Organic Bioelectronics: Materials and Biocompatibility
Abstract
:1. Introduction
2. Biocompatibility
- -
- Toxic: biomaterial has adverse effects on surrounding tissue e.g., cell death, immunological response, organ failure and inflammation.
- -
- Bioinert: non-toxic, biologically inactive. The material has no or minimal interaction with the living host. However, an adverse response may still occur as fibrous tissue may encapsulate the device, thus loosening and then severing the interface of the device with target cells resulting in device failure.
- -
- Bioactive: material is non-toxic and biologically active. The device forms an intimate connection with the host tissue.
- -
- Bioresorbable: non-toxic material dissolves in the host tissue. The bio-electronic device only functions temporarily. The surrounding host tissue can eventually replace the synthetic device.
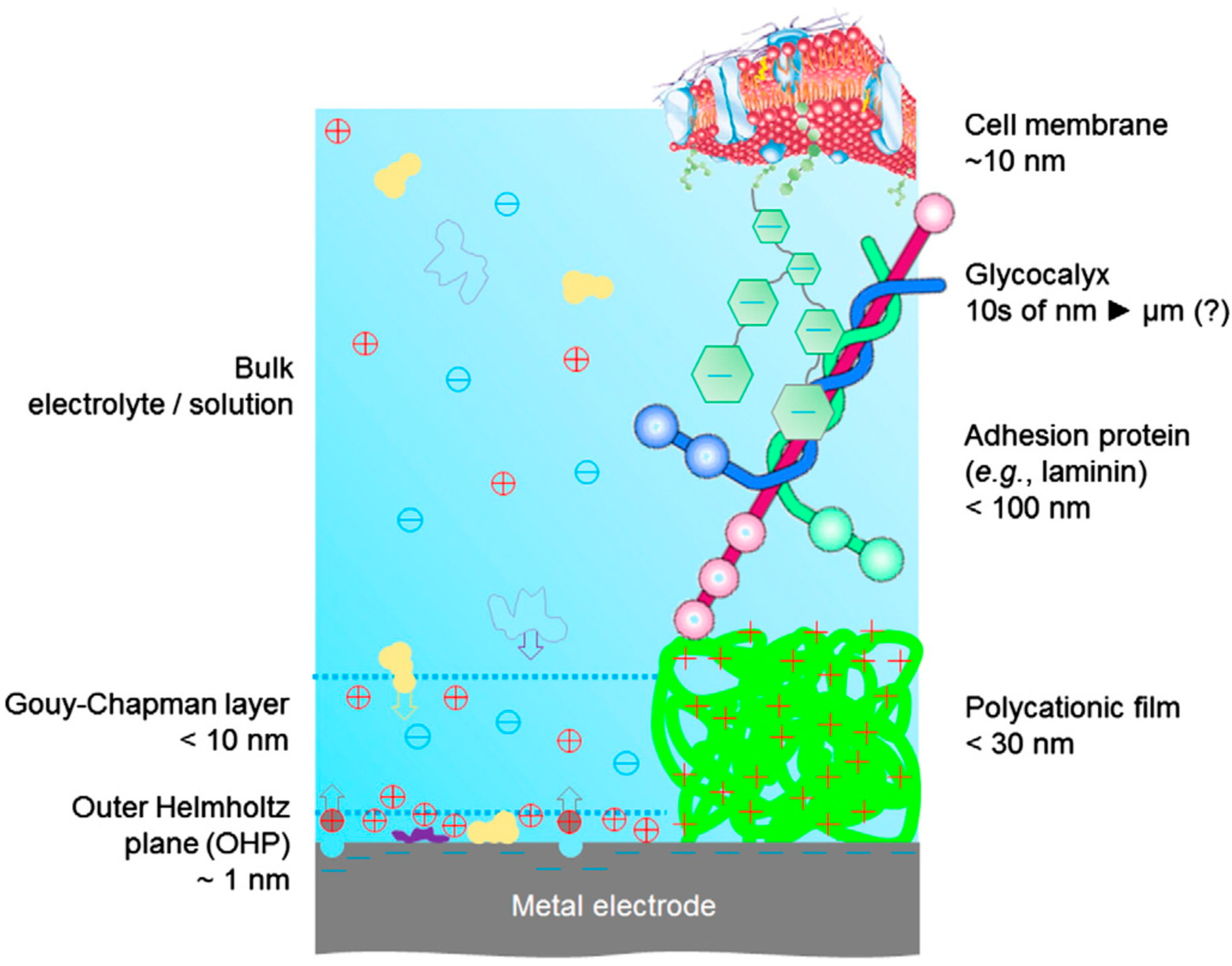
3. Cell Adhesion
4. Organic Semiconductors for Bioelectronic Application
4.1. Materials for Electroactive Scaffolds
4.2. Materials for Neural Interface Electrodes
4.3. Materials for Photostimulation
4.4. Materials for Nerve Growth and Guidance
4.5. Materials for Drug Delivery
4.6. Materials for Biosensing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gimsa, J.; Habel, B.; Schreiber, U.; Van Rienen, U.; Strauss, U.; Gimsa, U. Choosing electrodes for deep brain stimulation experiments–electrochemical considerations. J. Neurosci. Methods 2005, 142, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, P.; Yang, G.; Kim, G.; Abidian, M.R. A review of organic and inorganic biomaterials for neural interfaces. Adv. Mater. 2014, 26, 1846–1885. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.S.; Dorman, M.F. Cochlear implants: A remarkable past and a brilliant future. Hear. Res. 2008, 242, 3–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hageman, K.N.; Kalayjian, Z.K.; Tejada, F.; Chiang, B.; Rahman, M.A.; Fridman, G.Y.; Dai, C.; Pouliquen, P.O.; Georgiou, J.; Della Santina, C.C.; et al. A CMOS Neural Interface for a Multichannel Vestibular Prosthesis. IEEE Trans. Biomed. Circuits Syst. 2016, 10, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Weiland, J.D.; Roska, B.; Humayun, M.S. Retinal stimulation strategies to restore vision: Fundamentals and systems. Prog. Retin. Eye Res. 2016, 53, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Velliste, M.; Perel, S.; Spalding, M.C.; Whitford, A.S.; Schwartz, A.B. Cortical control of a prosthetic arm for self-feeding. Nature 2008, 453, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Asplund, M.; Nyberg, T.; Inganäs, O. Electroactive polymers for neural interfaces. Polym. Chem. 2010, 1, 1374–1391. [Google Scholar] [CrossRef]
- Someya, T.; Bao, Z.; Malliaras, G.G. The rise of plastic bioelectronics. Nature 2016, 540, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Löffler, S.; Libberton, B.; Richter-Dahlfors, A. Organic Bioelectronic Tools for Biomedical Applications. Electronics 2015, 4, 879–908. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.C. Molecular design, synthesis, and characterization of conjugated polymers for interfacing electronic biomedical devices with living tissue. MRS Commun. 2015, 5, 131–153. [Google Scholar] [CrossRef]
- Rivnay, J.; Owens, R.M.; Malliaras, G.G. The rise of organic bioelectronics. Chem. Mater. 2014, 26, 679–685. [Google Scholar] [CrossRef]
- Voskerician, G.; Shive, M.S.; Shawgo, R.S.; Von Recum, H.; Anderson, J.M.; Cima, M.J.; Langer, R. Biocompatibility and biofouling of MEMS drug delivery devices. Biomaterials 2003, 24, 1959–1967. [Google Scholar] [CrossRef]
- Ghezzi, D.; Antognazza, M.R.; Maccarone, R.; Bellani, S.; Lanzarini, E.; Martino, N.; Mete, M.; Pertile, G.; Bisti, S.; Lanzani, G.; et al. A polymer optoelectronic interface restores light sensitivity in blind rat retinas. Nat. Photonics 2013, 7, 400–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghezzi, D.; Antognazza, M.R.; Dal Maschio, M.; Lanzarini, E.; Benfenati, F.; Lanzani, G. A hybrid bioorganic interface for neuronal photoactivation. Nat. Commun. 2011, 2, 166. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.R.; Wallace, G.G. Organic Electrodes and Communications with Excitable Cells. Adv. Funct. Mater. 2018, 28, 1700587. [Google Scholar] [CrossRef]
- Rivnay, J.; Inal, S.; Collins, B.A.; Sessolo, M.; Stavrinidou, E.; Strakosas, X.; Tassone, C.; Delongchamp, D.M.; Malliaras, G.G. Structural control of mixed ionic and electronic transport in conducting polymers. Nat. Commun. 2016, 7, 11287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.; Zhang, M.; Yao, M.Y.; Hua, T.; Li, L.; Yan, F. Flexible Organic Electronics in Biology: Materials and Devices. Adv. Mater. 2015, 27, 7493–7527. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Lovell, N.H.; Wallace, G.G.; Poole-Warren, L.A. Conducting polymers for neural interfaces: Challenges in developing an effective long-term implant. Biomaterials 2008, 29, 3393–3399. [Google Scholar] [CrossRef] [PubMed]
- Spector, M. 4.401—The Concept of Biocompatibility; Elsevier Ltd.: New York, NY, USA, 2011; ISBN 978-0-08-055294-1. [Google Scholar]
- Gad, S.C. Safety Evaluation of Pharmaceuticals and Medical Devices; Springer: Boston, MA, USA, 2011; ISBN 978-1-44-197448-8. [Google Scholar]
- Shi, D. (Ed.) Introduction to Biomaterials; World Scientific Publishing Co.: Singapore; Pte. Ltd.: Singapore; Tsinghua University Press: Beijing, China, 2005; ISBN 978-9-81-270085-8. [Google Scholar]
- Gautam, V.; Rand, D.; Hanein, Y.; Narayan, K.S. A Polymer Optoelectronic Interface Provides Visual Cues to a Blind Retina. Adv. Mater. 2014, 26, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Kung, T.A.; Langhals, N.B.; Martin, D.C.; Johnson, P.J.; Cederna, P.S.; Urbanchek, M.G. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast. Reconstr. Surg. 2014, 133, 1380–1394. [Google Scholar] [CrossRef] [PubMed]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Szarowski, D.H.; Andersen, M.D.; Retterer, S.; Spence, A.J.; Isaacson, M.; Craighead, H.G.; Turner, J.N.; Shain, W. Brain responses to micro-machined silicon devices. Brain Res. 2003, 983, 23–35. [Google Scholar] [CrossRef]
- Turner, J.N.; Shain, W.; Szarowski, D.H.; Andersen, M.; Martins, S.; Isaacson, M.; Craighead, H. Cerebral astrocyte response to micromachined silicon implants. Exp. Neurol. 1999, 156, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Edell, D.J.; Van Toi, V.; McNeil, V.M.; Clark, L.D. Factors Influencing the Biocompatibility of Insertable Silicon Microshafts in Cerebral Cortex. IEEE Trans. Biomed. Eng. 1992, 39, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Garipcan, B.; Odabas, S.; Demirel, G.; Burger, J.; Nonnenmann, S.S.; Coster, M.T.; Gallo, E.M.; Nabet, B.; Spanier, J.E.; Piskin, E. In Vitro Biocompatibility of n-Type and Undoped Silicon Nanowires. Adv. Eng. Mater. 2011, 13, B3–B9. [Google Scholar] [CrossRef]
- Wang, A.; Finlayson, P.G.; Li, J.; Brabant, K.; Black, C.A.; McAllister, J.P., II; Cao, T.; Tang, H.; Liang, X.; Salley, S.O.; et al. Is silicon suitable for making implantable biomedical devices. In Proceedings of the 2005 AIChE Annual Meeting, Cincinnati, OH, USA, 30 October–4 November 2005; pp. 5182–5183. [Google Scholar]
- Keong, L.C.; Halim, A.S. In Vitro Models in Biocompatibility Assessment for Biomedical-Grade Chitosan Derivatives in Wound Management. Int. J. Mol. Sci. 2009, 10, 1300–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M. Biocompatibility and the Relationship to Standards: Meaning and Scope of Biomaterials Testing; Elsevier Ltd.: New York, NY, USA, 2011; Volume 4, ISBN 978-0-08-055294-1. [Google Scholar]
- Li, N.; Zhang, X.; Song, Q.; Su, R.; Zhang, Q.; Kong, T.; Liu, L.; Jin, G.; Tang, M.; Cheng, G. The promotion of neurite sprouting and outgrowth of mouse hippocampal cells in culture by graphene substrates. Biomaterials 2011, 32, 9374–9382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzatenta, A.; Giugliano, M.; Campidelli, S.; Gambazzi, L.; Businaro, L.; Markram, H.; Prato, M.; Ballerini, L. Interfacing Neurons with Carbon Nanotubes: Electrical Signal Transfer and Synaptic Stimulation in Cultured Brain Circuits. J. Neurosci. 2007, 27, 6931–6936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blau, A. Cell adhesion promotion strategies for signal transduction enhancement in microelectrode array in vitro electrophysiology: An introductory overview and critical discussion. Curr. Opin. Colloid Interface Sci. 2013, 18, 481–492. [Google Scholar] [CrossRef]
- Cui, X.; Lee, V.A.; Raphael, Y.; Wiler, J.A.; Hetke, J.F.; Anderson, D.J.; Martin, D.C. Surface modification of neural recording electrodes with conducting polymer/biomolecule blends. J. Biomed. Mater. Res. 2001, 56, 261–272. [Google Scholar] [CrossRef]
- Bonetti, S.; Pistone, A.; Brucale, M.; Karges, S.; Favaretto, L.; Zambianchi, M.; Posati, T.; Sagnella, A.; Caprini, M.; Toffanin, S.; et al. A Lysinated Thiophene-Based Semiconductor as a Multifunctional Neural Bioorganic Interface. Adv. Healthc. Mater. 2015, 4, 1190–1202. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, G.; Idzko, A.L.; Götz, S.; Thalhammer, S. Biocompatibility Studies of Functionalized Regioregular Poly(3-hexylthiophene) Layers for Sensing Applications. Macromol. Biosci. 2010, 10, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Doublet, T.; Quilichini, P.; Gurfinkel, M.; Leleux, P.; Ghestem, A.; Ismailova, E.; Hervé, T.; Sanaur, S.; Bernard, C.; et al. In vivo recordings of brain activity using organic transistors. Nat. Commun. 2013, 4, 1575. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.A.; Uram, J.D.; Yang, J.; Martin, D.C.; Kipke, D.R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 2006, 3, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Gerwig, R.; Fuchsberger, K.; Schroeppel, B.; Link, G.S.; Heusel, G.; Kraushaar, U.; Schuhmann, W.; Stett, A.; Stelzle, M. PEDOT–CNT Composite Microelectrodes for Recording and Electrostimulation Applications: Fabrication, Morphology, and Electrical Properties. Front. Neuroeng. 2012, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Lensen, M.C.; Schulte, V.A.; Salber, J.; Diez, M.; Menges, F.; Möller, M. Cellular responses to novel, micropatterned biomaterials. Pure Appl. Chem. 2008, 80, 2479–2487. [Google Scholar] [CrossRef]
- Abdullaeva, O.S.; Schulz, M.; Balzer, F.; Parisi, J.; Lützen, A.; Dedek, K.; Schiek, M. Photoelectrical Stimulation of Neuronal Cells by an Organic Semiconductor-Electrolyte Interface. Langmuir 2016, 32, 8533–8542. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Tian, Y.; Jiang, L. Bio-inspired superoleophobic and smart materials: Design, fabrication, and application. Prog. Mater. Sci. 2013, 58, 503–564. [Google Scholar] [CrossRef]
- Zhu, B.; Luo, S.C.; Zhao, H.; Lin, H.A.; Sekine, J.; Nakao, A.; Chen, C.; Yamashita, Y.; Yu, H.H. Large enhancement in neurite outgrowth on a cell membrane-mimicking conducting polymer. Nat. Commun. 2014, 5, 4523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodagholy, D.; Gelinas, J.N.; Thesen, T.; Doyle, W.; Devinsky, O.; Malliaras, G.G.; Buzsáki, G. NeuroGrid: Recording action potentials from the surface of the brain. Nat. Neurosci. 2015, 18, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.T.; Kurup, S.; Larsson, K.C.; Hori, R.; Tybrandt, K.; Goiny, M.; Jager, E.W.H.; Berggren, M.; Canlon, B.; Richter-Dahlfors, A. Organic electronics for precise delivery of neurotransmitters to modulate mammalian sensory function. Nat. Mater. 2009, 8, 742–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantione, D.; del Agua, I.; Sanchez-Sanchez, A.; Mecerreyes, D. Poly(3,4-ethylenedioxythiophene) (PEDOT) derivatives: Innovative conductive polymers for bioelectronics. Polymers 2017, 9, 354. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson-Burns, S.M.; Hendricks, J.L.; Foster, B.; Povlich, L.K.; Kim, D.H.; Martin, D.C. Polymerization of the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) around living neural cells. Biomaterials 2007, 28, 1539–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benfenati, V.; Toffanin, S.; Bonetti, S.; Turatti, G.; Pistone, A.; Chiappalone, M.; Sagnella, A.; Stefani, A.; Generali, G.; Ruani, G.; et al. A transparent organic transistor structure for bidirectional stimulation and recording of primary neurons. Nat. Mater. 2013, 12, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, H.; Kim, I.J.; Kim, J.R.; Lee, J.I.; Ree, M. Bacterial adhesion, cell adhesion and biocompatibility of nafion films. J. Biomater. Sci. Polym. Ed. 2009, 20, 1687–1707. [Google Scholar] [CrossRef] [PubMed]
- Bystrenova, E.; Jelitai, M.; Tonazzini, I.; Lazar, A.N.; Huth, M.; Stoliar, P.; Dionigi, C.; Cacace, M.G.; Nickel, B.; Madarasz, E.; et al. Neural Networks Grown on Organic Semiconductors. Adv. Funct. Mater. 2008, 18, 1751–1756. [Google Scholar] [CrossRef]
- Kamalesh, S.; Tan, P.; Wang, J.; Lee, T.; Kang, E.T.; Wang, C.H. Biocompatibility of electroactive polymers in tissues. J. Biomed. Mater. Res. 2000, 52, 467–478. [Google Scholar] [CrossRef]
- Zaquen, N.; Lu, H.; Chang, T.; Mamdooh, R.; Lutsen, L.; Vanderzande, D.; Stenzel, M.; Junkers, T. Profluorescent PPV-Based Micellar System as a Versatile Probe for Bioimaging and Drug Delivery. Biomacromolecules 2016, 17, 4086–4094. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.; Zaquen, N.; D’Olieslaeger, L.; Bové, H.; Vanderzande, D.; Hellings, N.; Junkers, T.; Ethirajan, A. PPV-Based Conjugated Polymer Nanoparticles as a Versatile Bioimaging Probe: A Closer Look at the Inherent Optical Properties and Nanoparticle-Cell Interactions. Biomacromolecules 2016, 17, 2562–2571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Huang, L.; Ito, Y.; Wang, Z.; Shi, X.; Wei, Y.; Jing, X.; Zhang, P. Intracellular calcium ions and morphological changes of cardiac myoblasts response to an intelligent biodegradable conducting copolymer. Mater. Sci. Eng. C 2018, 90, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Rivers, T.J.; Hudson, T.W.; Schmidt, C.E. Synthesis of a Novel, Biodegradable Electrically Conducting Polymer for Biomedical Applications. Adv. Funct. Mater. 2002, 12, 33–37. [Google Scholar] [CrossRef]
- Knight, E.; Przyborski, S. Advances in 3D cell culture technologies enabling tissue-like structures to be created in vitro. J. Anat. 2015, 227, 746–756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunatillake, P.A.; Adhikari, R.; Gadegaard, N. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Eisenbarth, E. Biomaterials for tissue engineering. Adv. Eng. Mater. 2007, 9, 1051–1060. [Google Scholar] [CrossRef]
- Inal, S.; Hama, A.; Ferro, M.; Pitsalidis, C.; Oziat, J.; Iandolo, D.; Pappa, A.-M.; Hadida, M.; Huerta, M.; Marchat, D.; et al. Conducting Polymer Scaffolds for Hosting and Monitoring 3D Cell Culture. Adv. Biosyst. 2017, 1, 1700052. [Google Scholar] [CrossRef]
- Wan, A.M.-D.; Inal, S.; Williams, T.; Wang, K.; Leleux, P.; Estevez, L.; Giannelis, E.P.; Fischbach, C.; Malliaras, G.G.; Gourdon, D. 3D conducting polymer platforms for electrical control of protein conformation and cellular functions. J. Mater. Chem. B 2015, 3, 5040–5048. [Google Scholar] [CrossRef] [PubMed]
- Mawad, D.; Lauto, A.; Wallace, G.G. Polymeric Hydrogels as Smart Biomaterials; Kalia, S., Ed.; Springer Series on Polymer and Composite Materials; Springer: Cham, Switzerland, 2016; ISBN 978-3-31-925320-6. [Google Scholar]
- Javadi, M.; Gu, Q.; Naficy, S.; Farajikhah, S.; Crook, J.M.; Wallace, G.G.; Beirne, S.; Moulton, S.E. Conductive Tough Hydrogel for Bioapplications. Macromol. Biosci. 2017, 18, 1700270. [Google Scholar] [CrossRef] [PubMed]
- Cheong, G.L.M.; Lim, K.S.; Jakubowicz, A.; Martens, P.J.; Poole-Warren, L.A.; Green, R.A. Conductive hydrogels with tailored bioactivity for implantable electrode coatings. Acta Biomater. 2014, 10, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Goding, J.; Gilmour, A.; Martens, P.; Poole-Warren, L.; Green, R. Interpenetrating Conducting Hydrogel Materials for Neural Interfacing Electrodes. Adv. Healthc. Mater. 2017, 6, 1601177. [Google Scholar] [CrossRef] [PubMed]
- Mihic, A.; Cui, Z.; Wu, J.; Vlacic, G.; Miyagi, Y.; Li, S.H.; Lu, S.; Sung, H.W.; Weisel, R.D.; Li, R.K. A conductive polymer hydrogel supports cell electrical signaling and improves cardiac function after implantation into myocardial infarct. Circulation 2015, 132, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Goding, J.; Gilmour, A.; Robles, U.A.; Poole-Warren, L.; Lovell, N.; Martens, P.; Green, R. A living electrode construct for incorporation of cells into bionic devices. MRS Commun. 2017, 7, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Glavas, L.; Albertsson, A.C. Biodegradable and electrically conducting polymers for biomedical applications. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Henze, D.A.; Borhegyi, Z.; Csicsvari, J.; Mamiya, A.; Harris, K.D.; Buzsáki, G. Intracellular features predicted by extracellular recordings in the hippocampus in vivo. J. Neurophysiol. 2000, 84, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G. Large-scale recording of neuronal ensembles. Nat. Neurosci. 2004, 7, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ma, M.; Zhang, N.; Langer, R.; Anderson, D.G. Stretchable polymeric multielectrode array for conformal neural interfacing. Adv. Mater. 2014, 26, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.K.; Kundu, S.C.; Yadavalli, V.K. Biosensing using photolithographically micropatterned electrodes of PEDOT:PSS on ITO substrates. Sens. Actuators B Chem. 2017, 242, 140–147. [Google Scholar] [CrossRef]
- Khodagholy, D.; Doublet, T.; Gurfinkel, M.; Quilichini, P.; Ismailova, E.; Leleux, P.; Herve, T.; Sanaur, S.; Bernard, C.; Malliaras, G.G. Highly conformable conducting polymer electrodes for in vivo recordings. Adv. Mater. 2011, 23, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Strakosas, X.; Wei, B.; Martin, D.C.; Owens, R.M. Biofunctionalization of polydioxythiophene derivatives for biomedical applications. J. Mater. Chem. B 2016, 4, 4952–4968. [Google Scholar] [CrossRef] [Green Version]
- Picaud, S.; Sahel, J.-A. Retinal prostheses: Clinical results and future challenges. C. R. Biol. 2014, 337, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Stavrinidou, E.; Leleux, P.; Rajaona, H.; Khodagholy, D.; Rivnay, J.; Lindau, M.; Sanaur, S.; Malliaras, G.G. Direct measurement of ion mobility in a conducting polymer. Adv. Mater. 2013, 25, 4488–4493. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Wiler, J.; Dzaman, M.; Altschuler, R.A.; Martin, D.C. In vivo studies of polypyrrole/peptide coated neural probes. Biomaterials 2003, 24, 777–787. [Google Scholar] [CrossRef]
- George, P.M.; Lyckman, A.W.; Lavan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Richardson-Burns, S.M.; Hendricks, J.L.; Sequera, C.; Martin, D.C. Effect of Immobilized Nerve Growth Factor on Conductive Polymers: Electrical Properties and Cellular Response. Adv. Funct. Mater. 2007, 17, 79–86. [Google Scholar] [CrossRef]
- Richardson, R.T.; Wise, A.K.; Thompson, B.C.; Flynn, B.O.; Atkinson, P.J.; Fretwell, N.J.; Fallon, J.B.; Wallace, G.G.; Shepherd, R.K.; Clark, G.M.; et al. Polypyrrole-coated electrodes for the delivery of charge and neurotrophins to cochlear neurons. Biomaterials 2009, 30, 2614–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, B.C.; Richardson, R.T.; Moulton, S.E.; Evans, A.J.; O’Leary, S.; Clark, G.M.; Wallace, G.G. Conducting polymers, dual neurotrophins and pulsed electrical stimulation—Dramatic effects on neurite outgrowth. J. Control. Release 2010, 141, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Gkoupidenis, P.; Schaefer, N.; Garlan, B.; Malliaras, G.G. Neuromorphic Functions in PEDOT:PSS Organic Electrochemical Transistors. Adv. Mater. 2015, 27, 7176–7180. [Google Scholar] [CrossRef] [PubMed]
- Antognazza, M.R.; Scherf, U.; Monti, P.; Lanzani, G. Organic-based tristimuli colorimeter. Appl. Phys. Lett. 2007, 90, 163509. [Google Scholar] [CrossRef]
- Maya-Vetencourt, J.F.; Ghezzi, D.; Antognazza, M.R.; Colombo, E.; Mete, M.; Feyen, P.; Desii, A.; Buschiazzo, A.; Di Paolo, M.; Di Marco, S.; et al. A fully organic retinal prosthesis restores vision in a rat model of degenerative blindness. Nat. Mater. 2017, 16, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Feron, K.; Belcher, W.; Fell, C.; Dastoor, P. Organic Solar Cells: Understanding the Role of Förster Resonance Energy Transfer. Int. J. Mol. Sci. 2012, 13, 17019–17047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, R.A.; Mashburn, D.N.; Sidorov, V.Y.; Wikswo, J.P. Quantification of transmembrane currents during action potential propagation in the heart. Biophys. J. 2013, 104, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Martino, N.; Feyen, P.; Porro, M.; Bossio, C.; Zucchetti, E.; Ghezzi, D.; Benfenati, F.; Lanzani, G.; Antognazza, M.R. Photothermal cellular stimulation in functional bio-polymer interfaces. Sci. Rep. 2015, 5, 8911. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.L.H.; Lee, E.-J.; Humayun, M.S.; Weiland, J.D. Both electrical stimulation thresholds and SMI-32-immunoreactive retinal ganglion cell density correlate with age in S334ter line 3 rat retina. J. Neurophysiol. 2011, 105, 2687–2697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, V.; Bag, M.; Narayan, K.S. Single-pixel, single-layer polymer device as a tricolor sensor with signals mimicking natural photoreceptors. J. Am. Chem. Soc. 2011, 133, 17942–17949. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.; Lee, J.Y.; Nickels, J.D.; Schmidt, C.E. Micropatterned polypyrrole: A combination of electrical and topographical characteristics for the stimulation of cells. Adv. Funct. Mater. 2007, 17, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Gu, X.; Yuan, C.; Chen, S.; Zhang, P.; Zhang, T.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. A 2004, 68, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Gomez, N.; Schmidt, C.E. Nerve growth factor-immobilized polypyrrole: Bioactive electrically conducting polymer for enhanced neurite extension. J. Biomed. Mater. Res. Part A 2007, 81, 135–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Yue, Z.; Higgins, M.J.; Wallace, G.G. Conducting polymers with immobilised fibrillar collagen for enhanced neural interfacing. Biomaterials 2011, 32, 7309–7317. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.C.; Moulton, S.E.; Ding, J.; Richardson, R.; Cameron, A.; O’Leary, S.; Wallace, G.G.; Clark, G.M. Optimising the incorporation and release of a neurotrophic factor using conducting polypyrrole. J. Control. Release 2006, 116, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.T.; Thompson, B.; Moulton, S.; Newbold, C.; Lum, M.G.; Cameron, A.; Wallace, G.; Kapsa, R.; Clark, G.; O’Leary, S. The effect of polypyrrole with incorporated neurotrophin-3 on the promotion of neurite outgrowth from auditory neurons. Biomaterials 2007, 28, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uguz, I.; Proctor, C.M.; Curto, V.F.; Pappa, A.M.; Donahue, M.J.; Ferro, M.; Owens, R.M.; Khodagholy, D.; Inal, S.; Malliaras, G.G. A Microfluidic Ion Pump for In Vivo Drug Delivery. Adv. Mater. 2017, 29, 1701217. [Google Scholar] [CrossRef] [PubMed]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Hutchison, A.S.; Lewis, T.W.; Moulton, S.E.; Spinks, G.M.; Wallace, G.G. Development of polypyrrole-based electromechanical actuators. Synth. Met. 2000, 113, 121–127. [Google Scholar] [CrossRef]
- Madden, J.D.; Cush, R.A.; Kanigan, T.S.; Hunter, I.W. Fast contracting polypyrrole actuators. Synth. Met. 2000, 113, 185–192. [Google Scholar] [CrossRef]
- Hodgson, A.J.; John, M.J.; Campbell, T.; Georgevich, A.; Woodhouse, S.; Aoki, T.; Ogata, N.; Wallace, G.G. Integration of biocomponents with synthetic structures: Use of conducting polymer polyelectrolyte composites. In Smart Structures and Materials 1996: Smart Materials Technologies and Biomimetics; Crowson, A., Ed.; International Society for Optics and Photonics: Bellingham, WA, USA, 1996; 164p. [Google Scholar]
- Esrafilzadeh, D.; Razal, J.M.; Moulton, S.E.; Stewart, E.M.; Wallace, G.G. Multifunctional conducting fibres with electrically controlled release of ciprofloxacin. J. Control. Release 2013, 169, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkington, D.; Cooling, N.; Belcher, W.; Dastoor, P.; Zhou, X. Organic Thin-Film Transistor (OTFT)-Based Sensors. Electronics 2014, 3, 234–254. [Google Scholar] [CrossRef]
- Elkington, D.; Belcher, W.J.; Dastoor, P.C.; Zhou, X.J. Detection of saliva-range glucose concentrations using organic thin-film transistors. Appl. Phys. Lett. 2014, 105, 43303. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Darwis, D.; Elkington, D.; Ulum, S.; Bryant, G.; Belcher, W.; Dastoor, P.; Zhou, X. Novel low voltage and solution processable organic thin film transistors based on water dispersed polymer semiconductor nanoparticulates. J. Colloid Interface Sci. 2013, 401, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Morrin, A.; Shepherd, R.; Crowley, K.; Killard, A.J.; Innis, P.C.; Wallace, G.G. Wholly printed polypyrrole nanoparticle-based biosensors on flexible substrate. J. Mater. Chem. B 2014, 2, 793–799. [Google Scholar] [CrossRef]
- Sokolov, A.N.; Roberts, M.E.; Bao, Z. Fabrication of low-cost electronic biosensors. Mater. Today 2009, 12, 12–20. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Kougianos, E. Biosensors: A tutorial review. IEEE Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]




| Aspect | Abiotic Electronic Biomedical Devices | Conjugated Polymers | Biotic Living Tissue |
|---|---|---|---|
| Composition | Inorganic metals, semiconductors | Organic molecules, including functionalized polythiophenes, copolymers, and dopants | Complicated, dynamic mixture of water, electrolytes, proteins, lipids, nucleic acids |
| Physical State | Hard solids | Soft solids | Extremely soft solids |
| Morphology | Single crystal, polycrystalline, or amorphous | Semicrystalline or amorphous | Complicated and dynamic; cells, intercellular spaces |
| Surface structure | Nearly flat | Can be tailored from nearly flat to rough and fuzzy | Complicated and dynamic |
| Mechanics: Young’s modulus | ~100 GPa | 10 MPa–3 GPa (as solids) 20 kPa–2 MPa (as gels) | ~10 kPa (cortex) |
| Charge carriers | Electrons, holes | Electrons, holes, and ions | Ions |
| Mass transport | Relatively limited at the molecular scale (solids), but can potentially incorporate microfluidic channels at large length scales | Facilitate ion transport with appropriate counterions, bicontinuous structures, deposition into hydrogels | Locally liquid-like biological environment |
| Material | Assay Environment | Cell Type | Cell Adhesion | Reference |
|---|---|---|---|---|
| poly(3-hexylthiophene-2,5-diyl), P3HT | Ex vivo, in vitro | Hippocampal neuron from embryonic 18-day rat embryos. Retinal neurons from 13–15 day chick embryos. | poly-l-lysine | [13,14,22,37] |
| phenyl-C61-butyric-acid-methyl ester, PCBM | Ex vivo | Hippocampal neuron from embryonic 18-day rat embryos | poly-l-lysine | [14,22] |
| Quaterthiophene, T4 | In vitro | Primary dorsal root ganglion (DRG) neurons, postnatal Sprague Dawley rats | poly-l-lysine | [36] |
| Lysinated quaterthiophene, T4Lys | In vitro | DRG neurons, postnatal Sprague Dawley rats | Inherently good | [36] |
| 2,4-bis [4-(N,Ndiisobutylamino)-2,6dihydroxyphenyl] squaraine, DIBSq | In vitro | N2A cells | Inherently good | [42] |
| Polypyrole, PPy | In vitro, ex vivo, in vivo | PC-12 cells, primary chicken sciatic nerve explants, subcutaneous and intramuscular sites, adult male Lewis rats | Poly-l-lysine | [48] |
| poly(3,4-ethylenedioxythiophene), PEDOT | In vitro | Primary cortical cells, embryonic (18–20 days) mice. | Inherently good | [49] |
| poly(3,4-ethylenedioxythiophene) doped with poly(styrenesulfonate), PEDOT:PSS | In vivo | Hippocampal and cortex neurons, male Long Evans rats | Inherently good | [45] |
| N,N′-ditridecylperylene-3,4,9,10-tetracarboxylic diimide, P13 | In vitro | Dorsal root ganglion neurons, post-natal rat | Poly-d-lysine + laminin | [50] |
| C60 | In vitro | Dorsal root ganglion neurons, mice | Poly-d-lysine | This work—Supplementary Information |
| poly(2,3-bis-(3-octyloxyphenyl)-quinoxaline-5,8-dyl-alt-thiophene-2,5-diyl), TQ1 | In vitro | Dorsal root ganglion neurons, mice | Poly-d-lysine | This work—Supplementary Information |
| 16,17-Bis(n-octyloxy) anthra [9,1,2-cde] benzo[rst]pentaphene-5,10-dione, Violanthrone-79 | In vitro | Dorsal root ganglion neurons, mice | Poly-d-lysine | This work—Supplementary Information |
| Nafion | In vitro, In vivo | HEp-2 cells. Male ICR mice | Inherently good | [51] |
| Pentacene | In vitro | Neurons from forebrain of mouse embryos | Poly-l-lysine, laminin | [52] |
| Graphene | In vitro | Brain tissue from postnatal mice | Poly-l-lysine | [32] |
| Carbon nanotubes | In vitro | Hippocampal cells from Sprague Dawley rats | Inherently good | [33] |
| poly{[N,N′-bis(2-octyldodecyl)-naphthalene-1,4,5,8-bis(dicarboximide)-2,6-diyl]-alt-5,5′-(2,2′-bithiophene), N2200 | In vitro | Retina from chick eyes at embryonic day 13–15 | l-ornithin, laminin | [22] |
| Poly(aniline), PANI | In vivo | Subcutaneous implantation into male Sprague-Dawley rats beneath the dorsal skin | Inherently good | [53] |
| Ethylene-vinyl acetate, EVAc | In vivo | Subcutaneous implantation into male Sprague-Dawley rats beneath the dorsal skin | Inherently good | [53] |
| Polyethylene, PE | In vivo | Subcutaneous implantation into male Sprague-Dawley rats beneath the dorsal skin | Inherently good | [53] |
| poly(p-phenylenevinylene) derivatives, PPV | In vitro | AsPC-1, HMEC-1, BV-2 and C8-D1A cells | Inherently good | [54,55] |
| PLA-b-AP-b-PLA copolymer, PAP | In vitro | H9c2 cells | Inherently good | [56] |
| Pyrrole-thiophene based polymer, BECP | In vitro, in vivo | Human neuroblastoma cells, subcutaneous implantation into rats | Inherently good | [57] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feron, K.; Lim, R.; Sherwood, C.; Keynes, A.; Brichta, A.; Dastoor, P.C. Organic Bioelectronics: Materials and Biocompatibility. Int. J. Mol. Sci. 2018, 19, 2382. https://doi.org/10.3390/ijms19082382
Feron K, Lim R, Sherwood C, Keynes A, Brichta A, Dastoor PC. Organic Bioelectronics: Materials and Biocompatibility. International Journal of Molecular Sciences. 2018; 19(8):2382. https://doi.org/10.3390/ijms19082382
Chicago/Turabian StyleFeron, Krishna, Rebecca Lim, Connor Sherwood, Angela Keynes, Alan Brichta, and Paul C. Dastoor. 2018. "Organic Bioelectronics: Materials and Biocompatibility" International Journal of Molecular Sciences 19, no. 8: 2382. https://doi.org/10.3390/ijms19082382





