, W.; Boyle, C. Life cycle of Mycosphaerella brassicicola (duby) lindau and ascospore production in vitro. J. Phytopathol. 1993, 139, 298–308. [Google Scholar] [CrossRef]
- Van Den Ende, J.; Frinking, H. Comparison of inoculation methods with Mycosphaerella brassicicola on brassica oleracea var. Capitata: Ascospores versus mycelial fragments. Neth. J. Plant Pathol. 1993, 99, 69. [Google Scholar] [CrossRef]
- Zornbach, W. Untersuchungen Zur Pathogenese, Epidemiologie und BekäMpfbarkeit Von Mycosphaerella Brassicicila (Duby) Lindau, dem Erreger der Ringfleckenkrankheit an Cruciferen; Kommissionsverlag P. Parey: Berlin, Germany, 1990. [Google Scholar]
- Frinking, H.; van den Ende, J.; Geerds, C. Mycosphaerella brassicicola op kool: Een epidemiologische beschouwing. Gewasbescherming 1986, 17, 12. [Google Scholar]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef] [Green Version]
- Kliebenstein, D. Secondary metabolites and plant/environment interactions: A view through Arabidopsis thaliana tinged glasses. Plant Cell Environ. 2004, 27, 675–684. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochem. Adv. Res. 2006, 661, 23–67. [Google Scholar]
- Wink, M. Plant breeding: Importance of plant secondary metabolites for protection against pathogens and herbivores. Theor. Appl. Genet. 1988, 75, 225–233. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Mithen, R. Glucosinolates–biochemistry, genetics and biological activity. Plant Growth Regul. 2001, 34, 91–103. [Google Scholar] [CrossRef]
- Bekaert, M.; Edger, P.P.; Hudson, C.M.; Pires, J.C.; Conant, G.C. Metabolic and evolutionary costs of herbivory defense: Systems biology of glucosinolate synthesis. New Phytol. 2012, 196, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Mota, V.; Saavedra, M.; Rosa, E.; Bennett, R. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009, 106, 2086–2095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giamoustaris, A.; Mithen, R. The effect of modifying the glucosinolate content of leaves of oilseed rape (Brassica napus ssp. oleifera) on its interaction with specialist and generalist pests. Ann. Appl. Biol. 1995, 126, 347–363. [Google Scholar]
- Manici, L.M.; Lazzeri, L.; Palmieri, S. In vitro fungitoxic activity of some glucosinolates and their enzyme-derived products toward plant pathogenic fungi. J. Agric. Food Chem. 1997, 45, 2768–2773. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E.; Sørensen, H. Initial and final products, nitriles, and ascorbigens produced in myrosinase-catalyzed hydrolysis of indole glucosinolates. J. Agric. Food Chem. 1998, 46, 1563–1571. [Google Scholar] [CrossRef]
- Brader, G.; Tas, E.; Palva, E.T. Jasmonate-dependent induction of indole glucosinolates in arabidopsis by culture filtrates of the nonspecific pathogenerwinia carotovora. Plant Physiol. 2001, 126, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Tierens, K.F.-J.; Thomma, B.P.; Brouwer, M.; Schmidt, J.; Kistner, K.; Porzel, A.; Mauch-Mani, B.; Cammue, B.P.; Broekaert, W.F. Study of the role of antimicrobial glucosinolate-derived isothiocyanates in resistance of arabidopsis to microbial pathogens. Plant Physiol. 2001, 125, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.; Jander, G. Arabidopsis myrosinases tgg1 and tgg2 have redundant function in glucosinolate breakdown and insect defense. Plant J. 2006, 46, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Stotz, H.U.; Sawada, Y.; Shimada, Y.; Hirai, M.Y.; Sasaki, E.; Krischke, M.; Brown, P.D.; Saito, K.; Kamiya, Y. Role of camalexin, indole glucosinolates, and side chain modification of glucosinolate-derived isothiocyanates in defense of arabidopsis against sclerotinia sclerotiorum. Plant J. 2011, 67, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Calmes, B.; N’Guyen, G.; Dumur, J.; Brisach, C.A.; Campion, C.; Iacomi, B.; Pigné, S.; Dias, E.; Macherel, D.; Guillemette, T. Glucosinolate-derived isothiocyanates impact mitochondrial function in fungal cells and elicit an oxidative stress response necessary for growth recovery. Front. Plant Sci. 2015, 6, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogge, L.; Reed, D.; Underhill, E.; Haughn, G. Hplc separation of glucosinolates from leaves and seeds of arabidopsis thaliana and their identification using thermospray liquid chramatography/mass spectrometry. J. Chromatogr. Sci. 1988, 26, 551–556. [Google Scholar] [CrossRef]
- Mithen, R.; Raybould, A.; Giamoustaris, A. Divergent selection for secondary metabolites between wild populations of brassica oleracea and its implications for plant-herbivore interactions. Heredity 1995, 75, 472. [Google Scholar] [CrossRef]
- Benderoth, M.; Textor, S.; Windsor, A.J.; Mitchell-Olds, T.; Gershenzon, J.; Kroymann, J. Positive selection driving diversification in plant secondary metabolism. Proc. Natl Acad. Sci. USA 2006, 103, 9118–9123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J. Role of glucosinolates in insect-plant relationships and multitrophic interactions. Ann. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef] [PubMed]
- Schnug, E.; Haneklaus, S.; Murphy, D. Impact of sulphur fertilization on fertilizer nitrogen efficiency. Sulphur Agric. 1993, 17, 12. [Google Scholar]
- Krumbein, A.; Schonhof, I.; Rühlmann, J.; Widell, S. Influence of sulphur and nitrogen supply on flavour and health-affecting compounds in brassicaceae. In Plant Nutrition; Springer: New York, NY, USA, 2001; pp. 294–295. [Google Scholar]
- Salac, I.; Haneklaus, S.; Bloem, E.; Booth, E.; Sutherland, K.; Walker, K.; Schnug, E. Influence of sulfur fertilization on sulfur metabolites, disease incidence and severity of fungal pathogens in oilseed rape in scotland. Landbauforsch. Vôlkenrode 2006, 56, 1–4. [Google Scholar]
- Schonhof, I.; Blankenburg, D.; Müller, S.; Krumbein, A. Sulfur and nitrogen supply influence growth, product appearance, and glucosinolate concentration of broccoli. J. Plant Nutr. Soil Sci. 2007, 170, 65–72. [Google Scholar] [CrossRef]
- Wittstock, U.; Halkier, B.A. Glucosinolate research in the Arabidopsis era. Trends Plant Sci. 2002, 7, 263–270. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Kurashige, N.S. A role for isothiocyanates in plant resistance against the specialist herbivore pieris rapae. J. Chem. Ecol. 2003, 29, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Mewis, I.; Appel, H.M.; Hom, A.; Raina, R.; Schultz, J.C. Major signaling pathways modulate arabidopsis glucosinolate accumulation and response to both phloem-feeding and chewing insects. Plant Physiol. 2005, 138, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.; Pedersen, D.; Barker, B.; Mitchell-Olds, T. Comparative analysis of quantitative trait loci controlling glucosinolates, myrosinase and insect resistance in Arabidopsis thaliana. Genetics 2002, 161, 325–332. [Google Scholar] [PubMed]
- Mikkelsen, M.D.; Petersen, B.L.; Glawischnig, E.; Jensen, A.B.; Andreasson, E.; Halkier, B.A. Modulation of cyp79 genes and glucosinolate profiles in arabidopsis by defense signaling pathways. Plant Physiology 2003, 131, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.H.K.; Yi, G.-E.; Laila, R.; Hossain, M.R.; Park, J.-I.; Kim, H.R.; Nou, I.-S. Leptosphaeria maculans alters glucosinolate profiles in blackleg disease–resistant and-susceptible cabbage lines. Front. Plant Sci. 2017, 8, 1769. [Google Scholar] [CrossRef] [PubMed]
- Mithen, R.F.; Magrath, R. Glucosinolates and resistance to leptosphaeria-maculans in wild and cultivated brassica species. Plant Breed. 1992, 108, 60–68. [Google Scholar] [CrossRef]
- Sexton, A.C.; Kirkegaard, J.A.; Howlett, B.J. Glucosinolates in brassica juncea and resistance to australian isolates of Leptosphaeria maculans, the blackleg fungus. Australas. Plant Pathol. 1999, 28, 95–102. [Google Scholar] [CrossRef]
- Li, Y.; Kiddle, G.; Bennett, R.; Wallsgrove, R. Local and systemic changes in glucosinolates in chinese and european cultivars of oilseed rape (Brassica napus L.) after inoculation with sclerotinia sclerotiorum (stem rot). Ann. Appl. Biol. 1999, 134, 45–58. [Google Scholar] [CrossRef]
- Sotelo, T.; Lema, M.; Soengas, P.; Cartea, M.; Velasco, P. In vitro activity of glucosinolates and their degradation products against brassica pathogenic bacteria and fungi. Appl. Environ. Microbiol. 2015, 81, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Giamoustaris, A.; Mithen, R. Glucosinolates and disease resistance in oilseed rape (Brassica napus ssp. Oleifera). Plant Pathol. 1997, 46, 271–275. [Google Scholar] [CrossRef]
- Doughty, K.J.; Porter, A.J.R.; Morton, A.M.; Kiddle, G.; Bock, C.H.; Wallsgrove, R. Variation in the glucosinolate content of oilseed rape (Brassica napus L.) leaves. Ann. Appl. Biol. 1991, 118, 469–477. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Bennett, R.; Kiddle, G.; Ihmig, S.; Ruppel, M.; Hilgenberg, W. The host range of Plasmodiophora brassicae and its relationship to endogenous glucosinolate content. New Phytol. 1999, 141, 443–458. [Google Scholar] [CrossRef]
- Brader, G.; Mikkelsen, M.D.; Halkier, B.A.; Tapio Palva, E. Altering glucosinolate profiles modulates disease resistance in plants. Plant J. 2006, 46, 758–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Vallet, A.; Ramos, B.; Bednarek, P.; López, G.; Piślewska-Bednarek, M.; Schulze-Lefert, P.; Molina, A. Tryptophan-derived secondary metabolites in Arabidopsis thaliana confer non-host resistance to necrotrophic plectosphaerella cucumerina fungi. Plant J. 2010, 63, 115–127. [Google Scholar] [PubMed]
- Buxdorf, K.; Yaffe, H.; Barda, O.; Levy, M. The effects of glucosinolates and their breakdown products on necrotrophic fungi. PLoS ONE 2013, 8, e70771. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.; Piślewska-Bednarek, M.; Svatoš, A.; Schneider, B.; Doubský, J.; Mansurova, M.; Humphry, M.; Consonni, C.; Panstruga, R.; Sanchez-Vallet, A.; et al. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 2009, 323, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, K.; Fukunaga, S.; Bednarek, P.; Piślewska-Bednarek, M.; Watanabe, S.; Narusaka, Y.; Shirasu, K.; Takano, Y. Glutathione and tryptophan metabolism are required for Arabidopsis immunity during the hypersensitive response to hemibiotrophs. Proc. Natl Acad. Sci. USA 2013, 110, 9589–9594. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.H.K.; Yi, G.-E.; Laila, R.; Yang, K.; Park, J.-I.; Kim, H.R.; Nou, I.-S. Expression profiling of glucosinolate biosynthetic genes in Brassica oleracea L. var. capitata inbred lines reveals their association with glucosinolate content. Molecules 2016, 21, 787. [Google Scholar]
- Yi, G.-E.; Robin, A.H.K.; Yang, K.; Park, J.-I.; Hwang, B.H.; Nou, I.-S. Exogenous methyl jasmonate and salicylic acid induce subspecies-specific patterns of glucosinolate accumulation and gene expression in Brassica oleracea L. Molecules 2016, 21, 1417. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.H.K.; Hossain, M.R.; Park, J.-I.; Kim, H.R.; Nou, I.-S. Glucosinolate profiles in cabbage genotypes influence the preferential feeding of diamondback moth (Plutella xylostella). Front. Plant Sci. 2017, 8, 1244. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Bennett, R.; Kiddle, G.; Wallsgrove, R.; Huang, Y.J.; He, Y.H. Undefined. Breeding, inheritance, and biochemical studies on brassica napus cv. Zhongyou 821: Tolerance to Sclerotinia sclerotiorum (stem rot). In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia, 26 September 1999. [Google Scholar]
- Frerigmann, H.; Piślewska-Bednarek, M.; Sánchez-Vallet, A.; Molina, A.; Glawischnig, E.; Gigolashvili, T.; Bednarek, P. Regulation of pathogen-triggered tryptophan metabolism in arabidopsis thaliana by Myb transcription factors and indole glucosinolate conversion products. Mol. Plant 2016, 9, 682–695. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, Q.; Yang, Q.; Liu, H.; Li, Q.; Yi, X.; Cheng, Y.; Guo, L.; Fan, C.; Zhou, Y. Comparative transcriptomic analysis uncovers the complex genetic network for resistance to sclerotinia sclerotiorum in brassica napus. Sci. Rep. 2016, 6, 19007. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Gigolashvili, T. Myb34, myb51, and myb122 distinctly regulate indolic glucosinolate biosynthesis in Arabidopsis thaliana. Mol. Plant 2014, 7, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, T.; Velasco, P.; Soengas, P.; Rodríguez, V.M.; Cartea, M.E. Modification of leaf glucosinolate contents in Brassica oleracea by divergent selection and effect on expression of genes controlling glucosinolate pathway. Front. Plant Sci. 2016, 7, 1012. [Google Scholar] [CrossRef] [PubMed]
- Wretblad, S.; Dixelius, C. B-genome derived resistance to leptosphaeria maculans in near isogenic Brassica napus lines is independent of glucosinolate profile. Physiol. Plant. 2000, 110, 461–468. [Google Scholar] [CrossRef]
- Mithen, R.F.; Lewis, B.G.; Fenwick, G.R. In vitro activity of glucosinolates and their products against leptosphaeria maculans. Trans. Br. Mycol. Soc. 1986, 87, 433–440. [Google Scholar] [CrossRef]
- Lesur, C.; Monot, C.; Silué, D.; Guidet, F.; Tirilly, Y. Assessment by means of rapd markers of variability in Mycosphaerella brassicicola responsible for the ringspot disease of crucifers. J. Plant Pathol. 1998, 80, 105–113. [Google Scholar]
- Yi, G.-E.; Robin, A.H.K.; Yang, K.; Park, J.-I.; Kang, J.-G.; Yang, T.-J.; Nou, I.-S. Identification and expression analysis of glucosinolate biosynthetic genes and estimation of glucosinolate contents in edible organs of Brassica oleracea subspecies. Molecules 2015, 20, 13089–13111. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Hu, Z.; Zhang, Y.; Li, Y.; Zhou, S.; Chen, G. A putative functional myb transcription factor induced by low temperature regulates anthocyanin biosynthesis in purple kale (Brassica oleracea var. Acephala f. Tricolor). Plant Cell Rep. 2012, 31, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, I.; Iqbal, M.; Hakvoort, H.W.; Bliek, M.; de Boer, B.; Schat, H. Expression levels and promoter activities of candidate salt tolerance genes in halophytic and glycophytic brassicaceae. Environ. Exp. Bot. 2014, 99, 59–66. [Google Scholar] [CrossRef]
- Lee, J.; Yang, K.; Lee, M.; Kim, S.; Kim, J.; Lim, S.; Kang, G.-H.; Min, S.R.; Kim, S.-J.; Park, S.U. Differentiated cuticular wax content and expression patterns of cuticular wax biosynthetic genes in bloomed and bloomless broccoli (Brassica oleracea var. Italica). Process Biochem. 2015, 50, 456–462. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.; Zhao, M.; Ma, J.; Yu, J.; Huang, S. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef] [PubMed]



| Sl No. | Line Name | 24 DAI | 30 DAI |
|---|---|---|---|
| 1 | BN3122 | MS | MS |
| 2 | BN3270 | MS | VS |
| 3 | BN3273 | MS | VS |
| 4 | BN3305 | MS | MS |
| 5 | BN3328 | MS | MS |
| 6 | BN3383 | MS | MS |
| 7 | BN3412 | VS | VS |
| 8 | BN3414 | MS | VS |
| 9 | BN3449 | VS | VS |
| 10 | BN3631 | VS | VS |
| 11 | BN3637 | MS | MS |
| 12 | BN4066 | MS | MS |
| 13 | BN4071 | R | R |
| 14 | BN4072 | R | R |
| 15 | BN4073 | MS | MS |
| 16 | BN4074 | MS | VS |
| 17 | BN4085 | MS | VS |
| 18 | BN4098 | VS | VS |
| 19 | BN4105 | MS | VS |
| 20 | BN4118 | MS | VS |
| 21 | BN4161 | MS | VS |
| 22 | BN4168 | MS | VS |
| 23 | BN4303 | MS | VS |
| 24 | BN4320 | MS | VS |
| 25 | BN106 | MS | MS |
| 26 | BN107 | MS | VS |
| Gene Functions | Upregulated Genes in the R Line BN4072 | Upregulated Genes in the S Line BN3449 | Upregulated Genes in Both the R Line and S Line | |
|---|---|---|---|---|
| Higher in the R Line | Higher in the S Line | |||
| Transcription factor related genes | MYB34-Bol017062 (14–9.25) | MYB28-Bol017019 (1–6.1) MYB122-Bol026204 (1–8.0) | MYB34-Bol036262 (14–4.46) | MYB51-Bol030761 (1–4.9) MYB28-Bol036743 (1–4.0) MYB29-Bol008849 (1–11.0) |
| Aliphatic biosynthesis | AOP2-Bo2g102190 (1–8.3) FMOGS-OX2-Bol010993 (1–2.6) | ST5b-Bol026202 (14–14.07) ST5c-Bol030757 (14–27.31) | GSL-OH -Bol033373 (3–2.43) AOP2-Bo3g052110 (3–14.05) AOP2-Bo9g006240 (3–6.21) | |
| Indolic biosynthesis | ST5a-Bol039395 (1–3.1) CYP81F1-Bol028913 (1–3.9) CYP81F1-Bol028914 (3–30.43) CYP81F2-Bol014239 (1–1.8) IGMT1-Bol007029 (1–2.0) IGMT1-Bol020663 (1–11.4) IGMT2-Bol007030 (1–4.3) | CYP81F4-Bol032712 (1–15.44) CYP81F3-Bol032711 (3–9.26) CYP81F2-Bol026044 (14–16.08) CYP81F4-Bol032714 (1–15.35) | ST5a-Bol026200 (1–4.5) CYP81F3-Bol028919 (1–4.3) CYP81F4-Bol028918 (3–5.6) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuyusuf, M.; Robin, A.H.K.; Kim, H.-T.; Islam, M.R.; Park, J.-I.; Nou, I.-S. Altered Glucosinolate Profiles and Expression of Glucosinolate Biosynthesis Genes in Ringspot-Resistant and Susceptible Cabbage Lines. Int. J. Mol. Sci. 2018, 19, 2833. https://doi.org/10.3390/ijms19092833
Abuyusuf M, Robin AHK, Kim H-T, Islam MR, Park J-I, Nou I-S. Altered Glucosinolate Profiles and Expression of Glucosinolate Biosynthesis Genes in Ringspot-Resistant and Susceptible Cabbage Lines. International Journal of Molecular Sciences. 2018; 19(9):2833. https://doi.org/10.3390/ijms19092833
Chicago/Turabian StyleAbuyusuf, Md., Arif Hasan Khan Robin, Hoy-Taek Kim, Md. Rafiqul Islam, Jong-In Park, and Ill-Sup Nou. 2018. "Altered Glucosinolate Profiles and Expression of Glucosinolate Biosynthesis Genes in Ringspot-Resistant and Susceptible Cabbage Lines" International Journal of Molecular Sciences 19, no. 9: 2833. https://doi.org/10.3390/ijms19092833