Development of a Graphene Oxide-Incorporated Polydimethylsiloxane Membrane with Hexagonal Micropillars
Abstract
1. Introduction
2. Results
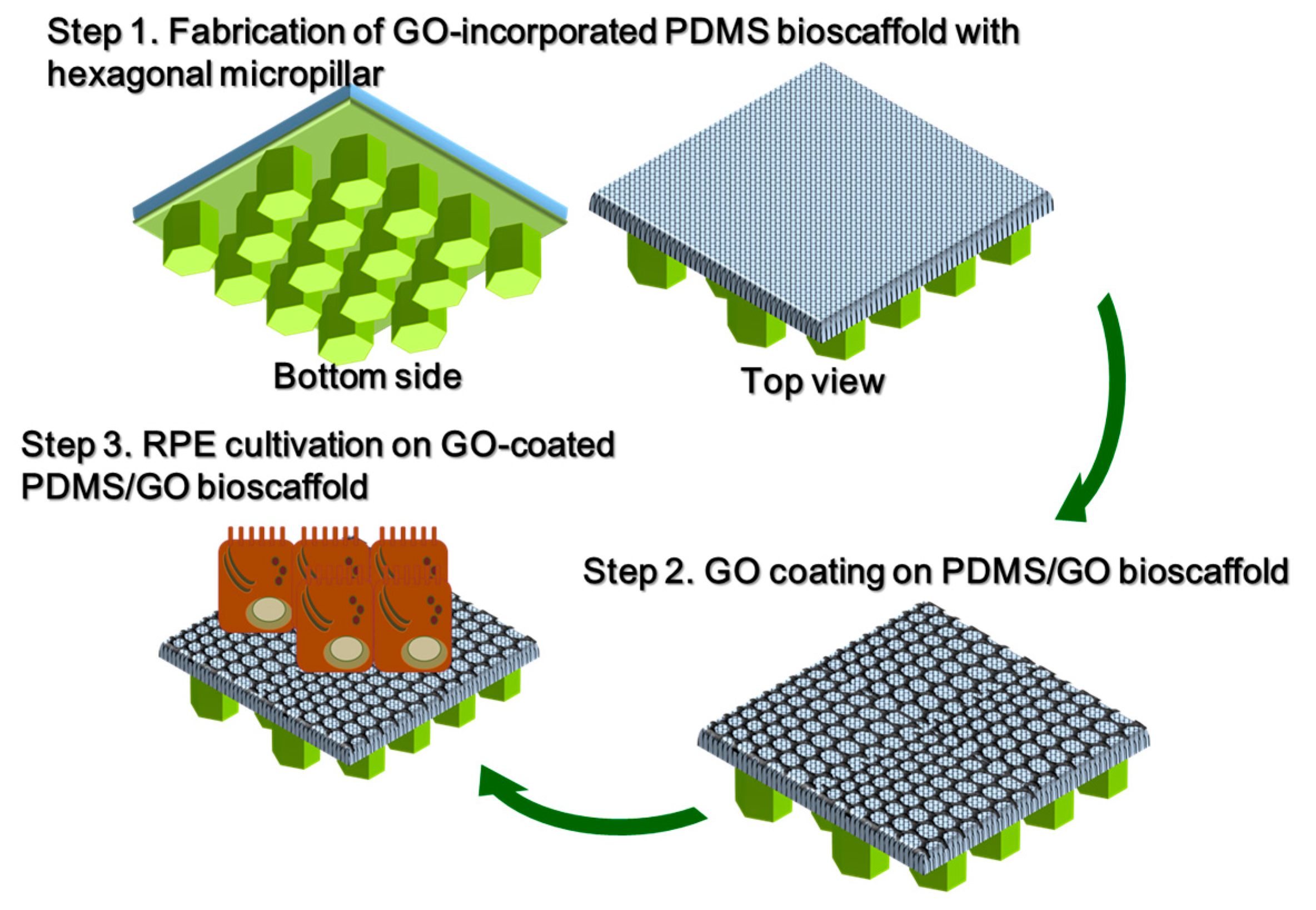
2.1. Manufacture of PDMS/GO Membrane
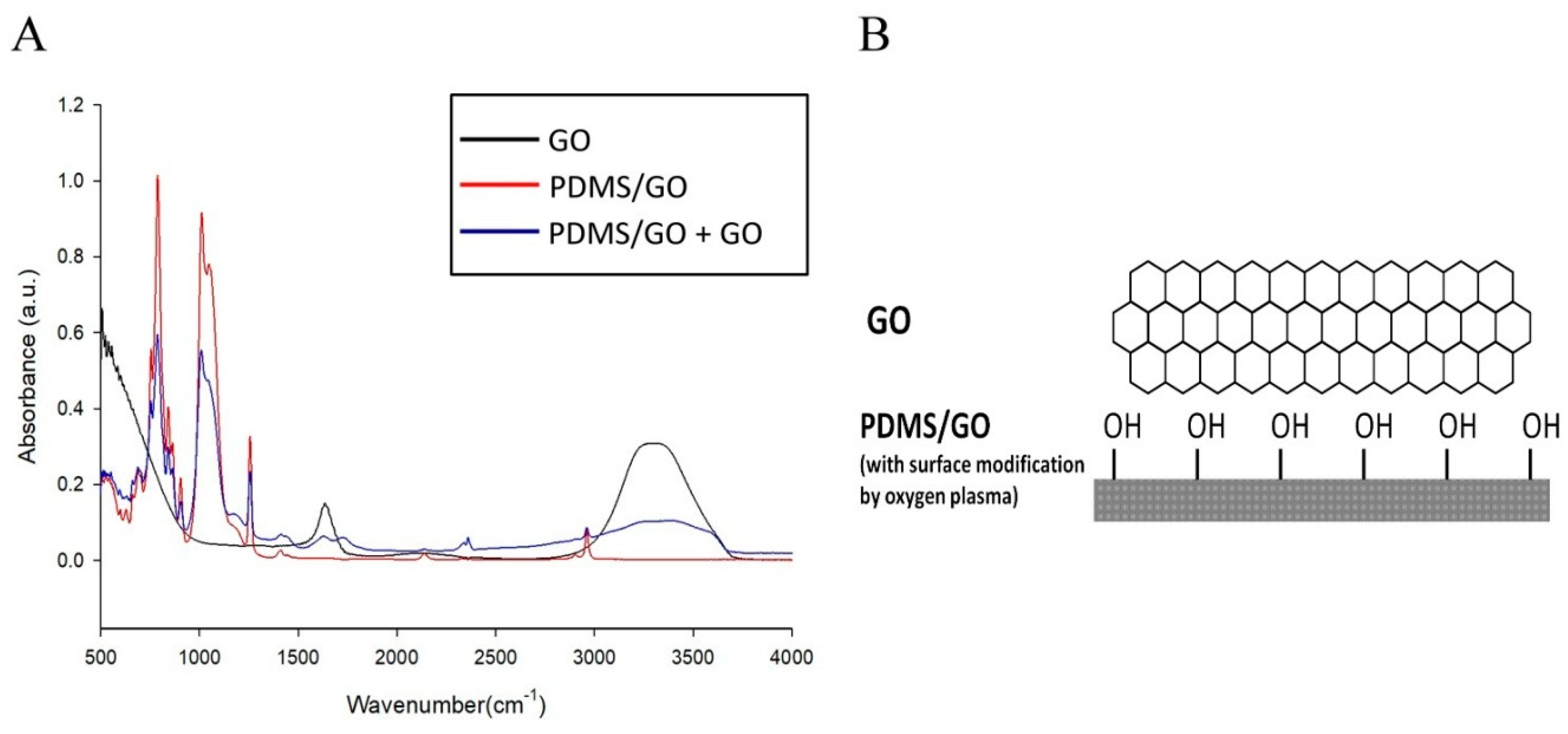
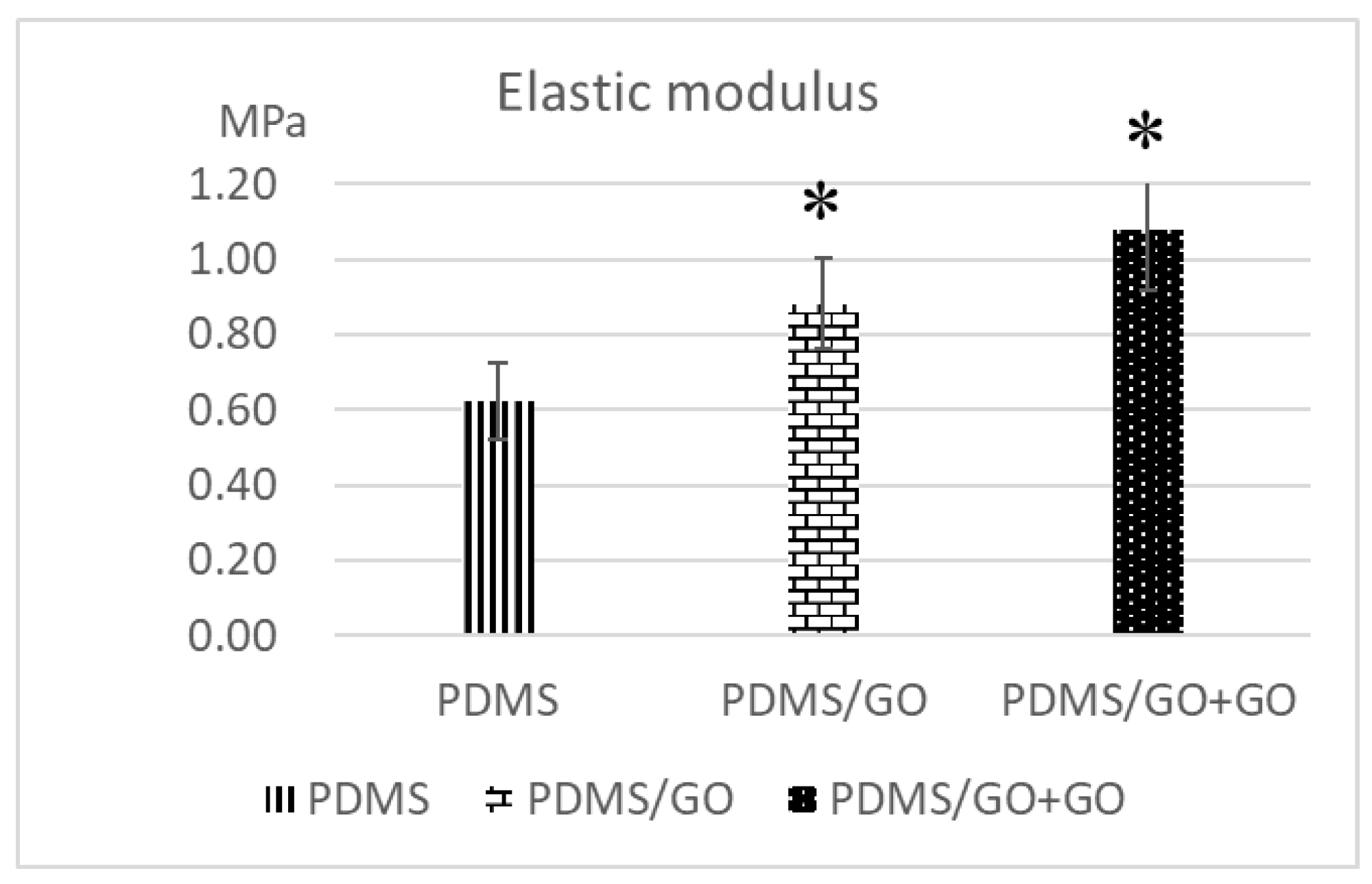
2.2. Comparison of the Characteristics of Pure PDMS and Other GO-Modified PDMS Elastomers
2.3. Introduction of Hexagonal Micropillars onto the GO-Enriched and GO-Coated PDMS Scaffolds
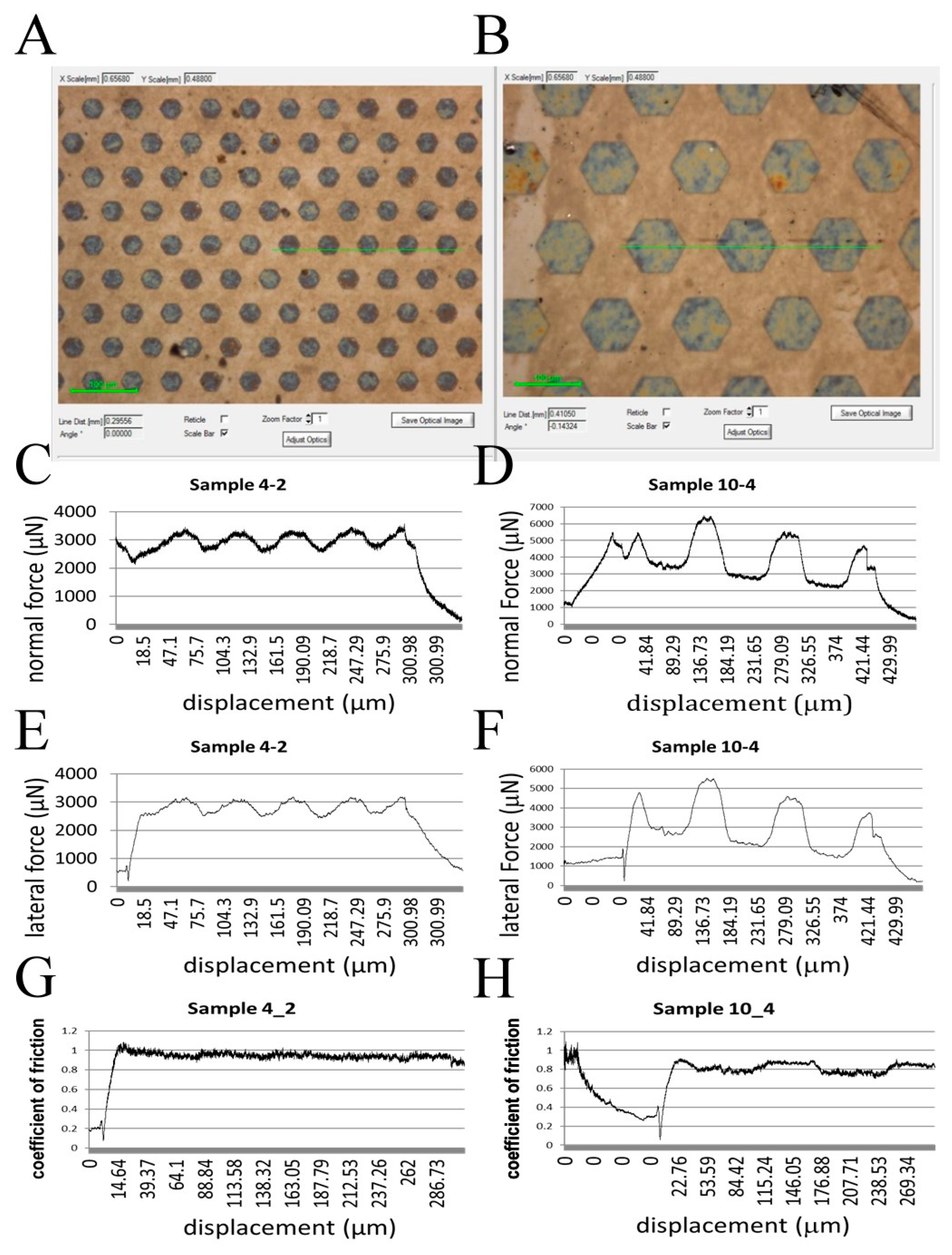
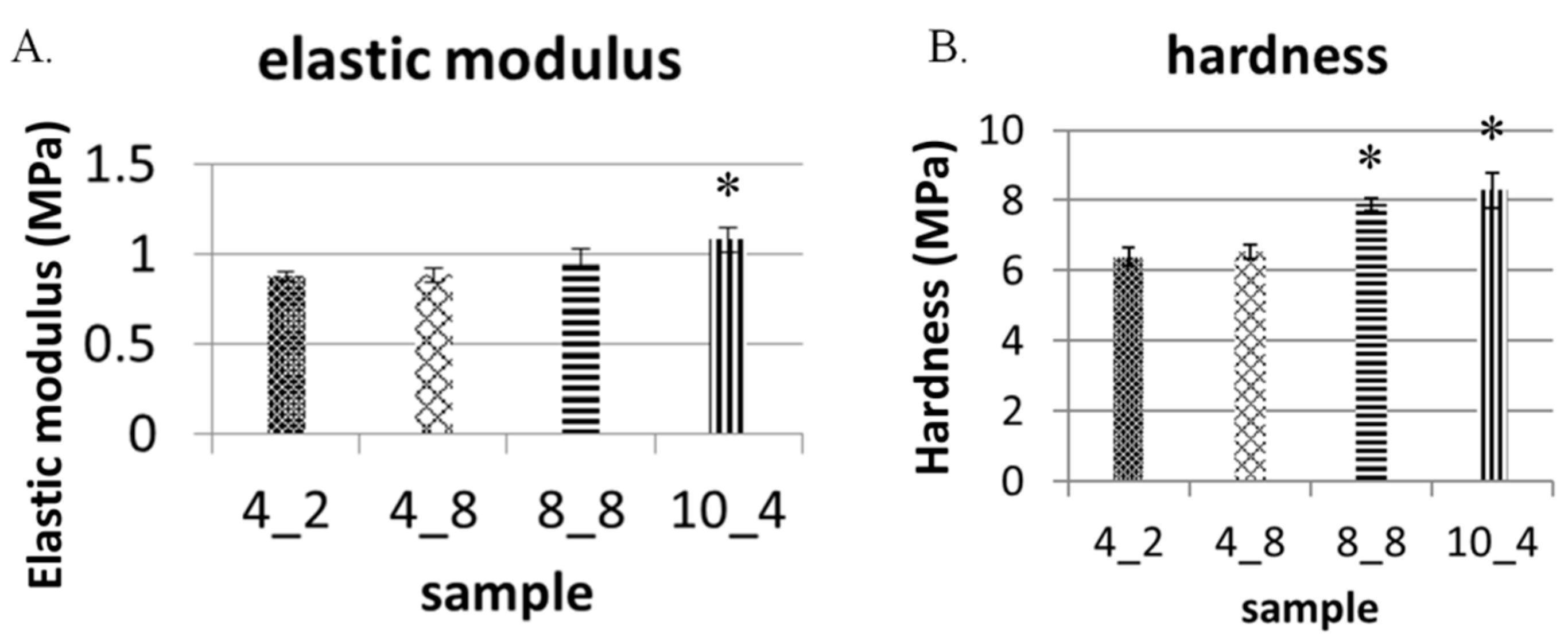
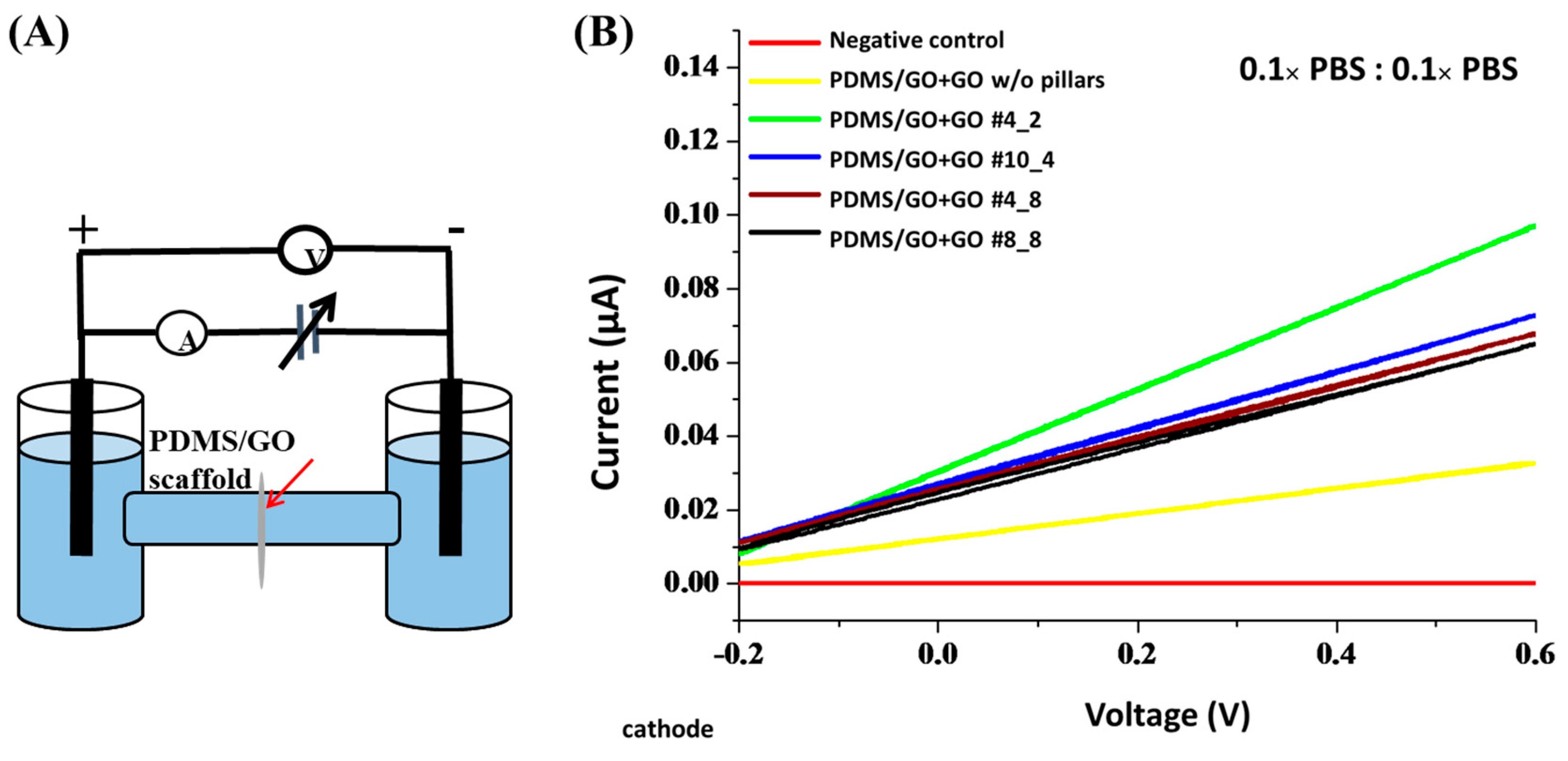
2.4. Physical Characteristics of the GO-Enriched and GO-Coated PDMS Scaffold with Hexagonal Micropillars
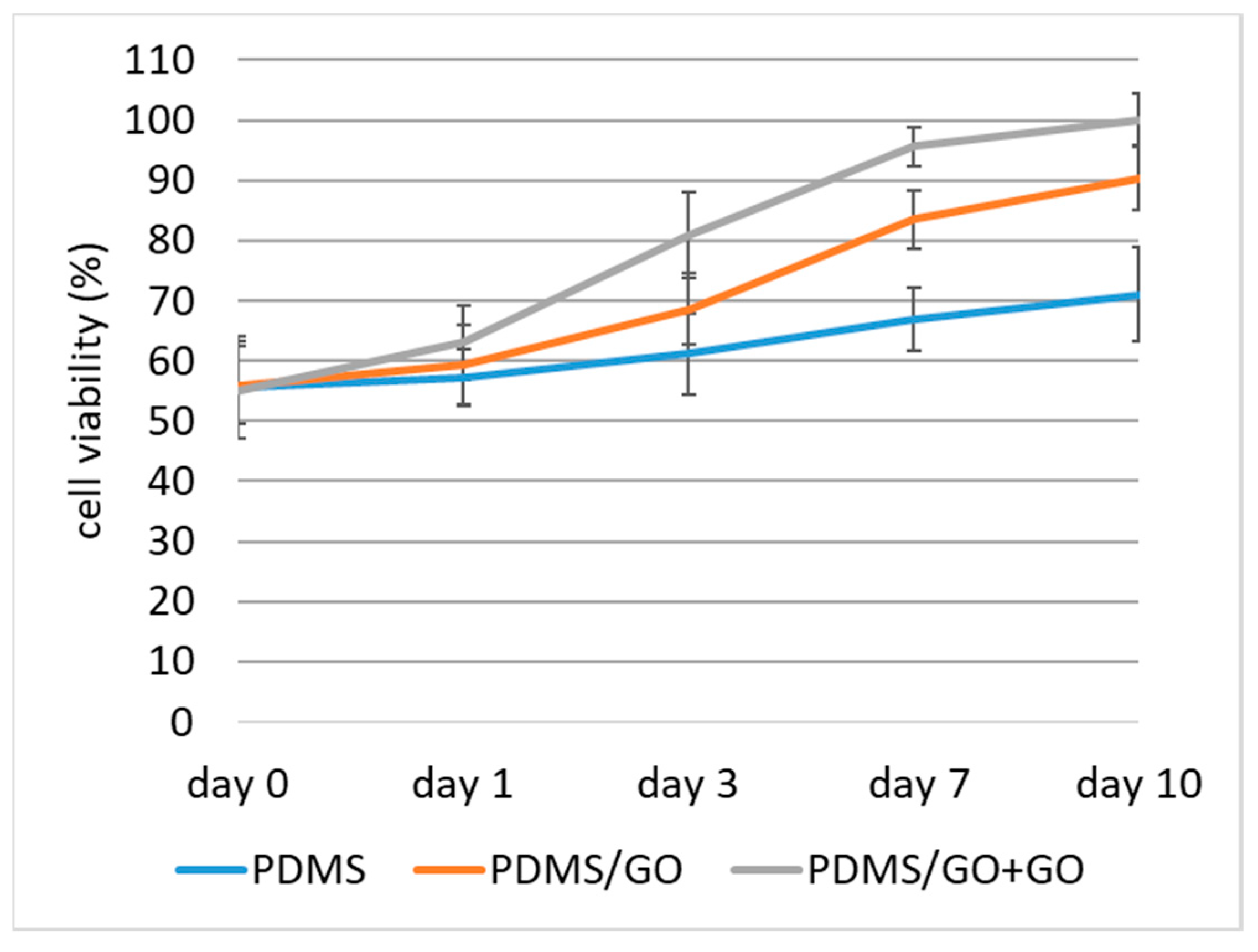
2.5. Biocompatibility of PDMS/GO Scaffold with hiPSC-RPE Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Fabrication of PDMS/GO Membrane
4.3. PDMS Scaffold Surface Modification
4.4. Atomic Force Microscopy
4.5. Contact Angle Measurement
4.6. FTIR Characterization
4.7. Mechanical Properties Measurement with Nanoindentation
4.8. Membrane Resistance Analysis (Ion Permeability Test)
4.9. Human Induced Pluripotent Stem Cells (hiPSCs) Cultivation and Differentiation to RPEs
4.10. Immunofluorescence Staining
4.11. Quantitative RT-PCR
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PDMS | polydimethylsiloxane |
| GO | graphene oxide |
| hiPSC | human induced pluripotent stem cell |
| hiPSC-RPEs | hiPSC-differentiated retinal pigment epithelial cells |
| ZO-1 | zonula occludens-1 |
| PEDF | pigment epithelium-derived factor |
| RPE65 | retinal pigmented epithelium-specific protein with molecular mass 65 kDa |
| BDNF | brain-derived neurotrophic factor |
| CCD | charge-coupled device |
| NEAA | nonessential amino acids |
| DKK1 | recombinant Dickkopf-related protein 1 |
References
- Karp, J.M.; Langer, R. Development and therapeutic applications of advanced biomaterials. Curr. Opin. Biotechnol. 2007, 18, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Peng, C.H.; Chuang, J.H.; Wang, M.L.; Jhan, Y.Y.; Chien, K.H.; Chung, Y.C.; Hung, K.H.; Chang, C.C.; Lee, C.K.; Tseng, W.L.; et al. Laminin modification subretinal bio-scaffold remodels retinal pigment epithelium-driven microenvironment in vitro and in vivo. Oncotarget 2016, 7, 64631–64648. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.N.; Jiang, X.; Ryan, D.; Whitesides, G.M. Compatibility of mammalian cells on surfaces of poly(dimethylsiloxane). Langmuir 2004, 20, 11684–11691. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.J.; Koh, Y.T.; Lim, K.; Menon, N.V.; Wu, Y.; Kang, Y. Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency. Sci. Rep. 2015, 5, 18162. [Google Scholar] [CrossRef] [PubMed]
- Pedraza, E.; Brady, A.-C.; Fraker, C.A.; Stabler, C.L. Synthesis of macroporous poly(dimethylsiloxane) scaffolds for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2013, 24, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.E.; Dunn, B. Oxygen diffusion in poly(dimethyl siloxane) using fluorescence quenching. I. Measurement technique and analysis. J. Polym. Sci. Part A Polym. Chem. 1986, 24, 621–636. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (pdms) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Yamamoto, T.; Kojima, N.; Kikuo, K.; Fujii, T.; Sakai, Y. Stable immobilization of rat hepatocytes as hemispheroids onto collagen-conjugated poly-dimethylsiloxane (pdms) surfaces: Importance of direct oxygenation through pdms for both formation and function. Biotechnol. Bioeng. 2008, 99, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Toworfe, G.K.; Composto, R.J.; Adams, C.S.; Shapiro, I.M.; Ducheyne, P. Fibronectin adsorption on surface-activated poly(dimethylsiloxane) and its effect on cellular function. J. Biomed. Mater. Res. A 2004, 71, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yang, H.; Guo, X.; Lu, J. Thermal degradation behaviors of some branched and linear polysiloxanes. Polym. Degrad. STable 2006, 91, 1471–1475. [Google Scholar] [CrossRef]
- Fuard, D.; Tzvetkova-Chevolleau, T.; Decossas, S.; Tracqui, P.; Schiavone, P. Optimization of poly-di-methyl-siloxane (pdms) substrates for studying cellular adhesion and motility. Microelectron. Eng. 2008, 85, 1289–1293. [Google Scholar] [CrossRef]
- Chuah, Y.J.; Kuddannaya, S.; Lee, M.H.; Zhang, Y.; Kang, Y. The effects of poly(dimethylsiloxane) surface silanization on the mesenchymal stem cell fate. Biomater. Sci. 2015, 3, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Noimark, S.; Colchester, R.J.; Blackburn, B.J.; Zhang, E.Z.; Alles, E.J.; Ourselin, S.; Beard, P.C.; Papakonstantinou, I.; Parkin, I.P.; Desjardins, A.E. Carbon-nanotube-pdms composite coatings on optical fibers for all-optical ultrasound imaging. Adv. Funct. Mater. 2016, 26, 8390–8396. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, W.; Gao, L.; Liu, B.; Pei, S.; Cheng, H.M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.-J.; Zang, J.; Zhang, G.-D.; Guan, L.-Z.; Li, S.-N.; Zhao, L.; Tang, L.-C. Efficient interfacial interaction for improving mechanical properties of polydimethylsiloxane nanocomposites filled with low content of graphene oxide nanoribbons. RSC Adv. 2017, 7, 22045–22053. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Khare, R.; Mielke, S.L.; Paci, J.T.; Zhang, S.; Ballarini, R.; Schatz, G.C.; Belytschko, T. Coupled quantum mechanical/molecular mechanical modeling of the fracture of defective carbon nanotubes and graphene sheets. Phys. Rev. B 2007, 75, 075412. [Google Scholar] [CrossRef]
- Sur, U.K. Graphene: A rising star on the horizon of materials science. Int. J. Electrochem. 2012, 2012, 12. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Z. Graphene in biomedicine: Opportunities and challenges. Nanomedicine 2011, 6, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Novoselov, K.S. Exploring the interface of graphene and biology. Science 2014, 344, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Cobos, M.; González, B.; Fernández, M.J.; Fernández, M.D. Study on the effect of graphene and glycerol plasticizer on the properties of chitosan-graphene nanocomposites via in situ green chemical reduction of graphene oxide. Int. J. Biol. Macromol. 2018, 114, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Girase, B.; Shah, J.S.; Misra, R.D.K. Structure–process–property relationship of the polar graphene oxide-mediated cellular response and stimulated growth of osteoblasts on hybrid chitosan network structure nanocomposite scaffolds. Acta Biomater. 2011, 7, 3432–3445. [Google Scholar] [CrossRef] [PubMed]
- Jangho, K.; Soon, C.K.; Yeonju, K.; Ki-Tack, L.; Hoon, S.; Yensil, P.; Deok-Ho, K.; Pill-Hoon, C.; Chong-Su, C.; Young, K.S.; et al. Bioactive effects of graphene oxide cell culture substratum on structure and function of human adipose-derived stem cells. J. Biomed. Mater. Res. Part A 2013, 101, 3520–3530. [Google Scholar]
- Ryu, S.; Kim, B.-S. Culture of neural cells and stem cells on graphene. Tissue Eng. Regener. Med. 2013, 10, 39–46. [Google Scholar] [CrossRef]
- Chen, G.Y.; Pang, D.W.; Hwang, S.M.; Tuan, H.Y.; Hu, Y.C. A graphene-based platform for induced pluripotent stem cells culture and differentiation. Biomaterials 2012, 33, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Abouei, E.; Hatamie, S.; Ghasemi, E. Accelerated differentiation of neural stem cells into neurons on ginseng-reduced graphene oxide sheets. Carbon 2014, 66, 395–406. [Google Scholar] [CrossRef]
- Hermenean, A.; Codreanu, A.; Herman, H.; Balta, C.; Rosu, M.; Mihali, C.V.; Ivan, A.; Dinescu, S.; Ionita, M.; Costache, M. Chitosan-graphene oxide 3d scaffolds as promising tools for bone regeneration in critical-size mouse calvarial defects. Sci. Rep. 2017, 7, 16641. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Chawla, A.; Vairamani, M.; Sastry, T.P.; Subramanian, K.S.; Selvamurugan, N. Scaffolds containing chitosan, gelatin and graphene oxide for bone tissue regeneration in vitro and in vivo. Int. J. Biol. Macromol. 2017, 104, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Sydlik, S.A. Graphene oxide as a scaffold for bone regeneration. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1437. [Google Scholar] [CrossRef] [PubMed]
- Nishida, E.; Miyaji, H.; Kato, A.; Takita, H.; Iwanaga, T.; Momose, T.; Ogawa, K.; Murakami, S.; Sugaya, T.; Kawanami, M. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int. J. Nanomed. 2016, 11, 2265–2277. [Google Scholar]
- Kawamoto, K.; Miyaji, H.; Nishida, E.; Miyata, S.; Kato, A.; Tateyama, A.; Furihata, T.; Shitomi, K.; Iwanaga, T.; Sugaya, T. Characterization and evaluation of graphene oxide scaffold for periodontal wound healing of class ii furcation defects in dog. Int. J. Nanomed. 2018, 13, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, S.; Shafiei, S.S.; Asadi-Eydivand, M.; Ardeshir, M.; Solati-Hashjin, M. Graphene oxide-enriched poly(ε-caprolactone) electrospun nanocomposite scaffold for bone tissue engineering applications. J. Bioact. Compat. Polym. 2016, 32, 325–342. [Google Scholar] [CrossRef]
- Han, L.; Sun, H.; Tang, P.; Li, P.; Xie, C.; Wang, M.; Wang, K.; Weng, J.; Tan, H.; Ren, F.; et al. Mussel-inspired graphene oxide nanosheet-enwrapped ti scaffolds with drug-encapsulated gelatin microspheres for bone regeneration. Biomater. Sci. 2018, 6, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Li, K.; Li, K.; Xian, B.; Liu, Y.; Yang, S.; Xu, C.; Fan, Z.; Lu, S.; Zhang, H.; et al. Establishing a surgical procedure for rhesus epiretinal scaffold implantation with hipsc-derived retinal progenitors. Stem Cells Int. 2018, 2018, 9437041. [Google Scholar] [CrossRef] [PubMed]
- Varley, M.C.; Neelakantan, S.; Clyne, T.W.; Dean, J.; Brooks, R.A.; Markaki, A.E. Cell structure, stiffness and permeability of freeze-dried collagen scaffolds in dry and hydrated states. Acta Biomater. 2016, 33, 166–175. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Tao, S.L.; Saint-Geniez, M. Porous poly(ε-caprolactone) scaffolds for retinal pigment epithelium transplantation. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Thomson, H.A.; Treharne, A.J.; Walker, P.; Grossel, M.C.; Lotery, A.J. Optimisation of polymer scaffolds for retinal pigment epithelium (rpe) cell transplantation. Br. J. Ophthalmol. 2011, 95, 563–568. [Google Scholar] [CrossRef] [PubMed]
- White, C.E.; Olabisi, R.M. Scaffolds for retinal pigment epithelial cell transplantation in age-related macular degeneration. J. Tissue Eng. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.J.; Spencer, C.; D’Amore, P.A.; Tao, S.L.; Saint-Geniez, M. A porous poly(-caprolactone) tissue engineering scaffold for rpe transplantation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3641. [Google Scholar]
- Binder, S. Scaffolds for retinal pigment epithelium (rpe) replacement therapy. Br. J. Ophthalmol. 2011, 95, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Polydimethylsiloxane Mechanical Properties Measured by Macroscopic Compression and Nanoindentation Techniques. Ph.D. Thesis, University of South Florida, Tampa, FL, USA, 2011. [Google Scholar]
- Johnston, I.D.; McCluskey, D.K.; Tan, C.K.L.; Tracey, M.C. Mechanical characterization of bulk sylgard 184 for microfluidics and microengineering. J. Micromech. Microeng. 2014, 24, 035017. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Bauer, W.A.; Fischlechner, M.; Abell, C.; Huck, W.T. Hydrophilic pdms microchannels for high-throughput formation of oil-in-water microdroplets and water-in-oil-in-water double emulsions. Lab Chip 2010, 10, 1814–1819. [Google Scholar] [CrossRef] [PubMed]
- Bodas, D.; Khan-Malek, C. Formation of more stable hydrophilic surfaces of pdms by plasma and chemical treatments. Microelectron. Eng. 2006, 83, 1277–1279. [Google Scholar] [CrossRef]
- Bekkers, J.E.J.; Tsuchida, A.I.; Malda, J.; Creemers, L.B.; Castelein, R.J.M.; Saris, D.B.F.; Dhert, W.J.A. Quality of scaffold fixation in a human cadaver knee model. Osteoarthr. Cartil. 2010, 18, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Zelle, S.; Zantop, T.; Schanz, S.; Petersen, W. Arthroscopic techniques for the fixation of a three-dimensional scaffold for autologous chondrocyte transplantation: Structural properties in an in vitro model. Arthroscopy 2007, 23, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Drobnič, M.; Radosavljevič, D.; Ravnik, D.; Pavlovčič, V.; Hribernik, M. Comparison of four techniques for the fixation of a collagen scaffold in the human cadaveric knee. Osteoarthr. Cartil. 2006, 14, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Shelyakova, T.; Casino, D.; Lopomo, N.F.; Strazzari, A.; Ortolani, A.; Visani, A.; Dediu, A.; Marcacci, M. A new approach to scaffold fixation by magnetic forces: Application to large osteochondral defects. Med. Eng. Phys. 2012, 34, 1287–1293. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, D.E.; Pennington, B.O.; Croze, R.H.; Hinman, C.R.; Coffey, P.J.; Clegg, D.O. Rapid and efficient directed differentiation of human pluripotent stem cells into retinal pigmented epithelium. Stem Cells Transl. Med. 2013, 2, 384–393. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-Y.; Chien, Y.; Chuang, J.-H.; Chang, C.-C.; Yang, Y.-P.; Lai, Y.-H.; Lo, W.-L.; Chien, K.-H.; Huo, T.-I.; Wang, C.-Y. Development of a Graphene Oxide-Incorporated Polydimethylsiloxane Membrane with Hexagonal Micropillars. Int. J. Mol. Sci. 2018, 19, 2517. https://doi.org/10.3390/ijms19092517
Lin Y-Y, Chien Y, Chuang J-H, Chang C-C, Yang Y-P, Lai Y-H, Lo W-L, Chien K-H, Huo T-I, Wang C-Y. Development of a Graphene Oxide-Incorporated Polydimethylsiloxane Membrane with Hexagonal Micropillars. International Journal of Molecular Sciences. 2018; 19(9):2517. https://doi.org/10.3390/ijms19092517
Chicago/Turabian StyleLin, Yi-Ying, Yueh Chien, Jen-Hua Chuang, Chia-Ching Chang, Yi-Ping Yang, Ying-Hsiu Lai, Wen-Liang Lo, Ke-Hung Chien, Teh-Ia Huo, and Chien-Ying Wang. 2018. "Development of a Graphene Oxide-Incorporated Polydimethylsiloxane Membrane with Hexagonal Micropillars" International Journal of Molecular Sciences 19, no. 9: 2517. https://doi.org/10.3390/ijms19092517
APA StyleLin, Y.-Y., Chien, Y., Chuang, J.-H., Chang, C.-C., Yang, Y.-P., Lai, Y.-H., Lo, W.-L., Chien, K.-H., Huo, T.-I., & Wang, C.-Y. (2018). Development of a Graphene Oxide-Incorporated Polydimethylsiloxane Membrane with Hexagonal Micropillars. International Journal of Molecular Sciences, 19(9), 2517. https://doi.org/10.3390/ijms19092517







