Molecular and Physio-Biochemical Characterization of Cotton Species for Assessing Drought Stress Tolerance
Abstract
1. Introduction
2. Results and Discussion
2.1. The Effect of Drought on Growth Parameters and Biomass Accumulation
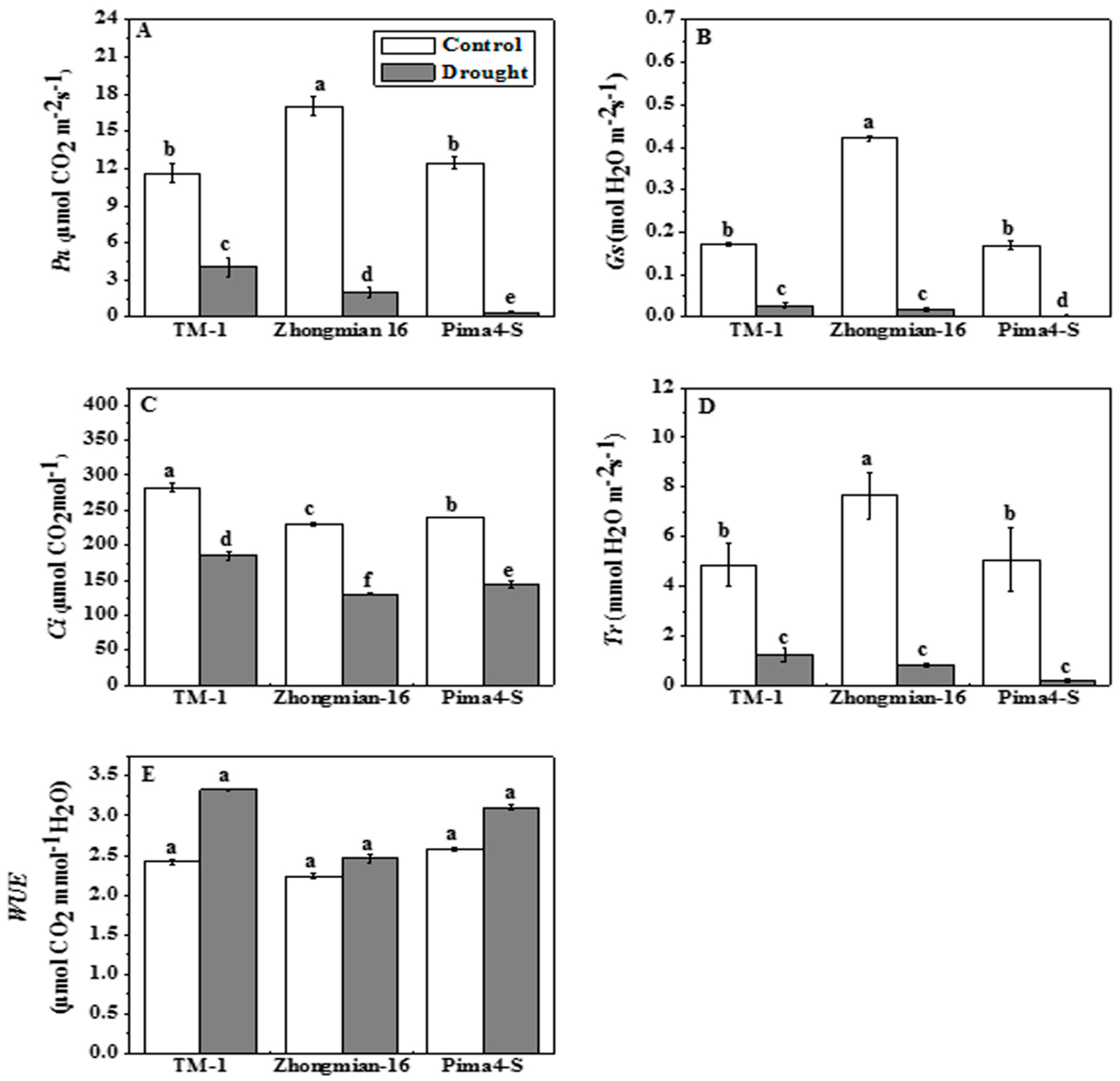
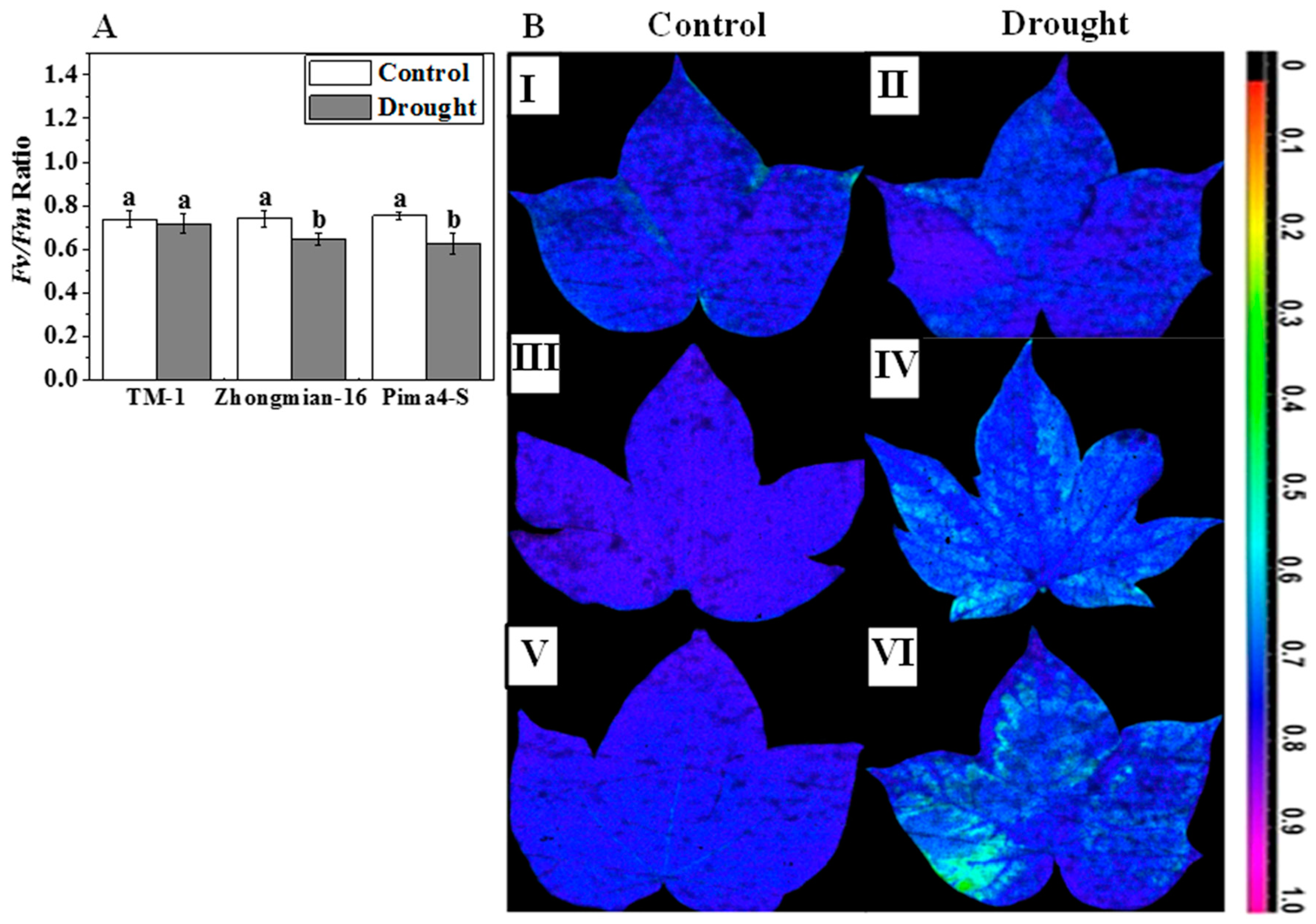
2.2. The Effect of Drought on Photosynthetic Parameters, Water Use Efficiency, Chlorophyll Content, and Fluorescence
2.3. The Effects of Drought on the Mineral Content, and Na+ and K+ Concentrations
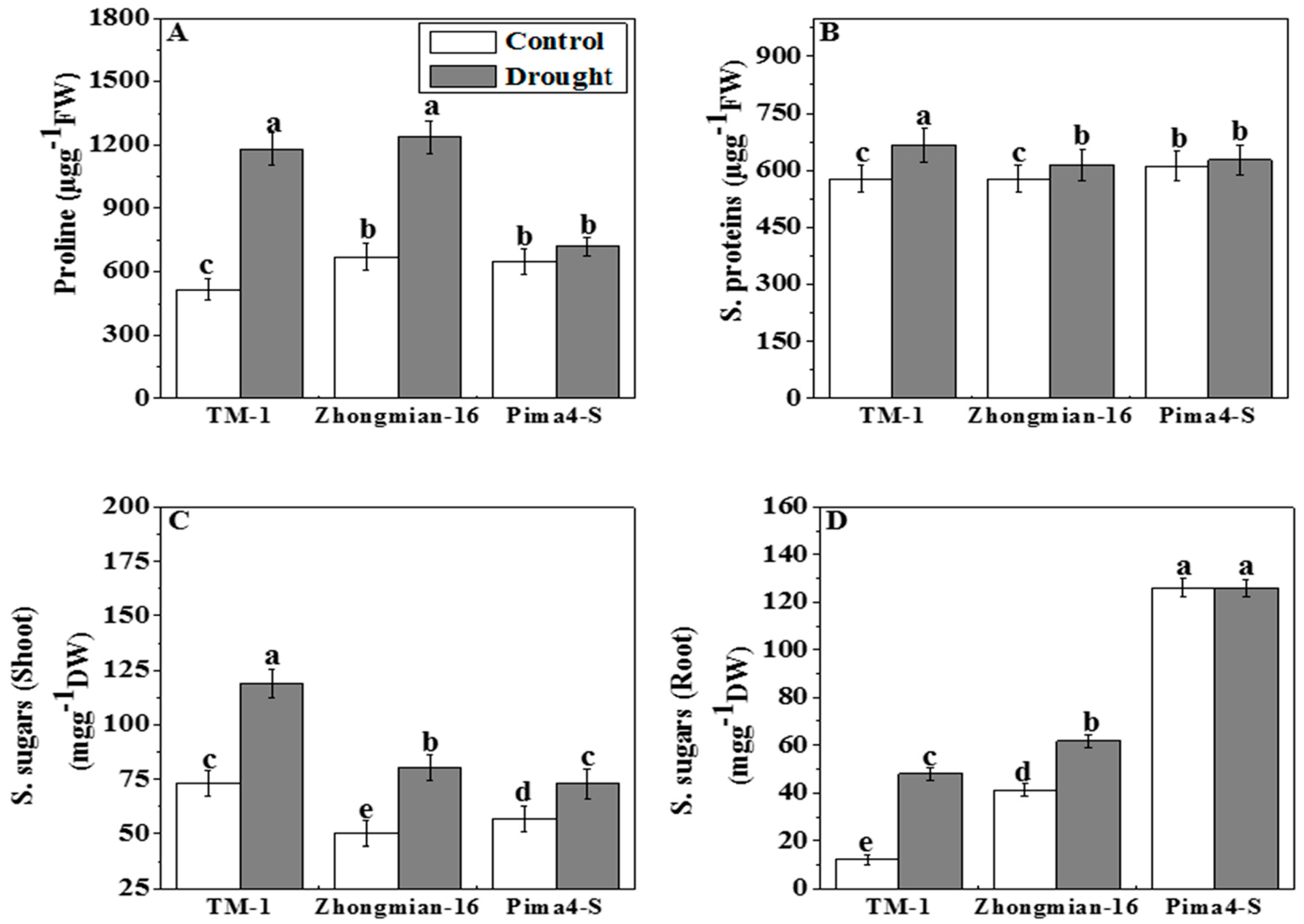
2.4. The Accumulation of Proline, Soluble Proteins, and Soluble Sugars during Drought Stress
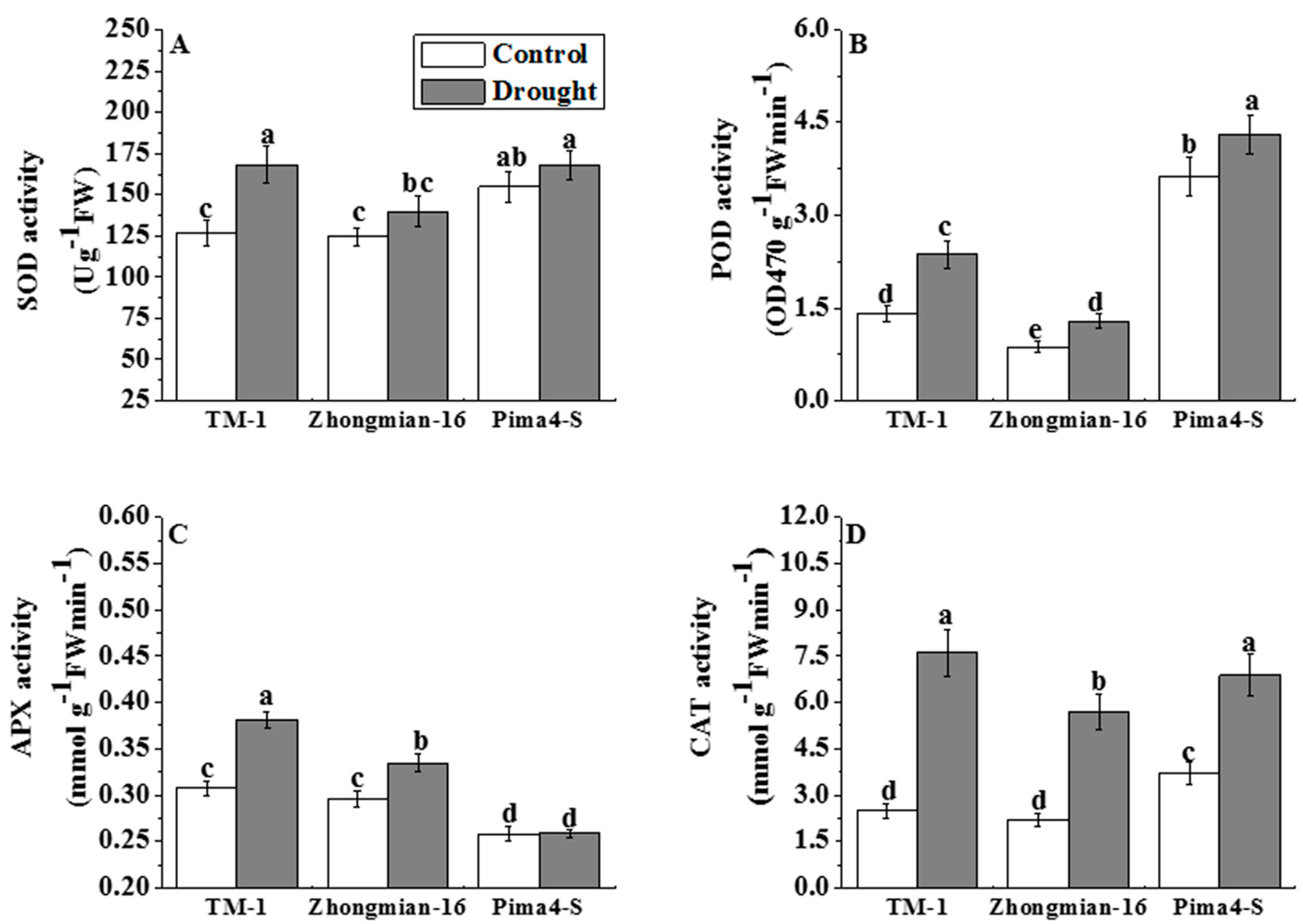
2.5. The Effect of Drought on Antioxidant Enzyme Activity
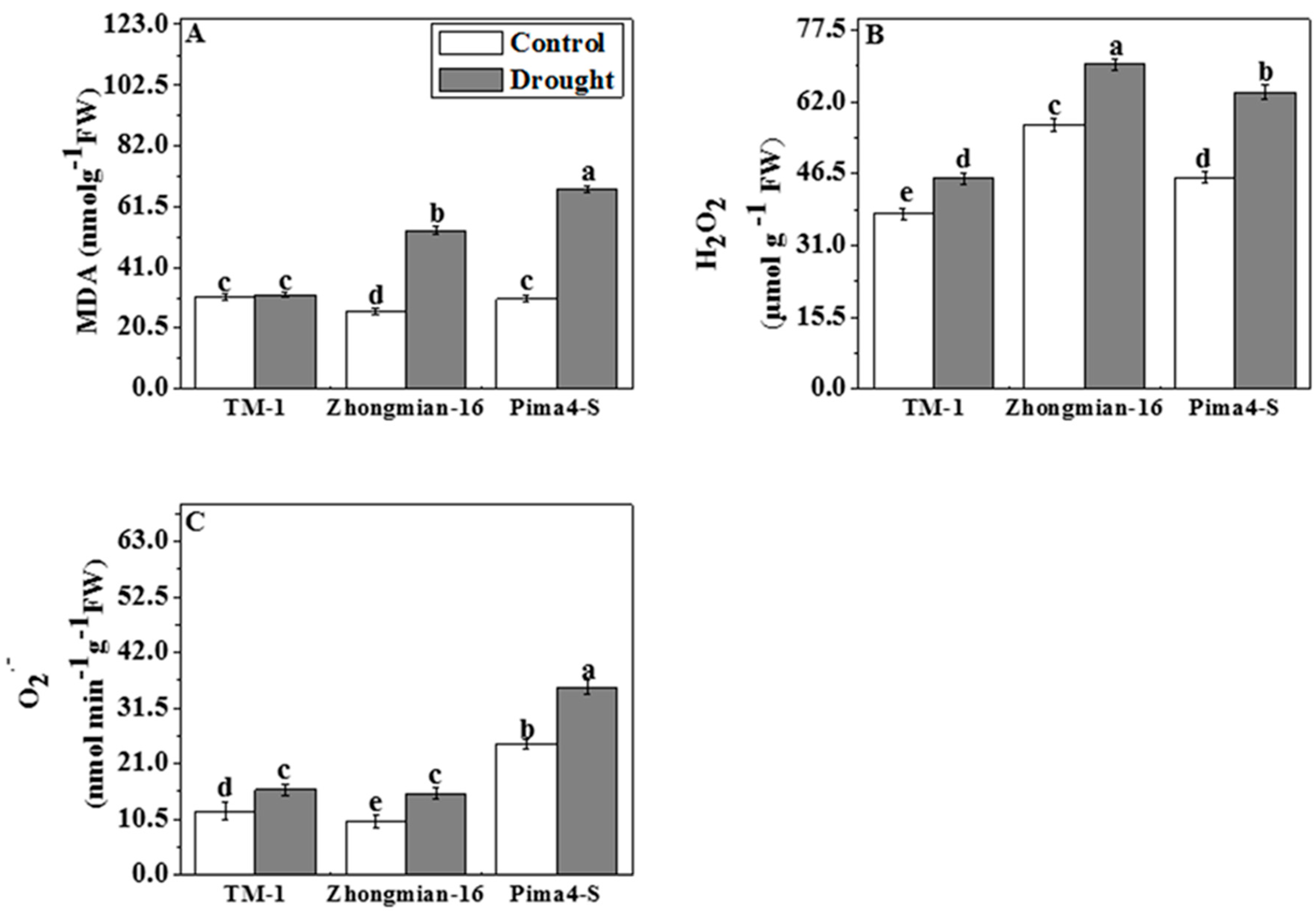
2.6. The Effect of Drought on Lipid Peroxidation, Hydrogen Peroxide (H2O2), and Superoxide Radical (O2•−) Content
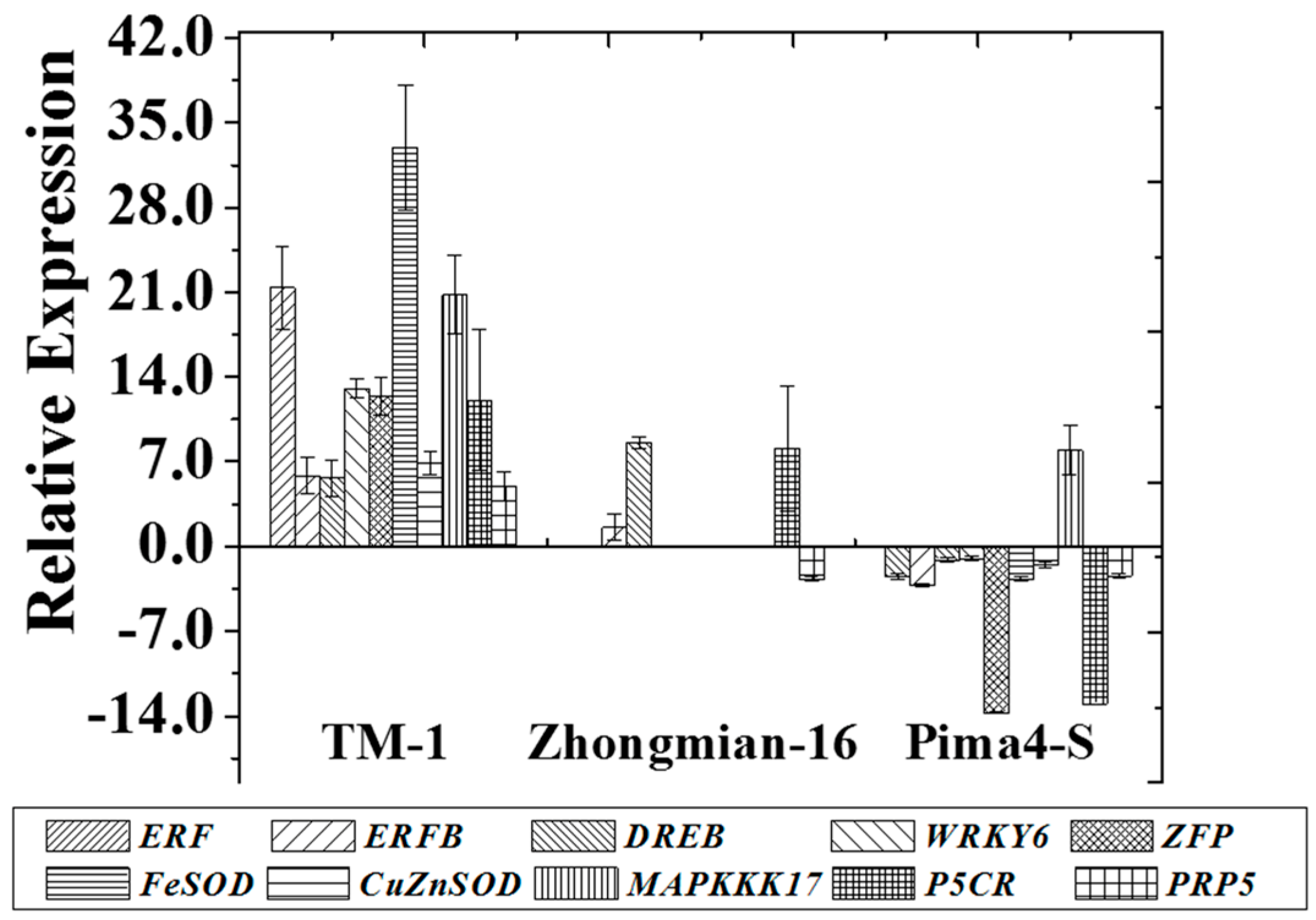
2.7. Changes in the Expression of Potential Genes during Drought
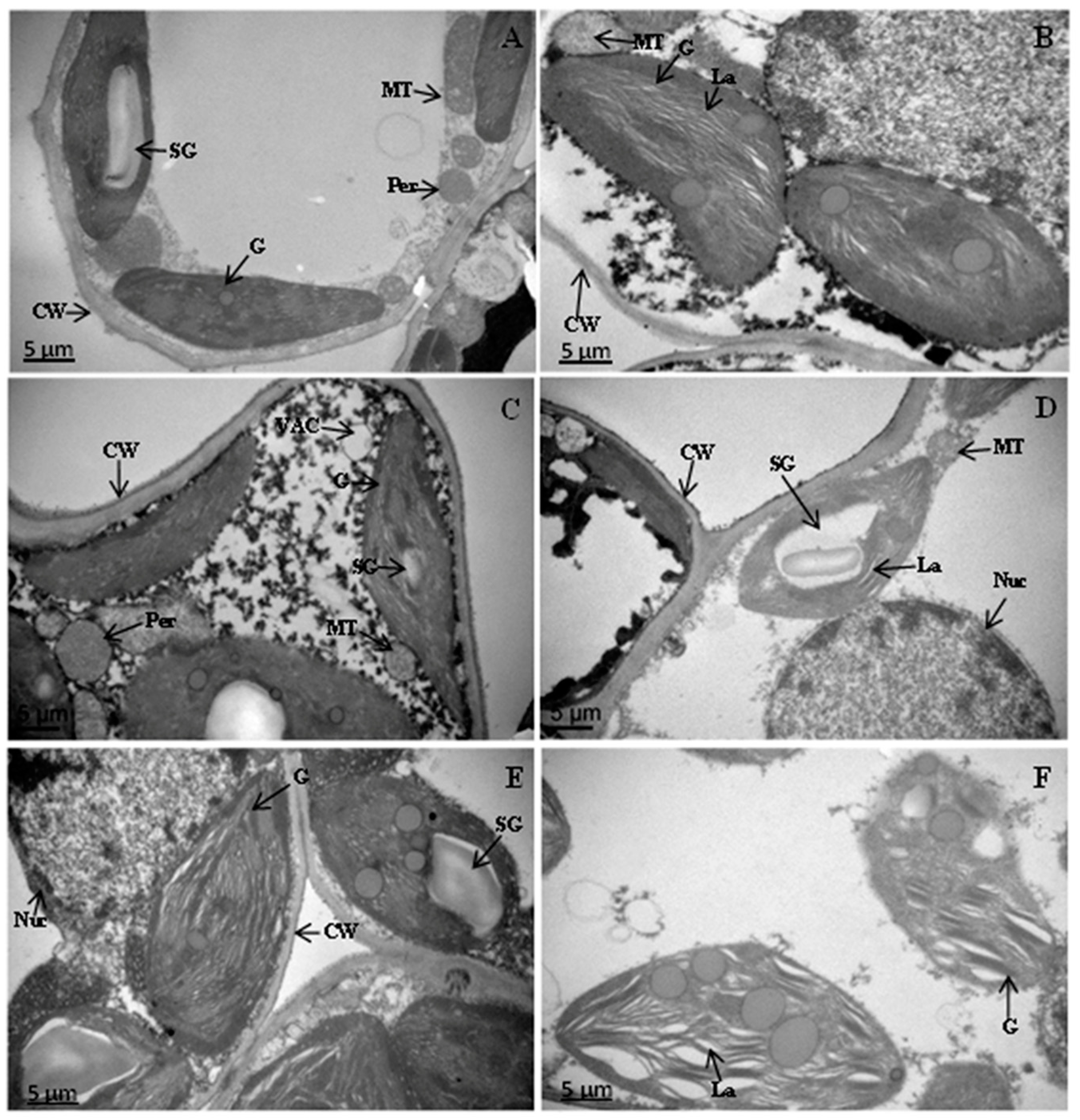
2.8. Modification in the Ultra-Structure of Chloroplasts in Drought Stress
3. Materials and Methods
3.1. Plant Material and Growth Conditions
3.2. Analysis of Na+, K+, and Other Mineral Elements
3.3. Determination of the Relative Water Content of the Leaves
3.4. Measurement of Chlorophyll Content, Chlorophyll Fluorescence, Photosynthesis Parameters, and Water Use Efficiency
3.5. Determination of Proline, Protein, and Soluble Sugar Contents
3.6. Assay of Lipid Peroxidation and Antioxidant Enzyme Activities
3.7. Determination of Hydrogen Peroxide, Superoxide Radical
3.8. Total RNA Extraction, cDNA Synthesis, and RT-PCR
3.9. Analysis of the Ultra-Structural Changes in Chloroplasts
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Sawant, K. Drought stress adaptation: Metabolic adjustment and regulation of gene expression. Plant Breed. 2013, 132, 21–32. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hufstetler, V.E.; Boerma, R.; Carter, T.E.J.; Earl, H.J. Genotypic variation for three physiological traits affecting drought tolerance in soybean. Crop Sci. 2007, 47, 25–35. [Google Scholar] [CrossRef]
- Afshari, R.T.; Angoshtari1, R.; Kalantari, S. Effects of light and different plant growth regulators on induction of callus growth in rapeseed (Brassica napus L.) genotypes. Plant Omics J. 2011, 4, 60–67. [Google Scholar]
- Kumar, N.; Nandwal, A.S.; Devi, S.; Sharma, K.D.; Yadav, A.; Waldia, R.S. Root characteristics, plant water status and CO2 exchange in relation to drought tolerance in chickpea. Ejournal ICRISAT Org. 2010, 8, 5. [Google Scholar]
- Sumartini, S.; Emy, S.; Sri, M.; Dan, A. Screening of Cotton line (Gossypium hirsutum L.) tolerance to drought at germination stage with PEG-6000. J. Littri 2013, 3, 139–146. [Google Scholar]
- Abdalla, M.M.; El-Khoshiban, N.H. The influence of water stress on growth relative water content, photosynthetic pigments, some metabolic and hormonal contents of two Triticum aestivum cultivars. J. Appl. Sci. Res. 2007, 3, 2062–2074. [Google Scholar]
- Akhtar, I.; Nazir, N. Effect of waterlogging and drought stress in plants. Int. J. Water Res. Environ. Sci. 2013, 2, 34–40. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Sapeta, H.; Costa, M.; Lourenc, T.; Marocod, J.; Van der Linde, P.; Oliveiraa, M.M. Drought stress response in Jatropha curcas: Growth and physiology. Environ. Exp. Bot. 2013, 85, 76–84. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.B.; Mao, W.H.; Zhang, G.P.; Zhou, M.X. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Dalton, D.A.; Joyner, S.L.; Becana, M.; Iturbe-Ormaetxe, I.; Chatfield, J.M. Antioxidant defenses in the peripheral cell layers of legume root nodules. Plant Physiol. 1998, 116, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, P.; Jaleel, C.A.; Sankar, B.; Kishore kumar, A.; Somasundaram, R.; Lakshmanan, G.A.; Panneerselvam, R. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf. B Biointerfaces 2007, 59, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Alharby, H.F.; Razafindrabe, B.H.; Fujita, M. Hydrogen peroxide pretreatment mitigates cadmium-induced oxidative stress in Brassica napus L.: An intrinsic study on antioxidant defense and glyoxalase systems. Front. Plant Sci. 2017, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, S.; Zhou, X.; Shen, X.; Deng, L.; Zhu, H.; Shao, J.; Shi, Y.; Dai, S.; Fritz, E.; et al. Ionic homeostasis and reactive oxygen species control in leaves and xylem sap of two poplars subjected to NaCl stress. Tree Physiol. 2008, 28, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.M.; Lin, H.; Maple, J.; Björkblom, B.; Alves, G.; Larsen, J.P.; Møller, S.G. The Arabidopsis DJ-1a protein confers stress protection through cytosolic SOD activation. J. Cell Sci. 2010, 123, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ocana, A.; Chaki, M.; Luque, F.; Gomez-Rodriguez, M.V.; Carreras, A.; Valderrama, R.; Begara-Morales, J.C.; Hernández, L.E.; Corpas, F.J. Functional analysis of superoxide dismutases (SODs) in sunflower under biotic and abiotic stress conditions. Identification of two new genes of mitochondrial Mn-SOD. J. Plant Physiol. 2011, 168, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Descourvieres, P.; Kunert, K.J. Protection against oxygen radicals–an important defense mechanism studied in transgenic plants. Plant Cell Environ. 1994, 17, 507–523. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trend Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Iannone, M.F.; Rosales, E.P.; Groppa, M.D.; Benavides, M.P. Reactive oxygen species formation and cell death in catalase-deficient tobacco leaf discs exposed to paraquat. Biol. Trace Elem. Res. 2012, 146, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Tadayyon, A.; Nikneshan, P.; Pessarakli, M. Effects of drought stress on concentration of macro and micronutrients in Castor (Ricinus Communis L.) Plant. J. Plant Nutr. 2017, 41, 304–310. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Dai, H.; Zheng, W.; Cao, F.; Zhang, G.; Sun, D.; Wu, F. Genotypic differences in physiological characteristics in the tolerance to drought and salinity combined stress between Tibetan wild and cultivated barley. Plant Physiol. Biochem. 2013, 63, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Türkan, I.; Bor, M.; Ozdemer, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. augustifolius Gray and drought sensitive P. vulgaris subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Ajum, N.A. Approaches in modeling proline metabolism in plants for salt and drought tolerance: Phytohoromes, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Turchetto-Zolet, A.C.; Margis-Pinheiro, M.; Margis, R. The evolution of pyrroline-5 carboxylate synthase in plants: A key enzyme in proline synthesis. Mol. Gen. Genom. 2009, 281, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Alyemeni, M.N.; Hasan, S.A. Foliar spray of brassinosteroid enhances yield and quality of Solanum lycopersicum under cadmium stress. Saudi J. Biol. Sci. 2012, 19, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Szabados, L.; Savoure, A. Proline: Multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Kishor, K.P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue. Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Man, D.; Bao, Y.X.; Han, L.B. Drought tolerance associated with proline and hormone metabolism in two tall fescue cultivars. Hort. Sci. 2011, 1, 1027–1032. [Google Scholar]
- Sultan, M.A.R.F.; Hui, L.; Yang, L.J.; Xian, Z.H. Assessment of drought tolerance of some Triticum L. species through physiological indices. Czech J. Genet. Plant Breed. 2012, 48, 178–184. [Google Scholar] [CrossRef]
- Hanson, A.D.; Nelson, C.E.; Everson, E.H. Evaluation of free proline accumulation as an index of drought resistance using two contrasting barley cultivars. Crop Sci. 1977, 17, 720–726. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, T. Expansion and stress responses of the AP2/EREBP superfamily in cotton. BMC Genom. 2017, 18, 118. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, S.J.; Zarka, D.G.; Stockinger, E.J. Low temperature regulation of the Arabidopsis CBF family of AP2 transcription activators as an early step in cold induced cor gene expression. Plant J. 1998, 16, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, G.; Sánchez-Bermejo, E.; De Lorenzo, L.; Crevillén, P.; Fraile-Escanciano, A.; Mohan, T.C.; Mouriz, A.; Catarecha, P.; Sobrino-Plata, J.; Olsson, S.; et al. WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell 2013, 25, 2944–2957. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.H.; Yu, Y.P.; Wang, D.; Wu, C.A.; Yang, G.D.; Huang, J.G.; Zheng, C.C. GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol. 2009, 183, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Negi, N.P.; Shrivastava, D.C.; Sharma, V.; Sarin, N.B. Overexpression of CuZnSOD from Arachis hypogaea alleviates salinity and drought stress in tobacco. Plant Cell Rep. 2015, 34, 1109–1126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zou, D.; Li, Y.; Sun, X.; Wang, N.N.; Gong, S.Y.; Zheng, Y.; Li, X.B. GhMPK17, a cotton mitogen-activated protein kinase, is involved in plant response to high salinity and osmotic stresses and ABA signaling. PLoS ONE 2014, 9, e95642. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Villarroel, R.; Van Montagu, M. Osmoregulation of a pyrroline-5-late reductase gene in Arabidopsis thaliana. Plant Physiol. 1993, 103, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Sahito, A.; Baloch, Z.A.; Mahar, A.; Otho, S.A.; Kalhoro, S.A.; Ali, A.; Kalhoro, F.A.; Soomro, R.N.; Ali, F. Effect of water stress on the growth and yield of cotton crop (Gossypium hirsutum L.). Am. J. Plant Sci. 2015, 6, 1027–1039. [Google Scholar] [CrossRef]
- Basal, H.; Bebeli, P.; Smith, C.W.; Thaxton, P. Root growth parameters of converted races of upland cotton (Gossypium hirsutum L.) and two BC2F2 populations. Crop Sci. 2003, 43, 1983–1988. [Google Scholar] [CrossRef]
- Ullah, I.; Ashraf, M.; Zafar, Y. Genotypic variation for drought tolerance in cotton (Gossypium hirsutum L.): Leaf gas exchange and productivity. Flora-Morphol. Distrib. Funct. Ecol. Plants 2008, 203, 105–115. [Google Scholar] [CrossRef]
- Hafeez, M.N.; Sadique, S.; Hassan, S.; Sarwar, M.B.; Rashid, B.; Ali, Q.; Husnain, T. Physiological, morphological, biochemical and molecular basis of drought tolerance in cotton. Int. J. Biol. Pharm. Allied Sci. 2015, 4, 1091–1112. [Google Scholar]
- Rodriguez-Uribe, L.; Abdelraheem, A.; Tiwari, R.; Sengupta-Gopalan, C.; Hughs, S.E.; Zhang, J. Identification of drought-responsive genes in a drought-tolerant cotton (Gossypium hirsutum L.) cultivar under reduced irrigation field conditions and development of candidate gene markers for drought tolerance. Mol. Breed. 2014, 34, 1777–1796. [Google Scholar] [CrossRef]
- Singh, R.; Pandey, N.; Naskar, J.; Shirke, P.A. Physiological performance and differential expression profiling of genes associated with drought tolerance in contrasting varieties of two Gossypium species. Protoplasma 2015, 252, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Sun, H.; Yang, X.; Zhang, X. Drought coping strategies in cotton: Increased crop per drop. Plant Biotechnol. J. 2017, 15, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Trovato, M.; Mattioli, R.; Costantino, P. Multiple roles of proline in plant stress tolerance and development. Rendiconti Lincei 2008, 19, 325–346. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. J. Ann. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Zhang, Q.; Alfarra, M.R.; Worsnop, D.; Allan, J.D.; Coe, H. Deconvolution and quantification of hydrocarbon-like and oxygenated organic aerosols based on aerosol mass spectrometry. Environ. Sci. Technol. 2005, 39, 4938–4952. [Google Scholar] [CrossRef] [PubMed]
- Percival, G.C.; Sheriffs, C.N. Identification of drought tolerance woody perennials using chlorophyll fluorescence. J. Arboric. 2002, 28, 215–223. [Google Scholar]
- Valladares, F.; Dobarro, I.; Sanchez-Gomez, D.; Pearcy, R.W. Photoinhibition and drought in Mediterranean woody saplings: Scaling effects and interactions in sun and shade phenotypes. J. Exp. Bot. 2005, 56, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A.; Farooq, M.; Qadir, M.; Schubert, S. Potassium substitution by sodium in plants. Crit. Rev. Plant Sci. 2011, 30, 401–413. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef] [PubMed]
- Sucre, B.; Suarez, N. Effect of salinity and PEG-induced water stress on water status, gas exchange, solute accumulation, and leaf growth in Ipomoea pescaprae. Environ. Exp. Bot. 2011, 70, 192–203. [Google Scholar] [CrossRef]
- Urbina, I.; Sardans, J.; Beierkuhnlein, C.; Jentsch, A.; Backhaus, S.; Grant, K.; Kreyling, J.; Penuelas, J. Shifts in the elemental composition of plants during a very severe drought. Environ. Exp. Bot. 2015, 111, 63–73. [Google Scholar] [CrossRef]
- Nikam, V.; Chavan, P. Influence of water deficit and water logging on the mineral status of a medicinal plant Chlorophytum borivilianum. Acta Biol. Hung. 2009, 51, 105–113. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Sanchez, F.J.; Manzanares, M.; de Andres, E.F.; Tenorio, J.L.; Ayerbe, L. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res. 1998, 59, 225–235. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Cao, K.F. Gas exchange, chlorophyll fluorescence, and osmotic adjustment in two mango cultivars under drought stress. Acta Physiol. Plant. 2008, 30, 769–777. [Google Scholar] [CrossRef]
- Singh, N.K.; Bracker, C.A.; Hasegawa, P.M.; Handa, A.K.; Buckel, S.; Hermodson, M.A. Characterization of osmotin: A thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987, 85, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 2003, 119, 355–364. [Google Scholar] [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Ashraf, M. Induction of drought tolerance in Maize (Zea mays L.) due to exogenous application of Trehalose: Growth, photosynthesis, water relations and oxidative defence mechanism. J. Agric. Crop Sci. 2011, 197, 258–271. [Google Scholar] [CrossRef]
- Petridis, A.; Therios, I.; Samouris, G.; koundouras, S.; Giannakoula, A. Effect of water deficit on leaf phenolic composition, gas exchange, oxidative damage and antioxidant activity of four Greek olive (Olea europaea L.) cultivars. Plant Physiol. Biochem. 2012, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hameed, A.; Bibi, N.; Akhter, J.; Iqbal, N. Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol. Biochem. 2011, 49, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Cheng, Y.; Kanwar, M.K.; Chu, X.Y.; Ahammed, G.J.; Qi, Z.Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed]
- Shin, R.; Park, J.M.; An, J.M.; Peak, K.H. Ectopic expression of Tsi1 in transgenic hot pepper plants enhances host resistance to viral, bacterial, and oomycete pathogens. Mol. Plant-Microbe Interact. 2002, 15, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Sakuma, Y.; Tran, L.S.P.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress-responsive gene expression. Plant Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef] [PubMed]
- Nishiuchi, T.; Suzuki, K.; Kitajima, S.; Sato, H.; Shinshi, H. Wounding activates immediate early transcription of genes for ERFs in tobacco plants. Plant Mol. Biol. 2002, 49, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Katsura, K.; Maruyama, K.; Taji, T.; Kobayashi, M.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of rice DREB1/CBF-type transcription factors involved in cold-responsive gene expression in transgenic rice. Plant Cell Physiol. 2006, 47, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, C.Z.; Ye, Q.; Wu, W.H.; Chen, Y.F. Arabidopsis WRKY6 transcription factor acts as a positive regulator of abscisic acid signaling during seed germination and early seedling development. PLoS Genet. 2016, 12, e1005833. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Sun, H.; Hakim; Yang, X.; Zhang, X. A novel cotton WRKY gene, GhWRKY6-like, improves salt tolerance by activating the ABA signaling pathway and scavenging of reactive oxygen species. Physiol. Plant. 2018, 162, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Romman, S.A.; Shatnawi, M. Isolation and expression analysis of chloroplastic copper/zinc superoxide dismutase gene in barley. S. Afr. J. Bot. 2011, 77, 328–334. [Google Scholar] [CrossRef]
- Morita, S.; Tsukamoto, S.; Sakamoto, A.; Makino, H.; Nakauji, E.; Kaminaka, H.; Masumura, T.; Ogihara, Y.; Satoh, S.; Tanaka, K. Differences in intron-mediated enhancement of gene expression by the first intron of cytosolic superoxide dismutase gene from rice in monocot and dicot plants. Plant Biotechnol. 2012, 29, 115–119. [Google Scholar] [CrossRef]
- Wu, L.C. ZAS: C2H2 zinc finger proteins involved in growth and development. Gene Exp. 2002, 10, 137–152. [Google Scholar] [CrossRef]
- Ciftci-Yilmaz, S.; Mittler, R. The zinc finger network of plants. Cell Mol. Life Sci. 2008, 65, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Bai, X.; Zhu, D.; Li, Y.; Ji, W.; Cai, H.; Wu, J.; Liu, B.; Zhu, Y. GsZFP1, a new Cys2/His2-type zinc-finger protein, is a positive regulator of plant tolerance to cold and drought stress. Planta 2012, 235, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Schwacke, R.; Grallath, S.; Breitkreuz, K.E.; Stransky, H.; Frommer, W.B.; Rentsch, D. LeProT1, a transporter for proline, glycine betaine, and γ-amino butyric acid in tomato pollen. Plant Cell 1999, 11, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Simon-Sarkadi, L.; Kocsy, G.; Varhegyi, A.; Galiba, G.; De Ronde, J.A. Stress-induced changes in the free amino acid composition in transgenic soybean plants having increased proline content. Biol. Plant 2006, 50, 793–796. [Google Scholar] [CrossRef]
- Yamchi, A.; Jazii, F.R.; Mousav, A.; Karkhane, A.; Renu, A. Proline accumulation in transgenic tobacco as a result of expression of Arabidopsis Δ1-pyrroline-5-carboxylate synthetase (P5CS) during osmotic stress. J. Plant Biochem. Biotechnol. 2007, 16, 9–15. [Google Scholar] [CrossRef]
- Kiyosue, T.; Yoshiba, Y.; Yamagichi-Shinozaki, K.; Shinozaki, K. A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is up-regulated by proline but down-regulated by dehydration in Arabidopsis. Plant Cell 1996, 8, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Ali, S.; Farooq, M.A.; Wang, J.; Gill, R.A.; Zhu, J.; Ali, B.; Zhou, W. Butachlor-induced alterations in ultrastructure, antioxidant, and stress-responsive gene regulations in rice cultivars. Clean Soil Air Water 2016, 45, 1500851. [Google Scholar] [CrossRef]
- Shao, R.X.; Xin, L.F.; Zheng, H.F.; Li, L.L.; Ran, W.L.; Mao, J.; Yang, Q.H. Changes in chloroplast ultrastructure in leaves of drought-stressed maize inbred lines. Photosynthetica 2016, 54, 74–80. [Google Scholar] [CrossRef]
- Ferreira, A.L.; Lima-Costa, M.E. Growth and ultrastructural characteristics of Citrus cells grown in medium containing NaCl. Biol. Plant 2008, 52, 129–132. [Google Scholar] [CrossRef]
- Wu, F.B.; Wu, H.X.; Zhang, G.P.; Bachir, D.M.L. Differences in growth and yield in response to cadmium toxicity in cotton genotypes. J. Plant Nutr. Soil Sci. 2004, 167, 85–90. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, G.P.; Yao, H.; Dominy, P.; Wu, W.; Wang, R. Possibility of predicting heavy-metal contents in rice grains based on DTPA-extracted levels in soil. Commun. Soil Sci. Plant Anal. 2004, 35, 2731–2745. [Google Scholar] [CrossRef]
- Jones, M.M.; Turner, T.C. Osmotic adjustment in leaves of sorghum on response to water deficits. Plant Physiol. 1978, 25, 591–597. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.; Ries, S. Superoxide dismutase I. occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.J.; Leul, M. Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul. 1999, 27, 99–104. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen-peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Willekens, H.; Chamnongpol, S.; Davey, M.; Schraudner, M.; Langebartels, C.; VanMontagu, M. Catalase is a sink for H2O2 and is indispensable for stress defense in C3 plants. EMBO J. 1997, 16, 4806–4816. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Scmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔCT) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Genotype | Treatment | Plant Height (cm) | Root Length (cm) | Total Fresh Weight (g) | Total Dry Weight (g) | Root Shoot Ratio | Relative Water Content |
|---|---|---|---|---|---|---|---|
| TM-1 | Control | 37.25 ± 1.50 b | 19.25 ± 1.50 d | 21.73 ± 0.21 b | 5.38 ± 0.07 b | 0.18 ± 0.02 a | 83.84 ± 1.61 b |
| Drought | 33.38 ± 1.11 d | 22.50 ± 1.08 b | 18.07 ± 0.81 c | 4.89 ± 0.02 c | 0.15 ± 0.01 b | 67.23 ± 2.47 d | |
| Zhongmian-16 | Control | 41.00 ± 1.15 a | 22.25 ± 1.26 b,c | 18.69 ± 0.69 c | 5.31 ± 0.16 b | 0.14 ± 0.01 b | 77.81 ± 2.29 c |
| Drought | 34.50 ± 1.00 c,d | 25.63 ± 0.75 a | 14.77 ± 0.65 e | 4.57 ± 0.14 d | 0.12 ± 0.01 c | 39.38 ± 2.29 e | |
| Pima4-S | Control | 36.25 ± 1.26 b,c | 19.75 ± 1.71 d | 26.34 ± 0.10 a | 6.14 ± 0.12 a | 0.18 ± 0.01 a | 89.81 ± 4.75 a |
| Drought | 29.25 ± 1.50 e | 20.38 ± 1.11 c,d | 16.15 ± 0.75 d | 4.72 ± 0.12 d | 0.13 ± 0.01 b,c | 36.92 ± 0.69 e | |
| LSD0.05 between species | 1.86 | 2.10 | 1.15 | 0.17 | 0.02 | 5.22 | |
| Genotype | Treatments | Chlorophyll a | Chlorophyll b | Total Chlorophyll | Chlorophyll Ratio |
|---|---|---|---|---|---|
| TM-1 | Control | 1.85 ± 0.05 c | 0.72 ± 0.09 b | 2.56 ± 0.14 c | 2.61 ± 0.24 a |
| Drought | 1.53 ± 0.02 d | 0.63 ± 0.02 c | 2.16 ± 0.02 d | 2.42 ± 0.09 a,b | |
| Zhongmian-16 | Control | 2.29 ± 0.02 a | 0.96 ± 0.09 a | 3.24 ± 0.09 a | 2.39 ± 0.22 a,b,c |
| Drought | 1.57 ± 0.02 d | 0.74 ± 0.02 b | 2.31 ± 0.03 d | 2.14 ± 0.03 c | |
| Pima4-S | Control | 1.97 ± 0.07 b | 0.87 ± 0.07 a | 2.84 ± 0.14 b | 2.27 ± 0.14 b,c |
| Drought | 1.17 ± 0.01 e | 0.65 ± 0.01 b,c | 1.82 ± 0.02 e | 1.81 ± 0.03 d | |
| LSD0.05 between species | 0.07 | 0.11 | 0.17 | 0.27 | |
| Gene Name | Accession No | Forward Primmer (5′ to 3′) | Reverse Primmer (5′ to 3′) |
|---|---|---|---|
| Ethylene responsive transcription factor (ERF) | KC222015.1 | TCGTGACCCAACGAGGAATG | TGACCGTTGCTTTTCTTGCG |
| Ethylene responsive element binding factor (ERFB) | AY181251.1 | CGACAACACCTTCTCGGTGA | TTGTTATCGGCGCTGGTTCT |
| Dehydration responsive element binding factor (DREB) | AF509502.1 | GAGGAAGTGGGGAAAGTGGG | CGTATGGAAGCGGCTGAGAT |
| WRKY6 | KF669821.1 | CCCCTTTCCCACCATAACCC | AATGGGAATGAAGCAGCGGA |
| Zinc-finger protein (ZFP) | AY887895.1 | TCCGTTACATCGTCGTCGTC | AATACGATCACCGCCGTACC |
| Iron Superoxide dismutase (FeSOD) | DQ088821.1 | GGCTTGTCAGCTCCTGCTAA | ACGATGATGCACACCCCAAT |
| CuZn Superoxide dismutase1(CuZnSOD1) | AF191342.1 | ATGGCTGCATGTCAACTGGA | TGCTCAGTTCATGACCACCC |
| Mitogen activated protein kinase kinase kinase17 (MAPKKK17) | XM_016855464 | GTCTCCGGTTTCTTCACGGT | ACCTCCGGTGCCATGTAAAG |
| pyrroline-5-carboxylate reductase | XM_016889238 | GCAACAGAACAGGATGGGGA | CACTAAGGCCAGTGACAGCA |
| Proline rich protein (PRP5) | EF095706.1 | TACGACGAGAAGGCCAACAC | TTGGGCTTTGGAGGTGGTTT |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, M.M.-U.; Ma, F.; Prodhan, Z.H.; Li, F.; Shen, H.; Chen, Y.; Wang, X. Molecular and Physio-Biochemical Characterization of Cotton Species for Assessing Drought Stress Tolerance. Int. J. Mol. Sci. 2018, 19, 2636. https://doi.org/10.3390/ijms19092636
Hasan MM-U, Ma F, Prodhan ZH, Li F, Shen H, Chen Y, Wang X. Molecular and Physio-Biochemical Characterization of Cotton Species for Assessing Drought Stress Tolerance. International Journal of Molecular Sciences. 2018; 19(9):2636. https://doi.org/10.3390/ijms19092636
Chicago/Turabian StyleHasan, Md Mosfeq-Ul, Fanglu Ma, Zakaria Hossain Prodhan, Feng Li, Hao Shen, Yadong Chen, and Xuede Wang. 2018. "Molecular and Physio-Biochemical Characterization of Cotton Species for Assessing Drought Stress Tolerance" International Journal of Molecular Sciences 19, no. 9: 2636. https://doi.org/10.3390/ijms19092636
APA StyleHasan, M. M.-U., Ma, F., Prodhan, Z. H., Li, F., Shen, H., Chen, Y., & Wang, X. (2018). Molecular and Physio-Biochemical Characterization of Cotton Species for Assessing Drought Stress Tolerance. International Journal of Molecular Sciences, 19(9), 2636. https://doi.org/10.3390/ijms19092636





