Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome
Abstract
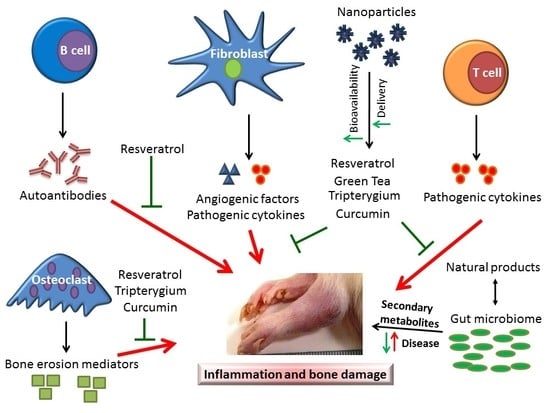
1. Introduction
2. The Cellular and Soluble Mediators of Arthritic Inflammation
3. Animal Models of RA for Studying Disease Pathogenesis and Testing Therapeutic Agents
4. Currently Used Drugs for Arthritis Therapy and Their Limitations
5. The Use of Plant Natural Products for Arthritis Therapy
5.1. Tripterygium wilfordii Hook F
5.1.1. Tripterygium wilfordii Hook F (TwHF)
5.1.2. Triptolide
5.1.3. Celastrol
5.2. Green Tea
5.3. Curcumin
5.4. Resveratrol
5.5. Other Natural Products
6. Nanoparticle-Based Delivery of Plant Natural Products and Other Drugs for Arthritis Therapy
6.1. Polymer Nanoparticles
6.2. Liposomes
6.3. Nanoemulsions
6.4. Nanomicelles
6.5. Lipid-Core Nanocapsules
7. Interplay between Dietary Products and the Host Microbiome for Maintenance of Health and Disease Modulation
8. Concluding Remarks
Acknowledgments
Conflicts of Interest
Declaration
References
- Du Montcel, S.T.; Michou, L.; Petit-Teixeira, E.; Osorio, J.; Lemaire, I.; Lasbleiz, S.; Pierlot, C.; Quillet, P.; Bardin, T.; Prum, B.; et al. New classification of HLA-DRB1 alleles supports the shared epitope hypothesis of rheumatoid arthritis susceptibility. Arthritis Rheumatol. 2005, 52, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Fugger, L.; Svejgaard, A. Association of MHC and rheumatoid arthritis. HLA-DR4 and rheumatoid arthritis: Studies in mice and men. Arthritis Res. 2000, 2, 208–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Makrygiannakis, D.; Hermansson, M.; Ulfgren, A.K.; Nicholas, A.P.; Zendman, A.J.; Eklund, A.; Grunewald, J.; Skold, C.M.; Klareskog, L.; Catrina, A.I. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann. Rheum. Dis. 2008, 67, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Snir, O.; Widhe, M.; von Spee, C.; Lindberg, J.; Padyukov, L.; Lundberg, K.; Engstrom, A.; Venables, P.J.; Lundeberg, J.; Holmdahl, R.; et al. Multiple antibody reactivities to citrullinated antigens in sera from patients with rheumatoid arthritis: Association with HLA-DRB1 alleles. Ann. Rheum. Dis. 2009, 68, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Van der Helm-van Mil, A.H.; Verpoort, K.N.; Breedveld, F.C.; Huizinga, T.W.; Toes, R.E.; de Vries, R.R. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheumatol. 2006, 54, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Alamanos, Y.; Drosos, A.A. Epidemiology of adult rheumatoid arthritis. Autoimmun. Rev. 2005, 4, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Crowson, C.S.; Matteson, E.L.; Myasoedova, E.; Michet, C.J.; Ernste, F.C.; Warrington, K.J.; Davis, J.M., III; Hunder, G.G.; Therneau, T.M.; Gabriel, S.E. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheumatol. 2011, 63, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Helmick, C.G.; Felson, D.T.; Lawrence, R.C.; Gabriel, S.; Hirsch, R.; Kwoh, C.K.; Liang, M.H.; Kremers, H.M.; Mayes, M.D.; Merkel, P.A.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheumatol. 2008, 58, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Silman, A.J.; Pearson, J.E. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res. 2002, 4, S265–S272. [Google Scholar] [CrossRef] [PubMed]
- Symmons, D.; Turner, G.; Webb, R.; Asten, P.; Barrett, E.; Lunt, M.; Scott, D.; Silman, A. The prevalence of rheumatoid arthritis in the United Kingdom: New estimates for a new century. Rheumatology 2002, 41, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hootman, J.M.; Helmick, C.G.; Barbour, K.E.; Theis, K.A.; Boring, M.A. Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 2016, 68, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, A.C.; Pereira, L.H.C.; Kahlow, B.S.; Silva, M.B.; Skare, T.L. Rheumatoid arthritis in elderly and young patients. Rev. Bras. Reumatol. 2017, 57, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.; Sawada, T.; Tahara, K.; Hayashi, H.; Tago, M.; Mori, H.; Nishino, J.; Matsui, T.; Tohma, S. The age at onset of rheumatoid arthritis is increasing in Japan: A nationwide database study. Int. J. Rheum. Dis. 2017, 20, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Kobak, S.; Bes, C. An autumn tale: Geriatric rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.D., Jr. Rheumatoid arthritis. Pathophysiology and implications for therapy. N. Engl. J. Med. 1990, 322, 1277–1289. [Google Scholar] [PubMed]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Yanni, G.; Whelan, A.; Feighery, C.; Bresnihan, B. Synovial tissue macrophages and joint erosion in rheumatoid arthritis. Ann. Rheum. Dis. 1994, 53, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Morco, S.; Bowden, A. Ulnar drift in rheumatoid arthritis: A review of biomechanical etiology. J. Biomech. 2015, 48, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, K.; Johnson, P.M.; Rydgren, L. The occurrence and significance of hand deformities in early rheumatoid arthritis. Br. J. Rheumatol. 1991, 30, 211–213. [Google Scholar] [CrossRef] [PubMed]
- Palmer, D.G.; Hogg, N.; Highton, J.; Hessian, P.A.; Denholm, I. Macrophage migration and maturation within rheumatoid nodules. Arthritis Rheumatol. 1987, 30, 728–736. [Google Scholar] [CrossRef]
- Sayah, A.; English, J.C., III. Rheumatoid arthritis: A review of the cutaneous manifestations. J. Am. Acad. Dermatol. 2005, 53, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Gavrila, B.I.; Ciofu, C.; Stoica, V. Biomarkers in Rheumatoid Arthritis, what is new? J. Med. Life 2016, 9, 144–148. [Google Scholar] [PubMed]
- Deane, K.D.; Norris, J.M.; Holers, V.M. Preclinical rheumatoid arthritis: Identification, evaluation, and future directions for investigation. Rheum. Dis. Clin. N. Am. 2010, 36, 213–241. [Google Scholar] [CrossRef] [PubMed]
- Chun-Lai, T.; Murad, S.; Erlandsson, M.C.; Hussein, H.; Sulaiman, W.; Dhaliwal, J.S.; Bokarewa, M.I. Recognizing rheumatoid arthritis: Oncoprotein survivin opens new possibilities: A population-based case-control study. Medicine 2015, 94, e468. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.; Knoechel, B.; Nagabhushanam, V.; Abbas, A.K. T-cell tolerance and autoimmunity to systemic and tissue-restricted self-antigens. Immunol. Rev. 2005, 204, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Ring, G.H.; Lakkis, F.G. Breakdown of self-tolerance and the pathogenesis of autoimmunity. Semin. Nephrol. 1999, 19, 25–33. [Google Scholar] [PubMed]
- Veale, D.J.; Orr, C.; Fearon, U. Cellular and molecular perspectives in rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Bluestone, J.A.; Mackay, C.R.; O’Shea, J.J.; Stockinger, B. The functional plasticity of T cell subsets. Nat. Rev. Immunol. 2009, 9, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Mucida, D.; Cheroutre, H. The many face-lifts of CD4 T helper cells. Adv. Immunol. 2010, 107, 139–152. [Google Scholar] [PubMed]
- Calabresi, E.; Petrelli, F.; Bonifacio, A.F.; Puxeddu, I.; Alunno, A. One year in review 2018: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2018, 36, 175–184. [Google Scholar] [PubMed]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.D.; Latham, K.A.; Rosloniec, E.F. Collagen-induced arthritis. Nat. Protoc. 2007, 2, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Bhatt, L.K.; Prabhavalkar, K.S. Experimental animal models for rheumatoid arthritis. Immunopharmacol. Immunotoxicol. 2018, 40, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Astry, B.; Venkatesha, S.H.; Laurence, A.; Christensen-Quick, A.; Garzino-Demo, A.; Frieman, M.B.; O’Shea, J.J.; Moudgil, K.D. Celastrol, a Chinese herbal compound, controls autoimmune inflammation by altering the balance of pathogenic and regulatory T cells in the target organ. Clin. Immunol. 2015, 157, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Dudics, S.; Venkatesha, S.H.; Moudgil, K.D. The micro-RNA expression profiles of autoimmune arthritis reveal novel biomarkers of the disease and therapeutic response. Int. J. Mol. Sci. 2018, 19, 2293. [Google Scholar] [CrossRef] [PubMed]
- De Castro Costa, M.; De Sutter, P.; Gybels, J.; Van Hees, J. Adjuvant-induced arthritis in rats: A possible animal model of chronic pain. Pain 1981, 10, 173–185. [Google Scholar] [CrossRef]
- Whitehouse, M.W. Adjuvant arthritis 50 years on: The impact of the 1956 article by CM Pearson, ‘Development of arthritis, periarthritis and periostitis in rats given adjuvants’. Inflamm. Res. 2007, 56, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Whiteley, P.E.; Dalrymple, S.A. Models of inflammation: Adjuvant-induced arthritis in the rat. Curr. Protoc. Pharmacol. 2001. [Google Scholar] [CrossRef]
- Kouskoff, V.; Korganow, A.S.; Duchatelle, V.; Degott, C.; Benoist, C.; Mathis, D. Organ-specific disease provoked by systemic autoimmunity. Cell 1996, 87, 811–822. [Google Scholar] [CrossRef]
- Christensen, A.D.; Haase, C.; Cook, A.D.; Hamilton, J.A. K/BxN serum-transfer arthritis as a model for human inflammatory arthritis. Front. Immunol. 2016, 7, 213. [Google Scholar] [CrossRef] [PubMed]
- Glant, T.T.; Radacs, M.; Nagyeri, G.; Olasz, K.; Laszlo, A.; Boldizsar, F.; Hegyi, A.; Finnegan, A.; Mikecz, K. Proteoglycan-induced arthritis and recombinant human proteoglycan aggrecan G1 domain-induced arthritis in BALB/c mice resembling two subtypes of rheumatoid arthritis. Arthritis Rheumatol. 2011, 63, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Nabozny, G.H.; Baisch, J.M.; Cheng, S.; Cosgrove, D.; Griffiths, M.M.; Luthra, H.S.; David, C.S. HLA-DQ8 transgenic mice are highly susceptible to collagen-induced arthritis: A novel model for human polyarthritis. J. Exp. Med. 1996, 183, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15 (Suppl. 3), S2. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Emery, P.; Tanaka, Y.; Burmester, G.; Pisetsky, D.S.; Naredo, E.; Fautrel, B.; van Vollenhoven, R. Tapering biologic and conventional DMARD therapy in rheumatoid arthritis: Current evidence and future directions. Ann. Rheum. Dis. 2016, 75, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Gerards, A.H.; de Lathouder, S.; de Groot, E.R.; Dijkmans, B.A.; Aarden, L.A. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology 2003, 42, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; Yin, Z.; Siegert, S.; Grolms, M.; Radbruch, A.; Braun, J.; Sieper, J. Response to methotrexate in early rheumatoid arthritis is associated with a decrease of T cell derived tumour necrosis factor alpha, increase of interleukin 10, and predicted by the initial concentration of interleukin 4. Ann. Rheum. Dis. 2000, 59, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.M.; Pratt, A.G.; Isaacs, J.D. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 2016, 12, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Tchetverikov, I.; Kraan, M.C.; van El, B.; Hanemaaijer, R.; DeGroot, J.; Huizinga, T.W. Leflunomide and methotrexate reduce levels of activated matrix metalloproteinases in complexes with α2 macroglobulin in serum of rheumatoid arthritis patients. Ann. Rheum. Dis. 2008, 67, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N. Going with the flow: Methotrexate, adenosine, and blood flow. Ann. Rheum. Dis. 2006, 65, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Hasko, G.; Cronstein, B. Regulation of inflammation by adenosine. Front. Immunol. 2013, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Olivo, M.A.; Siddhanamatha, H.R.; Shea, B.; Tugwell, P.; Wells, G.A.; Suarez-Almazor, M.E. Methotrexate for treating rheumatoid arthritis. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.C.; Cassia, F.F.; Lamy, F.; Chagas, V.L.; Ramos-e-Silva, M. Methotrexate and liver function: A study of 13 psoriasis cases treated with different cumulative dosages. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Rademaker, M. Monitoring methotrexate-induced liver fibrosis in patients with psoriasis: Utility of transient elastography. Psoriasis 2018, 8, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Conway, R.; Carey, J.J. Risk of liver disease in methotrexate treated patients. World J. Hepatol. 2017, 9, 1092–1100. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2015, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Weinblatt, M.E.; Keystone, E.C.; Furst, D.E.; Moreland, L.W.; Weisman, M.H.; Birbara, C.A.; Teoh, L.A.; Fischkoff, S.A.; Chartash, E.K. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: The ARMADA trial. Arthritis Rheumatol. 2003, 48, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef] [PubMed]
- Goh, L.; Jewell, T.; Laversuch, C.; Samanta, A. A systematic review of the influence of anti-TNF on infection rates in patients with rheumatoid arthritis. Rev. Bras. Reumatol. 2013, 53, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Lee, N.R.; Pi, R.H.; Lim, Y.S.; Lee, Y.M.; Lee, J.M.; Jeong, H.S.; Chung, S.H. IL-6 inhibitors for treatment of rheumatoid arthritis: Past, present, and future. Arch. Pharm. Res. 2015, 38, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, V.; Dhillon, S.; Plosker, G.L. Tocilizumab: A review of its use in the management of rheumatoid arthritis. Drugs 2009, 69, 609–632. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Millan, I.; Singh, J.A.; Curtis, J.R. Systematic review of tocilizumab for rheumatoid arthritis: A new biologic agent targeting the interleukin-6 receptor. Clin. Ther. 2012, 34, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Schoels, M.M.; Nishimoto, N.; Breedveld, F.C.; Burmester, G.R.; Dougados, M.; Emery, P.; Ferraccioli, G.; Gabay, C.; Gibofsky, A.; et al. Consensus statement on blocking the effects of interleukin-6 and in particular by interleukin-6 receptor inhibition in rheumatoid arthritis and other inflammatory conditions. Ann. Rheum. Dis. 2013, 72, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Hench, P.S.; Kendall, E.C.; Slocumb, C.H.; Polley, H.F. Effects of cortisone acetate and pituitary ACTH on rheumatoid arthritis, rheumatic fever and certain other conditions. Arch. Intern. Med. 1950, 85, 545–666. [Google Scholar] [CrossRef]
- Barnes, P.J. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 2006, 148, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Mundell, L.; Lindemann, R.; Douglas, J. Monitoring long-term oral corticosteroids. BMJ Open Qual. 2017, 6, e000209. [Google Scholar] [CrossRef] [PubMed]
- Youssef, J.; Novosad, S.A.; Winthrop, K.L. Infection risk and safety of corticosteroid use. Rheum. Dis. Clin. N. Am. 2016, 42, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.C.; Black, L.I.; Stussman, B.J.; Barnes, P.M.; Nahin, R.L. Trends in the Use of Complementary Health Approaches Among Adults: United States, 2002–2012; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2013.
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and Alternative Medicine Use Among Adults and Children: United States, 2007; National Health Statistics Reports; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics: Hyattsville, MD, USA, 2008; pp. 1–23.
- Rajesh, E.; Sankari, L.S.; Malathi, L.; Krupaa, J.R. Naturally occurring products in cancer therapy. J. Pharm. Bioallied Sci. 2015, 7, S181–S183. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Liu, X.; Komabayashi, T.; Jeong, S.I.; Selli, S. Natural products for infectious diseases. Evid.-Based Complement. Altern. Med. 2016, 2016, 9459047. [Google Scholar] [CrossRef] [PubMed]
- Shamsizadeh, A.; Roohbakhsh, A.; Ayoobi, F.; Moghaddamahmadi, A. The role of natural products in the prevention and treatment of multiple sclerosis. In Nutrition and Lifestyle in Neurological Autoimmune Diseases; Academic Press/Elsevier: Cambridge, MA, USA, 2017; pp. 249–260. ISBN 978-240-212-805298-805293. [Google Scholar]
- Chang, C.L.; Chen, Y.C.; Chen, H.M.; Yang, N.S.; Yang, W.C. Natural cures for type 1 diabetes: A review of phytochemicals, biological actions, and clinical potential. Curr. Med. Chem. 2013, 20, 899–907. [Google Scholar] [PubMed]
- Kapoor, B.; Gupta, R.; Gupta, M. Natural products in treatment of rheumatoid arthritis. Int. J. Green Pharm. 2017, 11, S356–S363. [Google Scholar]
- Venkatesha, S.H.; Rajaiah, R.; Berman, B.M.; Moudgil, K.D. Immunomodulation of autoimmune arthritis by herbal CAM. Evid.-Based Complement. Altern. Med. 2011, 2011, 986797. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.; Acharya, B.; Moudgil, K. Natural products as source of anti-inflammatory drugs. In Inflammation: From Molecular and Cellular Mechanisms to the Clinic; Cavaillo, J.-M., Singer, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; Chapter 64; pp. 1607–1635. [Google Scholar]
- Ben-Arye, E.; Frenkel, M.; Klein, A.; Scharf, M. Attitudes toward integration of complementary and alternative medicine in primary care: Perspectives of patients, physicians and complementary practitioners. Patient Educ. Couns. 2008, 70, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Cascao, R.; Vidal, B.; Raquel, H.; Neves-Costa, A.; Figueiredo, N.; Gupta, V.; Fonseca, J.E.; Moita, L.F. Effective treatment of rat adjuvant-induced arthritis by celastrol. Autoimmun. Rev. 2012, 11, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.H.; Dudics, S.; Astry, B.; Moudgil, K.D. Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog. Dis. 2016, 74, ftw059. [Google Scholar] [CrossRef] [PubMed]
- Nanjundaiah, S.M.; Venkatesha, S.H.; Yu, H.; Tong, L.; Stains, J.P.; Moudgil, K.D. Celastrus and its bioactive celastrol protect against bone damage in autoimmune arthritis by modulating osteoimmune cross-talk. J. Biol. Chem. 2012, 287, 22216–22226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.X.; Fan, A.Y.; Zhou, A.N.; Moudgil, K.D.; Ma, Z.Z.; Lee, D.Y.; Fong, H.H.; Berman, B.M.; Lao, L. Extract of the Chinese herbal formula Huo Luo Xiao Ling Dan inhibited adjuvant arthritis in rats. J. Ethnopharmacol. 2009, 121, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Busbee, P.B.; Rouse, M.; Nagarkatti, M.; Nagarkatti, P.S. Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev. 2013, 71, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Che, C.T.; Wong, M.S.; Lam, C.W. Natural products from chinese medicines with potential benefits to bone health. Molecules 2016, 21, 239. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.H.; Yu, H.; Rajaiah, R.; Tong, L.; Moudgil, K.D. Celastrus-derived celastrol suppresses autoimmune arthritis by modulating antigen-induced cellular and humoral effector responses. J. Biol. Chem. 2011, 286, 15138–15146. [Google Scholar] [CrossRef] [PubMed]
- Che, C.T.; Wong, M.S. Ligustrum lucidum and its Constituents: A mini-review on the anti-osteoporosis potential. Nat. Prod. Commun. 2015, 10, 2189–2194. [Google Scholar] [PubMed]
- Bao, J.; Dai, S.M. A Chinese herb Tripterygium wilfordii Hook F in the treatment of rheumatoid arthritis: Mechanism, efficacy, and safety. Rheumatol. Int 2011, 31, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Schulze-Koops, H.; Ma, L.; Cai, J.; Mao, Y.; Lipsky, P.E. Effects of Tripterygium wilfordii Hook F extracts on induction of cyclooxygenase 2 activity and prostaglandin E2 production. Arthritis Rheumatol. 1998, 41, 130–138. [Google Scholar] [CrossRef]
- Guo, W.; Ma, L.; Tao, X. In vitro inhibitive effects of Tripterygium wilforii on NO production, iNOS activity, and iNOS-mRNA expression in chondrocyrtes of patients with rheumatoid arthritis. Zhonghua Yi Xue Za Zhi 2001, 81, 1035–1037. [Google Scholar] [PubMed]
- Lin, N.; Sato, T.; Ito, A. Triptolide, a novel diterpenoid triepoxide from Tripterygium wilfordii Hook F, suppresses the production and gene expression of pro-matrix metalloproteinases 1 and 3 and augments those of tissue inhibitors of metalloproteinases 1 and 2 in human synovial fibroblasts. Arthritis Rheumatol. 2001, 44, 2193–2200. [Google Scholar]
- Sylvester, J.; Liacini, A.; Li, W.Q.; Dehnade, F.; Zafarullah, M. Tripterygium wilfordii Hook F extract suppresses proinflammatory cytokine-induced expression of matrix metalloproteinase genes in articular chondrocytes by inhibiting activating protein-1 and nuclear factor-κB activities. Mol. Pharmacol. 2001, 59, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Pandya, J.M.; Lundell, A.C.; Hallstrom, M.; Andersson, K.; Nordstrom, I.; Rudin, A. Circulating T helper and T regulatory subsets in untreated early rheumatoid arthritis and healthy control subjects. J. Leukoc. Biol. 2016, 100, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.A.; Kohlmeier, J.E.; Branden, M.; Jung, M.; Benedict, S.H. Triptolide is more effective in preventing T cell proliferation and interferon-γ production than is FK506. Phytother. Res. 1999, 13, 464–467. [Google Scholar] [CrossRef]
- Tong, K.K.; Yang, D.; Chan, E.Y.; Chiu, P.K.; Yau, K.S.; Lau, C.S. Downregulation of lymphocyte activity and human synovial fibroblast growth in rheumatoid arthritis by triptolide. Drug Dev. Res. 1999, 47, 144–153. [Google Scholar] [CrossRef]
- Li, X.W.; Weir, M.R. Radix Tripterygium wilfordii—A Chinese herbal medicine with potent immunosuppressive properties. Transplantation 1990, 50, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.J.; Chang, D.M.; Chang, M.L.; Kuo, S.Y.; Lai, J.H. Mechanism of immunosuppression of the antirheumatic herb TWHf in human T cells. J. Rheumatol. 1999, 26, 14–24. [Google Scholar] [PubMed]
- Jiang, M.; Zha, Q.; Zhang, C.; Lu, C.; Yan, X.; Zhu, W.; Liu, W.; Tu, S.; Hou, L.; Wang, C.; et al. Predicting and verifying outcome of Tripterygium wilfordii Hook F based therapy in rheumatoid arthritis: From open to double-blinded randomized trial. Sci. Rep. 2015, 5, 9700. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Tang, X.P.; Yuan, J.; Liu, X.; Liu, H.; Zhang, C.Y.; Wang, L.Y.; Jiang, Q. Effect of external applying compound Tripterygium wilfordii Hook F on joint pain of rheumatoid arthritis patients. Zhongguo Zhong Xi Yi Jie He Za Zhi 2016, 36, 29–34. [Google Scholar] [PubMed]
- Lv, Q.W.; Zhang, W.; Shi, Q.; Zheng, W.J.; Li, X.; Chen, H.; Wu, Q.J.; Jiang, W.L.; Li, H.B.; Gong, L.; et al. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): A randomised, controlled clinical trial. Ann. Rheum. Dis. 2015, 74, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Jiang, Q.; Feng, X.H.; Zhang, H.D.; Ge, L.; Luo, C.G.; Gong, X.; Li, B. Tripterygium wilfordii Hook F versus conventional synthetic disease-modifying anti-rheumatic drugs as monotherapy for rheumatoid arthritis: A systematic review and network meta-analysis. BMC Complement. Altern. Med. 2016, 16, 215. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, N.; Fang, L.; Feng, Z.; Li, G.; Mucelli, A.; Zhang, X.; Zhou, X. A systematic review about the efficacy and safety of Tripterygium wilfordii Hook F preparations used for the management of rheumatoid arthritis. Evid.-Based Complement. Altern. Med. 2018, 2018, 1567463. [Google Scholar]
- Wang, X.; Zu, Y.; Huang, L.; Yu, J.; Zhao, H.; Wen, C.; Chen, Z.; Xu, Z. Treatment of rheumatoid arthritis with combination of methotrexate and Tripterygium wilfordii: A meta-analysis. Life Sci. 2017, 171, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Xia, X.; Peng, W.K.; Wang, Q.H.; Peng, J.H.; Li, Y.L.; Wu, J.X.; Zhang, J.Y.; Zhao, Y.; Chen, X.M.; et al. The Effectiveness and Safety of Tripterygium wilfordii Hook F Extracts in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2018, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Goldbach-Mansky, R.; Wilson, M.; Fleischmann, R.; Olsen, N.; Silverfield, J.; Kempf, P.; Kivitz, A.; Sherrer, Y.; Pucino, F.; Csako, G.; et al. Comparison of Tripterygium wilfordii Hook F versus sulfasalazine in the treatment of rheumatoid arthritis: A randomized trial. Ann. Intern. Med. 2009, 151, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, T.; Chen, G.; Li, N.; Wang, J.; Ma, P.; Cao, X. Immunosuppressant triptolide inhibits dendritic cell-mediated chemoattraction of neutrophils and T cells through inhibiting Stat3 phosphorylation and NF-κB activation. Biochem. Biophys. Res. Commun. 2006, 345, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Z.; Tolosa, E.; Yang, J.; Li, L. Triptolide induces apoptotic death of T lymphocyte. Immunopharmacology 1998, 40, 139–149. [Google Scholar] [CrossRef]
- Chen, S.R.; Dai, Y.; Zhao, J.; Lin, L.; Wang, Y.; Wang, Y. A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front. Pharmacol. 2018, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, L.; Wu, H.; Pavlos, N.; Chim, S.M.; Liu, Q.; Zhao, J.; Xue, W.; Tan, R.X.; Ye, J.; et al. Triptolide inhibits osteoclast formation, bone resorption, RANKL-mediated NF-κB activation and titanium particle-induced osteolysis in a mouse model. Mol. Cell. Endocrinol. 2015, 399, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Huang, X.; Wang, D.; Li, M.; Liu, Z. Triptolide protects bone against destruction by targeting RANKL-mediated ERK/AKT signalling pathway in the collagen-induced rheumatoid arthritis. Biomed. Res. 2017, 28, 4111–4116. [Google Scholar]
- Li, Y.; Tian, Y.; Zhu, W.; Gong, J.; Zhang, W.; Yu, C.; Gu, L.; Li, N.; Li, J. Triptolide induces suppressor of cytokine signaling-3 expression and promotes lamina propria mononuclear cells apoptosis in Crohn’s colitis. Int. Immunopharmacol. 2013, 16, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, F.; Gao, W. Tripterygium wilfordii Inhibiting Angiogenesis for Rheumatoid Arthritis Treatment. J. Natl. Med. Assoc. 2017, 109, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Cascao, R.; Vidal, B.; Lopes, I.P.; Paisana, E.; Rino, J.; Moita, L.F.; Fonseca, J.E. Decrease of CD68 Synovial Macrophages in Celastrol Treated Arthritic Rats. PLoS ONE 2015, 10, e0142448. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Rajaiah, R.; Wu, Q.L.; Satpute, S.R.; Tan, M.T.; Simon, J.E.; Berman, B.M.; Moudgil, K.D. Green tea protects rats against autoimmune arthritis by modulating disease-related immune events. J. Nutr. 2008, 138, 2111–2116. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, G.; El-Beih, N.M.; Talaat, R.M.; Abd El-Ghffar, E.A. Anti-inflammatory activity of green versus black tea aqueous extract in a rat model of human rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Haqqi, T.M.; Anthony, D.D.; Gupta, S.; Ahmad, N.; Lee, M.S.; Kumar, G.K.; Mukhtar, H. Prevention of collagen-induced arthritis in mice by a polyphenolic fraction from green tea. Proc. Natl. Acad. Sci. USA 1999, 96, 4524–4529. [Google Scholar] [CrossRef] [PubMed]
- Bhutia Pemba, H.; Sharangi, A.B.; Lepcha, R.; Tamang, D. Bioactive Compounds and Antioxidant Properties of Tea: Status, Global Research and Potentialities. J. Tea Sci. Res. 2015, 5, 1–13. [Google Scholar]
- Ahmed, S.; Marotte, H.; Kwan, K.; Ruth, J.H.; Campbell, P.L.; Rabquer, B.J.; Pakozdi, A.; Koch, A.E. Epigallocatechin-3-gallate inhibits IL-6 synthesis and suppresses transsignaling by enhancing soluble gp130 production. Proc. Natl. Acad. Sci. USA 2008, 105, 14692–14697. [Google Scholar] [CrossRef] [PubMed]
- Fechtner, S.; Singh, A.; Chourasia, M.; Ahmed, S. Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1β signaling in rheumatoid arthritis synovial fibroblasts. Toxicol. Appl. Pharmacol. 2017, 329, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Umar, S.; Riegsecker, S.; Chourasia, M.; Ahmed, S. Regulation of transforming growth factor β-activated kinase activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts: suppression of K63-linked Autoubiquitination of tumor necrosis factor receptor-associated factor 6. Arthritis Rheumatol. 2016, 68, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Min, S.Y.; Yan, M.; Kim, S.B.; Ravikumar, S.; Kwon, S.R.; Vanarsa, K.; Kim, H.Y.; Davis, L.S.; Mohan, C. Green tea epigallocatechin-3-gallate suppresses autoimmune arthritis through indoleamine-2,3-dioxygenase expressing dendritic cells and the nuclear factor, erythroid 2-like 2 antioxidant pathway. J. Inflamm. 2015, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, Y.O.; Ryu, J.G.; Oh, H.J.; Son, H.J.; Lee, S.H.; Kwon, J.E.; Kim, E.K.; Park, M.K.; Park, S.H.; et al. Epigallocatechin-3-gallate ameliorates autoimmune arthritis by reciprocal regulation of T helper-17 regulatory T cells and inhibition of osteoclastogenesis by inhibiting STAT3 signaling. J. Leukoc. Biol. 2016, 100, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Oka, Y.; Iwai, S.; Amano, H.; Irie, Y.; Yatomi, K.; Ryu, K.; Yamada, S.; Inagaki, K.; Oguchi, K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 2012, 118, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Rambod, M.; Nazarinia, M.; Raieskarimian, F. The impact of dietary habits on the pathogenesis of rheumatoid arthritis: A case-control study. Clin. Rheumatol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kismali, G.; Aggarwal, B.B. Curcumin, a component of turmeric: From farm to pharmacy. Biofactors 2013, 39, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Saksena, A.K.; Khattri, S.; Kumar, S.; Dagur, R.S. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: A four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology 2016, 24, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Zdrojewicz, Z.; Szyca, M.; Popowicz, E.; Michalik, T.; Smieszniak, B. Turmeric—Not only spice. Polski Merkuriusz Lekarski 2017, 42, 227–230. [Google Scholar] [PubMed]
- Zheng, Z.; Sun, Y.; Liu, Z.; Zhang, M.; Li, C.; Cai, H. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 2015, 9, 4931–4942. [Google Scholar]
- Kumar, A.; Dhawan, S.; Hardegen, N.J.; Aggarwal, B.B. Curcumin (Diferuloylmethane) inhibition of tumor necrosis factor (TNF)-mediated adhesion of monocytes to endothelial cells by suppression of cell surface expression of adhesion molecules and of nuclear factor-κB activation. Biochem. Pharmacol. 1998, 55, 775–783. [Google Scholar] [CrossRef]
- Yeh, C.H.; Chen, T.P.; Wu, Y.C.; Lin, Y.M.; Jing Lin, P. Inhibition of NF-κB activation with curcumin attenuates plasma inflammatory cytokines surge and cardiomyocytic apoptosis following cardiac ischemia/reperfusion. J. Surg. Res. 2005, 125, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: An orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672–1692. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; John, T.; Schulze-Tanzil, G.; Lehmann, I.; Mobasheri, A. Suppression of NF-κB activation by curcumin leads to inhibition of expression of cyclo-oxygenase-2 and matrix metalloproteinase-9 in human articular chondrocytes: Implications for the treatment of osteoarthritis. Biochem. Pharmacol. 2007, 73, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.A.; Aznavoorian, S.; Engler, J.A.; Windsor, L.J. Induction of collagenase-3 (MMP-13) in rheumatoid arthritis synovial fibroblasts. Biochim. Biophys. Acta 2000, 1502, 307–318. [Google Scholar] [CrossRef]
- Xue, M.; McKelvey, K.; Shen, K.; Minhas, N.; March, L.; Park, S.Y.; Jackson, C.J. Endogenous MMP-9 and not MMP-2 promotes rheumatoid synovial fibroblast survival, inflammation and cartilage degradation. Rheumatology 2014, 53, 2270–2279. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.V. Regulation of COX and LOX by curcumin. Adv. Exp. Med. Biol. 2007, 595, 213–226. [Google Scholar] [PubMed]
- Kapil Kumar, A.K.R. Curcumin: A yellow magical spice of kitchen for treatment of rheumatoid arthritis. Int. Res. J. Pharm. 2011, 2, 29–31. [Google Scholar]
- Shang, W.; Zhao, L.J.; Dong, X.L.; Zhao, Z.M.; Li, J.; Zhang, B.B.; Cai, H. Curcumin inhibits osteoclastogenic potential in PBMCs from rheumatoid arthritis patients via the suppression of MAPK/RANK/c-Fos/NFATc1 signaling pathways. Mol. Med. Rep. 2016, 14, 3620–3626. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Coradini, K.; Friedrich, R.B.; Fonseca, F.N.; Vencato, M.S.; Andrade, D.F.; Oliveira, C.M.; Battistel, A.P.; Guterres, S.S.; da Rocha, M.I.; Pohlmann, A.R.; et al. A novel approach to arthritis treatment based on resveratrol and curcumin co-encapsulated in lipid-core nanocapsules: In vivo studies. Eur. J. Pharm. Sci. 2015, 78, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Xuzhu, G.; Komai-Koma, M.; Leung, B.P.; Howe, H.S.; McSharry, C.; McInnes, I.B.; Xu, D. Resveratrol modulates murine collagen-induced arthritis by inhibiting Th17 and B-cell function. Ann. Rheum. Dis. 2012, 71, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Koca, S.S.; Yolbas, S.; Yildirim, A.; Celik, Z.B.; Onalan, E.E.; Akin, M. AB0108 resveratrol inhibits canonical wnt signaling and ameliorates experimental arthritis. Ann. Rheum. Dis. 2016, 75, 933. [Google Scholar] [CrossRef]
- Cheon, Y.H.; Kim, H.O.; Suh, Y.S.; Hur, J.H.; Jo, W.; Lim, H.S.; Hah, Y.S.; Sung, M.J.; Kwon, D.Y.; Lee, S.I. Inhibitory effects for rheumatoid arthritis of dietary supplementation with resveratrol in collagen-induced arthritis. J. Rheum. Dis. 2015, 22, 93–101. [Google Scholar] [CrossRef]
- Miao, C.G.; Yang, Y.Y.; He, X.; Li, X.F.; Huang, C.; Huang, Y.; Zhang, L.; Lv, X.W.; Jin, Y.; Li, J. Wnt signaling pathway in rheumatoid arthritis, with special emphasis on the different roles in synovial inflammation and bone remodeling. Cell. Signal. 2013, 25, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Elmali, N.; Baysal, O.; Harma, A.; Esenkaya, I.; Mizrak, B. Effects of resveratrol in inflammatory arthritis. Inflammation 2007, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.; Savouret, J.F.; Widerak, M.; Corvol, M.T.; Rannou, F. Resveratrol, potential therapeutic interest in joint disorders: A critical narrative review. Nutrients 2017, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.H.; Nakahama, T.; Dang, T.T.; Chu, H.H.; Van Hoang, L.; Kishimoto, T.; Nguyen, N.T. Expression of aryl hydrocarbon receptor, inflammatory cytokines, and incidence of rheumatoid arthritis in Vietnamese dioxin-exposed people. J. Immunotoxicol. 2017, 14, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Nakahama, T.; Nguyen, C.H.; Tran, T.T.; Le, V.S.; Chu, H.H.; Kishimoto, T. Aryl hydrocarbon receptor antagonism and its role in rheumatoid arthritis. J. Exp. Pharmacol. 2015, 7, 29–35. [Google Scholar] [PubMed]
- Ogando, J.; Tardáguila, M.; Díaz-Alderete, A.; Usategui, A.; Miranda-Ramos, V.; Martínez-Herrera, D.J.; de la Fuente, L.; García-León, M.J.; Moreno, M.C.; Escudero, S.; et al. Notch-regulated miR-223 targets the aryl hydrocarbon receptor pathway and increases cytokine production in macrophages from rheumatoid arthritis patients. Sci. Rep. 2016, 6, 20223. [Google Scholar] [CrossRef] [PubMed]
- Almonte-Becerril, M.; Fernandez-Rodriguez, J.A.; Ramil-Gómez, O.; Riveiro-Naveira, R.R.; Hermida-Carballo, L.; Blanco, F.J.; Lopez-Armada, M.J. Resveratrol attenuates synovial hyperplasia in an acute antigen-induced arthritis model by augmenting autophagy and decreasing angiogenesis. Osteoarthr. Cartil. 2017, 25, S90–S91. [Google Scholar] [CrossRef]
- Abdel-Tawab, M.; Werz, O.; Schubert-Zsilavecz, M. Boswellia serrata: An overall assessment of in vitro, preclinical, pharmacokinetic and clinical data. Clin. Pharmacokinet. 2011, 50, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Ammon, H.P. Modulation of the immune system by Boswellia serrata extracts and boswellic acids. Phytomedicine 2010, 17, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, S.; Conradt, C.; Roufogalis, B.D. Effectiveness of Harpagophytum extracts and clinical efficacy. Phytother. Res. 2004, 18, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.; McBean, D.E.; Fyfe, L.; Warnock, A.M. A review of the biological and potential therapeutic actions of Harpagophytum procumbens. Phytother. Res. 2007, 21, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Chrubasik, C.; Roufogalis, B.D.; Muller-Ladner, U.; Chrubasik, S. A systematic review on the Rosa canina effect and efficacy profiles. Phytother. Res. 2008, 22, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Saaby, L.; Jager, A.K.; Moesby, L.; Hansen, E.W.; Christensen, S.B. Isolation of immunomodulatory triterpene acids from a standardized rose hip powder (Rosa canina L.). Phytother. Res. 2011, 25, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Erowele, G.I.; Kalejaiye, A.O. Pharmacology and therapeutic uses of cat’s claw. Am. J. Health Syst. Pharm. 2009, 66, 992–995. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, C.C.; O’Mathuna, D.P.; Chavez, M.; Shields, K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern. Ther. Health Med. 2010, 16, 32–40. [Google Scholar] [PubMed]
- Di Lorenzo, C.; Dell’Agli, M.; Badea, M.; Dima, L.; Colombo, E.; Sangiovanni, E.; Restani, P.; Bosisio, E. Plant food supplements with anti-inflammatory properties: A systematic review (II). Crit. Rev. Food Sci. Nutr. 2013, 53, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Randall, C.; Randall, H.; Dobbs, F.; Hutton, C.; Sanders, H. Randomized controlled trial of nettle sting for treatment of base-of-thumb pain. J. R. Soc. Med. 2000, 93, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Lakhan, S.E.; Ford, C.T.; Tepper, D. Zingiberaceae extracts for pain: A systematic review and meta-analysis. Nutr. J. 2015, 14, 50. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.J.; Lin, Y.; Huang, S.S.; Zhang, H.L.; Diao, Y.P.; Li, K. Quercetin: A potential natural drug for adjuvant treatment of rheumatoid arthritis. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 418–421. [Google Scholar] [PubMed]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The flavonoid quercetin in disease prevention and therapy: Facts and fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Kaina, B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Curr. Drug Metab. 2011, 12, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Byard, R.W. A review of the potential forensic significance of traditional herbal medicines. J. Forensic. Sci. 2010, 55, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Chen, Y.; Huang, J.Q.; Li, K.M.; Razmovski-Naumovski, V.; Poon, J.; Chan, K.; Roufogalis, B.D.; McLachlan, A.J.; Mo, S.L.; et al. Evidence-based toxicity evaluation and scheduling of Chinese herbal medicines. J. Ethnopharmacol. 2013, 146, 40–61. [Google Scholar] [CrossRef] [PubMed]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055–6074. [Google Scholar]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine 2012, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, B.V.; Silva, P.B.; Ramos, M.A.; Negri, K.M.; Bauab, T.M.; Chorilli, M. Nanotechnology-based drug delivery systems and herbal medicines: A review. Int. J. Nanomed. 2014, 9, 1–15. [Google Scholar]
- Obeid, M.A.; Al Qaraghuli, M.M.; Alsaadi, M.; Alzahrani, A.R.; Niwasabutra, K.; Ferro, V.A. Delivering natural products and biotherapeutics to improve drug efficacy. Ther. Deliv. 2017, 8, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.S.; Fenniri, H.; Singh, B. Nanotechnology-based drug delivery systems. J. Occup. Med. Toxicol. 2007, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J. Nanomater. 2016, 2016, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Pishko, M.V. Nanoparticle-based biocompatible and targeted drug delivery: Characterization and in vitro studies. Biomacromolecules 2011, 12, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Heeralal, B.; Swami, R.; Swarnakar, N.K.; Kushwah, V. Improved oral bioavailability, therapeutic efficacy, and reduced toxicity of tamoxifen-loaded liquid crystalline nanoparticles. AAPS PharmSciTech 2018, 19, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Spandana, G.; Agrawal, A.K.; Kushwah, V.; Thanki, K. Enhanced Antitumor Efficacy and Reduced Toxicity of Docetaxel Loaded Estradiol Functionalized Stealth Polymeric Nanoparticles. Mol. Pharm. 2015, 12, 3871–3884. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.L.; Fattahi, P.; Brown, J.L. Polymeric nanoparticles: The future of nanomedicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016, 8, 271–299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, T.; Li, Q.; Huang, J.; Xu, H.; Li, J.; Wang, Y.; Liang, Q. Fabrication of novel vesicles of triptolide for antirheumatoid activity with reduced toxicity in vitro and in vivo. Int. J. Nanomed. 2016, 11, 2663–2673. [Google Scholar]
- Zhang, L.; Chang, J.; Zhao, Y.; Xu, H.; Wang, T.; Li, Q.; Xing, L.; Huang, J.; Wang, Y.; Liang, Q. Fabrication of a triptolide-loaded and poly-γ-glutamic acid-based amphiphilic nanoparticle for the treatment of rheumatoid arthritis. Int. J. Nanomed. 2018, 13, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Hao, B.; Ju, D.; Liu, M.; Zhao, H.; Du, Z.; Xia, J. Pharmacokinetic and pharmacodynamic study of triptolide-loaded liposome hydrogel patch under microneedles on rats with collagen-induced arthritis. Acta Pharm. Sin. B 2015, 5, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Rajaiah, R.; Ruoslahti, E.; Moudgil, K.D. Peptides targeting inflamed synovial vasculature attenuate autoimmune arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 12857–12862. [Google Scholar] [CrossRef] [PubMed]
- Meka, R.; Venkatesha, S.; Moudgil, K.D. Peptide-directed liposomal delivery improves the therapeutic index of an immunomodulatory cytokine in controlling autoimmune arthritis. J. Control. Release 2018, 286, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Vadlapudi, A.D.; Mitra, A.K. Nanomicelles: An emerging platform for drug delivery to the eye. Ther. Deliv. 2013, 4, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, R.; Kompella, U.B. Nanomicellar formulations for sustained drug delivery: Strategies and underlying principles. Nanomedicine 2010, 5, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, J.; Liu, J.; Jiao, H.; Liu, B. Anti-inflammation and joint lubrication dual effects of a novel hyaluronic acid/curcumin nanomicelle improve the efficacy of rheumatoid arthritis therapy. ACS Appl. Mater. Interfaces 2018. [Google Scholar] [CrossRef] [PubMed]
- Venturini, C.G.; Jäger, E.; Oliveira, C.P.; Bernardi, A.; Battastini, A.M.; Guterres, S.S.; Pohlmann, A.R. Formulation of lipid core nanocapsules. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 200–208. [Google Scholar] [CrossRef]
- Frank, L.A.; Contri, R.V.; Beck, R.C.; Pohlmann, A.R.; Guterres, S.S. Improving drug biological effects by encapsulation into polymeric nanocapsules. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 2011, 19, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Fang, S.; Yang, H.; Gao, J.; He, M.; Ke, S.; Zhao, Y.; Chen, C.; Huang, L. Evaluating the contribution of gut microbiome to the variance of porcine serum glucose and lipid concentration. Sci. Rep. 2017, 7, 14928. [Google Scholar] [CrossRef] [PubMed]
- Riedl, R.A.; Atkinson, S.N.; Burnett, C.M.L.; Grobe, J.L.; Kirby, J.R. The Gut Microbiome, Energy Homeostasis, and Implications for Hypertension. Curr. Hypertens. Rep. 2017, 19, 27. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wright, K.; Davis, J.M.; Jeraldo, P.; Marietta, E.V.; Murray, J.; Nelson, H.; Matteson, E.L.; Taneja, V. An expansion of rare lineage intestinal microbes characterizes rheumatoid arthritis. Genome Med. 2016, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Haase, S.; Haghikia, A.; Wilck, N.; Muller, D.N.; Linker, R.A. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology 2018, 154, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Noto, D.; Kaga, N.; Chiba, A.; Miyake, S. The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS ONE 2017, 12, e0173032. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S.; Omata, Y.; Hofmann, J.; Bottcher, M.; Iljazovic, A.; Sarter, K.; Albrecht, O.; Schulz, O.; Krishnacoumar, B.; Kronke, G.; et al. Short-chain fatty acids regulate systemic bone mass and protect from pathological bone loss. Nat. Commun. 2018, 9, 55. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xia, S.; Gao, F.; Zhang, D.; Chen, J.; Zhang, J. 3,3′-Diindolylmethane attenuates experimental arthritis and osteoclastogenesis. Biochem. Pharmacol. 2010, 79, 715–721. [Google Scholar] [CrossRef] [PubMed]


| Plant Name | Bioactive Compounds | Mediators of Inflammation Targeted by the Natural Product | References |
|---|---|---|---|
| Boswellia serrata | Boswellic acids | Proinflammatory cytokines, 5-LOX | [148,149] |
| Harpagophytum procumbens | Harpagoside, Harpagide | COX-2, MAPK, NF-κB, NO | [150,151] |
| Rosa canina | Carotenoids, organic acids | COX-1, COX-2 | [152,153] |
| Uncaria tomentosa | Mitraphylline | Pro-inflammatory cytokines, anti-oxidant, NF-κB | [154,155] |
| Urtica dioica | Flavonoid glycosides, terpenoids | NF-κB, PLA2 | [156,157] |
| Zingiber officinale | Zingerone, Gingerol | COX-2, NF-κB | [158,159] |
| Several grains, vegetables, fruits | Quercetin | MAPK, NF-κB, PI3K/Akt, JAK3 | [160,161] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudics, S.; Langan, D.; Meka, R.R.; Venkatesha, S.H.; Berman, B.M.; Che, C.-T.; Moudgil, K.D. Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome. Int. J. Mol. Sci. 2018, 19, 2508. https://doi.org/10.3390/ijms19092508
Dudics S, Langan D, Meka RR, Venkatesha SH, Berman BM, Che C-T, Moudgil KD. Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome. International Journal of Molecular Sciences. 2018; 19(9):2508. https://doi.org/10.3390/ijms19092508
Chicago/Turabian StyleDudics, Steven, David Langan, Rakeshchandra R. Meka, Shivaprasad H. Venkatesha, Brian M. Berman, Chun-Tao Che, and Kamal D. Moudgil. 2018. "Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome" International Journal of Molecular Sciences 19, no. 9: 2508. https://doi.org/10.3390/ijms19092508
APA StyleDudics, S., Langan, D., Meka, R. R., Venkatesha, S. H., Berman, B. M., Che, C.-T., & Moudgil, K. D. (2018). Natural Products for the Treatment of Autoimmune Arthritis: Their Mechanisms of Action, Targeted Delivery, and Interplay with the Host Microbiome. International Journal of Molecular Sciences, 19(9), 2508. https://doi.org/10.3390/ijms19092508