Can Plasma Rich in Growth Factors Be Safe for Parental Use? A Safety Study in the Canine Model
Abstract
:1. Introduction
2. Results
2.1. Animals
2.2. IGF-1 Evaluation
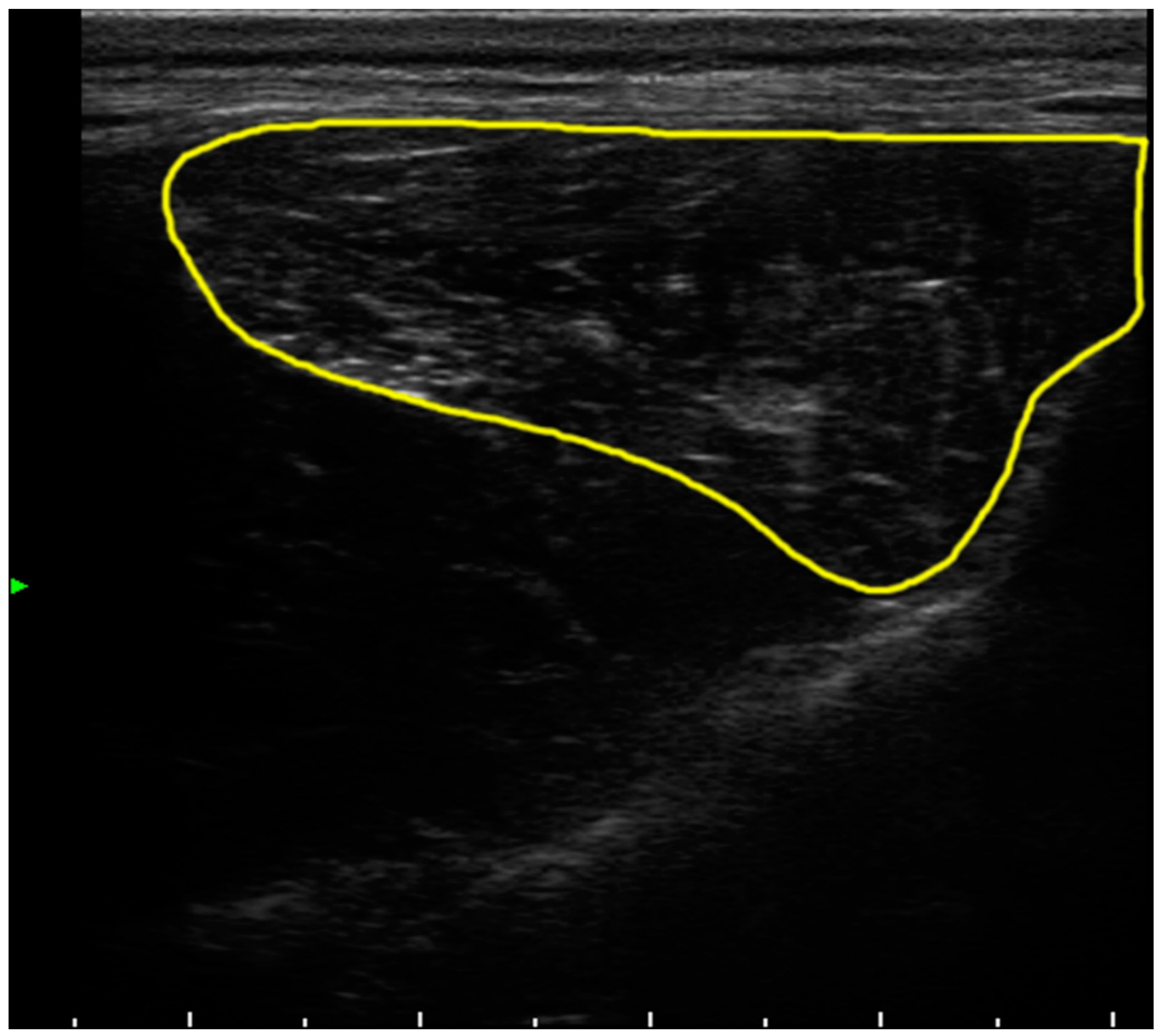
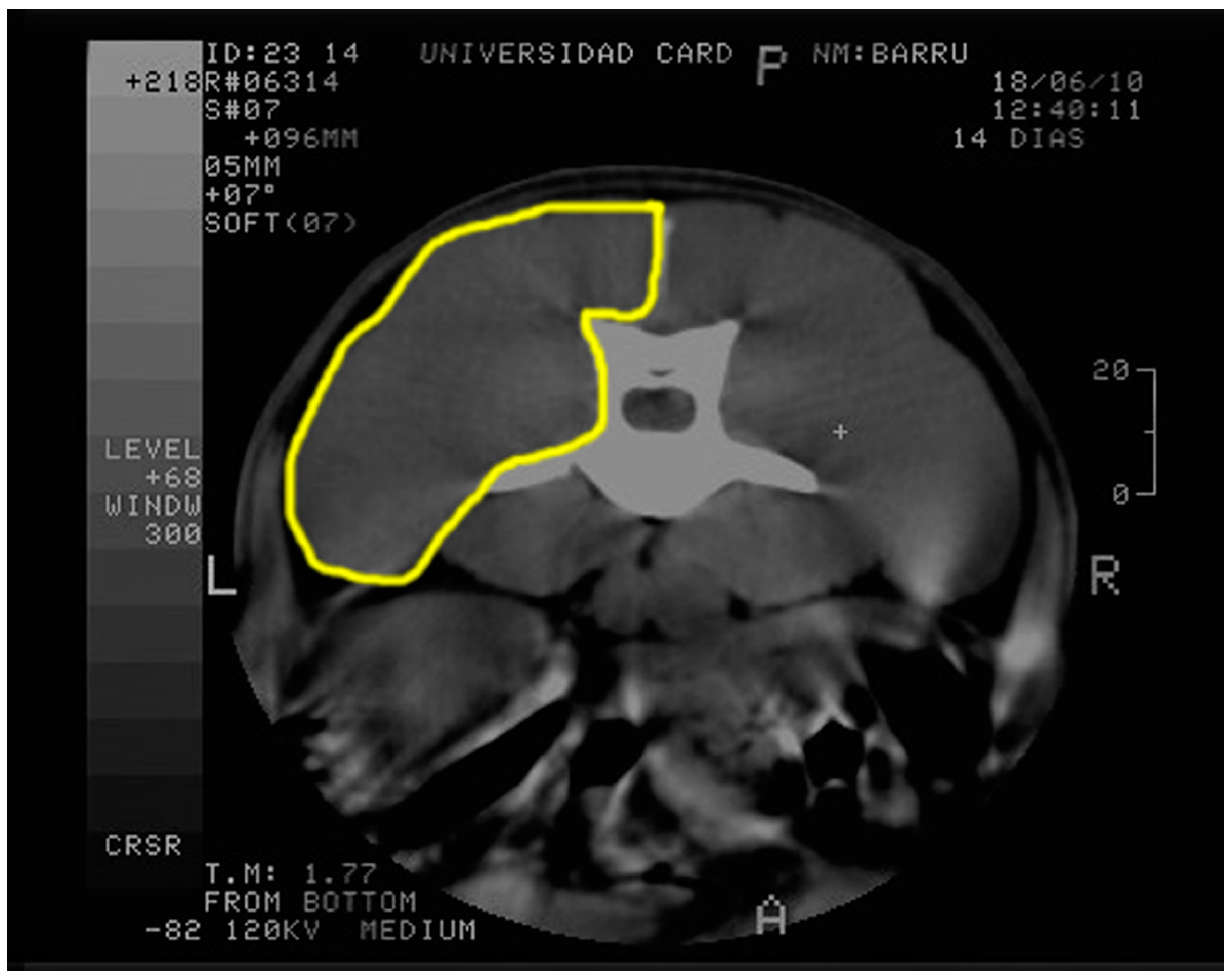
2.3. Computed Tomography and Echography Evaluation
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Plasma Rich in Growth Factors (PRGF) Preparation and Infiltration
- -
- Treatment 1: single dose of 1 mL sterile saline solution activated with 0.05 mL CaCl2 10% (control group) [40].
- -
- Treatment 2: single dose of 1 mL PRGF activated with 0.05 mL CaCl2 10% (PRGF group).
- -
- Treatment 3: single dose of 3 mL PRGF activated with 0.15 mL CaCl2 10% (HPRGF group).
4.3. Determination of IGF-1 Concentrations
4.4. Muscle Tissue Evaluation by Echography
4.5. Muscle Tissue Evaluation by Computed Tomography
4.6. Image Processing
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PRGF | plasma rich in growth factors |
| HPRGF | high-dose plasma rich in growth factors |
| PRP | platelet-rich plasma |
| IGF-1 | insulin-like growth factor-1 |
| CRP | C-reactive protein |
| WADA | World Anti-Doping Agency |
| VEGF | vascular endothelial growth factor |
| FGF | fibroblast growth factor |
| HGF | hepatocyte growth factor |
| PDGF | platelet-derived growth factor |
References
- Giannini, S.; Cielo, A.; Bonanome, L.; Rastelli, C.; Derla, C.; Corpaci, F.; Falisi, G. Comparison between PRP, PRGF and PRF: Lights and shadows in three similar but different protocols. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 927–930. [Google Scholar] [PubMed]
- Marques, L.F.; Stessuk, T.; Camargo, I.C.; Sabeh Junior, N.; dos Santos, L.; Ribeiro-Paes, J.T. Platelet-rich plasma (PRP): Methodological aspects and clinical applications. Platelets 2015, 26, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Andia, I.; Abate, M. Platelet-rich plasma: Underlying biology and clinical correlates. Reg. Med. 2013, 8, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.S.; Kumar, A.S.; Kirit, R.; Konathan, R.; Sivamani, R.K. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J. Cosmet. Dermatol. 2015, 14, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Justicz, N.; Lee, L.N. Platelet-Rich Plasma for the Treatment of Androgenic Alopecia: A Systematic Review. Facial Plast. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Ulusal, B.G. Platelet-rich plasma and hyaluronic acid–an efficient biostimulation method for face rejuvenation. J. Cosmet. Dermatol. 2017, 16, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Mlynarek, R.A.; Kuhn, A.W.; Bedi, A. Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine. Am. J. Orthop. (Belle Mead NJ) 2016, 45, 290–326. [Google Scholar] [PubMed]
- Del Corso, M.; Vervelle, A.; Simonpieri, A.; Jimbo, R.; Inchingolo, F.; Sammartino, G.; Dohan Ehrenfest, D.M. Current knowledge and perspectives for the use of platelet-rich plasma (PRP) and platelet-rich fibrin (PRF) in oral and maxillofacial surgery part 1: Periodontal and dentoalveolar surgery. Curr. Pharm. Biotechnol. 2012, 13, 1207–1230. [Google Scholar] [CrossRef] [PubMed]
- Alio, J.L.; Rodriguez, A.E.; Abdelghany, A.A.; Oliveira, R.F. Autologous Platelet-Rich Plasma Eye Drops for the Treatment of Post-LASIK Chronic Ocular Surface Syndrome. J. Ophthalmol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Borselli, C.; Storrie, H.; Benesch-Lee, F.; Shvartsman, D.; Cezar, C.; Lichtman, J.W.; Vandenburgh, H.H.; Mooney, D.J. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc. Natl. Acad. Sci. USA 2010, 107, 3287–3292. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.W.; Hinton, R.Y.; Curl, L.A.; Muriel, J.M.; Lovering, R.M. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am. J. Sports Med. 2009, 37, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Sanchez, M.; Orive, G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv. Drug Deliv. Rev. 2010, 62, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 2005, 37, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Tentori, L.; Graziani, G. Doping with growth hormone/IGF-1, anabolic steroids or erythropoietin: Is there a cancer risk? Pharmacol. Res. 2007, 55, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, M.A.; Tilling, K.; Holly, J.M.; Metcalfe, C.; Gunnell, D.; Lane, A.; Davis, M.; Donovan, J.; Hamdy, F.; Neal, D.E.; et al. Insulin-like growth factors (IGFs) and IGF-binding proteins in active monitoring of localized prostate cancer: A population-based observational study. Cancer Cause Control 2013, 24, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Grimberg, A. Mechanisms by which IGF-I. may promote cancer. Cancer Biol. Ther. 2003, 2, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Maniscalco, L.; Iussich, S.; Morello, E.; Martano, M.; Gattino, F.; Miretti, S.; Biolatti, B.; Accornero, P.; Martignani, E.; Sanchez-Cespedes, R.; et al. Increased expression of insulin-like growth factor-1 receptor is correlated with worse survival in canine appendicular osteosarcoma. Vet. J. 2015, 205, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Im, K.S.; Kim, N.H.; Kim, H.W.; Shin, J.I.; Yhee, J.Y.; Sur, J.H. Effects of Obesity and Obesity-Related Molecules on Canine Mammary Gland Tumors. Vet. Pathol. 2015, 52, 1045–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schippinger, G.; Oettl, K.; Fankhauser, F.; Spirk, S.; Domej, W.; Hofmann, P. Influence of Intramuscular Application of Autologous Conditioned Plasma on Systemic Circulating IGF-1. J. Sports Sci. Med. 2011, 10, 439–444. [Google Scholar] [PubMed]
- Wang, Y.; Cheng, Z.; Elalieh, H.Z.; Nakamura, E.; Nguyen, M.T.; Mackem, S.; Clemens, T.L.; Bikle, D.D.; Chang, W. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. J. Bone Miner. ResNLM 2011, 26, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Zanou, N.; Gailly, P. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell Mol. Life Sci. 2013, 70, 4117–4130. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Goldspink, G. Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett. 2002, 522, 156–160. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Feng, W.; Wu, Y.; Lv, H.; Jia, Y.; Jiang, D. Mechano-growth factor accelerates the proliferation and osteogenic differentiation of rabbit mesenchymal stem cells through the PI3K/AKT pathway. BMC Biochem. 2015, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Grahnen, A.; Kastrup, K.; Heinrich, U.; Gourmelen, M.; Preece, M.A.; Vaccarello, M.A.; Guevara-Aguirre, J.; Rosenfeld, R.G.; Sietnieks, A. Pharmacokinetics of recombinant human insulin-like growth factor I given subcutaneously to healthy volunteers and to patients with growth hormone receptor deficiency. Acta Paediatr. 1993, 82, 9–13. [Google Scholar] [CrossRef]
- World Anti-Doping Agency (WADA). The 2018 Prohibited List. 2018. Available online: https://www.wada-ama.org/en/content/what-is-prohibited (accessed on 1 January 2018).
- Wasterlain, A.S.; Braun, H.J.; Harris, A.H.; Kim, H.J.; Dragoo, J.L. The systemic effects of platelet-rich plasma injection. Am. J. Sports Med. 2013, 41, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, M.A.; Gunnell, D.; Harris, R.; Vatten, L.J.; Holly, J.M.; Martin, R.M. Circulating insulin-like growth factor peptides and prostate cancer risk: A systematic review and meta-analysis. Int. J. Cancer 2009, 124, 2416–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, K.; Price, P.; Harding, K.G.; Jiang, W.G. The molecular and clinical impact of hepatocyte growth factor, its receptor, activators, and inhibitors in wound healing. Wound Repair Regen. 2006, 14, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Andia, I.; Sanchez, M.; Azofra, J.; del Mar Zalduendo, M.; de la Fuente, M.; Nurden, P.; Nurden, A.T. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF production by human tendon cells in culture. J. Orthop. Res. 2005, 23, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, M.; Anitua, E.; Azofra, J.; Andia, I.; Padilla, S.; Mujika, I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. Am. J. Sports Med. 2007, 35, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Creaney, L.; Hamilton, B. Growth factor delivery methods in the management of sports injuries: The state of play. Br. J. Sports Med. 2008, 42, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.E.; Puskas, B.L.; Mandelbaum, B.R.; Gerhardt, M.B.; Rodeo, S.A. Platelet-rich plasma: From basic science to clinical applications. Am. J. Sports Med. 2009, 37, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Liu, S.; Xu, W.; Wang, X.; Zhao, W.; Wu, J. IGF-I and IGFBP-3 and the risk of lung cancer: A meta-analysis based on nested case-control studies. J. Exp. Clin. Cancer Res. 2009, 28, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marx, R.E. Platelet-rich plasma: Evidence to support its use. J. Oral Maxillofac. Surg. 2004, 62, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Vilar, J.M.; Damiá, E.; Rubio, M.; Santana, A.; Sopena, J.; Ceron, J.; Tvarijonaviciute, A.; Cugat, R.; Carrillo, J.M. Therapeutic doses of plasma rich in growth factors cannot provoke cancer by means of the IGF-1 pathway or inflammation in dogs. J. Appl. Anim. Res. 2016, 45, 490–493. [Google Scholar] [CrossRef]
- Favier, R.P.; Mol, J.A.; Kooistra, H.S.; Rijnberk, A. Large body size in the dog is associated with transient GH excess at a young age. J. Endocrinol. 2001, 170, 479–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard, G.; Nguyen, P.; Gayet, C.; Leriche, I.; Siliart, B.; Paragon, B.M. Rapid weight loss with a high-protein low-energy diet allows the recovery of ideal body composition and insulin sensitivity in obese dogs. J. Nutr. 2004, 134 (Suppl. 8), 2148S–2150S. [Google Scholar] [CrossRef] [PubMed]
- Coronado, R.; Diez, M.P.; Chévez, D. Correlación de edad, niveles séricos de IGF-1 e índice de masa muscular, y su influencia como determinantes de las variables isocinéticas en pacientes con osteoporosis. Cirugia y Cirujanos 2005, 73, 457–463. [Google Scholar]
- Greer, K.A.; Hughes, L.M.; Masternak, M.M. Connecting serum IGF-1, body size, and age in the domestic dog. Age (Dordr.) 2011, 33, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sarmiento, J.A.; Dominguez, J.M.; Granados, M.M.; Morgaz, J.; Navarrete, R.; Carrillo, J.M.; Gomez-Villamandos, R.J.; Munoz-Rascon, P.; Martin de Las Mulas, J.; Millan, Y.; et al. Histological study of the influence of plasma rich in growth factors (PRGF) on the healing of divided Achilles tendons in sheep. J. Bone Jt. Surg. Am. 2013, 95, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Tvarijonaviciute, A.; Tecles, F.; Carrillo, J.M.; Rubio, M.; Ceron, J.J. Serum insulin-like growth factor-1 measurements in dogs: Performance characteristics of an automated assay and study of some sources of variation. Can. J. Vet. Res. 2011, 75, 312–316. [Google Scholar] [PubMed]
- Connell, D.A.; Schneider-Kolsky, M.E.; Hoving, J.L.; Malara, F.; Buchbinder, R.; Koulouris, G.; Burke, F.; Bass, C. Longitudinal study comparing sonographic and MRI assessments of acute and healing hamstring injuries. AJR Am. J. Roentgenol. 2004, 183, 975–984. [Google Scholar] [CrossRef] [PubMed]
| Time | Group | Mean | SD |
|---|---|---|---|
| Baseline | Control | 166.3 | 31.1 |
| PRGF | 132.7 | 49.1 | |
| HPRGF | 95.6 | 31.5 | |
| 14 days | Control | 174.7 | 25.1 |
| PRGF | 114.5 | 40.2 | |
| HPRGF | 117.1 | 30.4 | |
| 28 days | Control | 157.9 | 26.4 |
| PRGF | 133.8 | 46.7 | |
| HPRGF | 127.5 | 34.0 | |
| 42 days | Control | 134.6 | 20.0 |
| PRGF | 143.9 | 48.5 | |
| HPRGF | 126.2 | 33.1 | |
| 56 days | Control | 155.6 | 24.3 |
| PRGF | 139.4 | 49.9 | |
| HPRGF | 117.2 | 38.5 |
| Time | Group | Mean | SD |
|---|---|---|---|
| Baseline | Control | 12.8 | 3.6 |
| PRGF | 12.5 | 3.1 | |
| HPRGF | 12.8 | 3.5 | |
| 14 days | Control | 13.4 | 3.3 |
| PRGF | 12.6 | 3.0 | |
| HPRGF | 13.1 | 3.3 | |
| 28 days | Control | 13.1 | 3.2 |
| PRGF | 12.7 | 3.0 | |
| HPRGF | 13.3 | 3.1 | |
| 42 days | Control | 13.0 | 3.1 |
| PRGF | 12.6 | 3.1 | |
| HPRGF | 13.0 | 3.1 | |
| 56 days | Control | 13.0 | 3.1 |
| PRGF | 12.4 | 3.1 | |
| HPRGF | 12.8 | 3.2 |
| Time | Group | Mean | SD |
|---|---|---|---|
| Baseline | Control | 13.3 | 2.6 |
| PRGF | 12.7 | 2.4 | |
| HPRGF | 12.8 | 2.9 | |
| 14 days | Control | 12.9 | 2.7 |
| PRGF | 12.2 | 2.5 | |
| HPRGF | 13.2 | 2.8 | |
| 28 days | Control | 12.5 | 2.5 |
| PRGF | 12.3 | 3.3 | |
| HPRGF | 12.8 | 2.6 | |
| 42 days | Control | 12.4 | 2.5 |
| PRGF | 12.1 | 2.7 | |
| HPRGF | 13.1 | 3.1 | |
| 56 days | Control | 12.9 | 2.6 |
| PRGF | 11.8 | 2.4 | |
| HPRGF | 12.4 | 3.1 |
| Correlations | |||
|---|---|---|---|
| L5 US | L5 CT scan | ||
| L5 US | Pearson correlation | 1 | 0.928 * |
| Sig. (2-tailed) | 0.000 | ||
| N | 210 | 105 | |
| L5 CT scan | Pearson correlation | 0.928 * | 1 |
| Sig. (2-tailed) | 0.000 | ||
| N | 105 | 105 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiá, E.; Chicharro, D.; Rubio, M.; Carrillo, J.M.; Sopena, J.; Cuervo, B.; López, S.; Vilar, J.M. Can Plasma Rich in Growth Factors Be Safe for Parental Use? A Safety Study in the Canine Model. Int. J. Mol. Sci. 2018, 19, 2701. https://doi.org/10.3390/ijms19092701
Damiá E, Chicharro D, Rubio M, Carrillo JM, Sopena J, Cuervo B, López S, Vilar JM. Can Plasma Rich in Growth Factors Be Safe for Parental Use? A Safety Study in the Canine Model. International Journal of Molecular Sciences. 2018; 19(9):2701. https://doi.org/10.3390/ijms19092701
Chicago/Turabian StyleDamiá, Elena, Deborah Chicharro, Mónica Rubio, José María Carrillo, Joaquín Sopena, Belén Cuervo, Sergio López, and José Manuel Vilar. 2018. "Can Plasma Rich in Growth Factors Be Safe for Parental Use? A Safety Study in the Canine Model" International Journal of Molecular Sciences 19, no. 9: 2701. https://doi.org/10.3390/ijms19092701