Modulation of Apoptosis by Cytotoxic Mediators and Cell-Survival Molecules in Sjögren’s Syndrome
Abstract
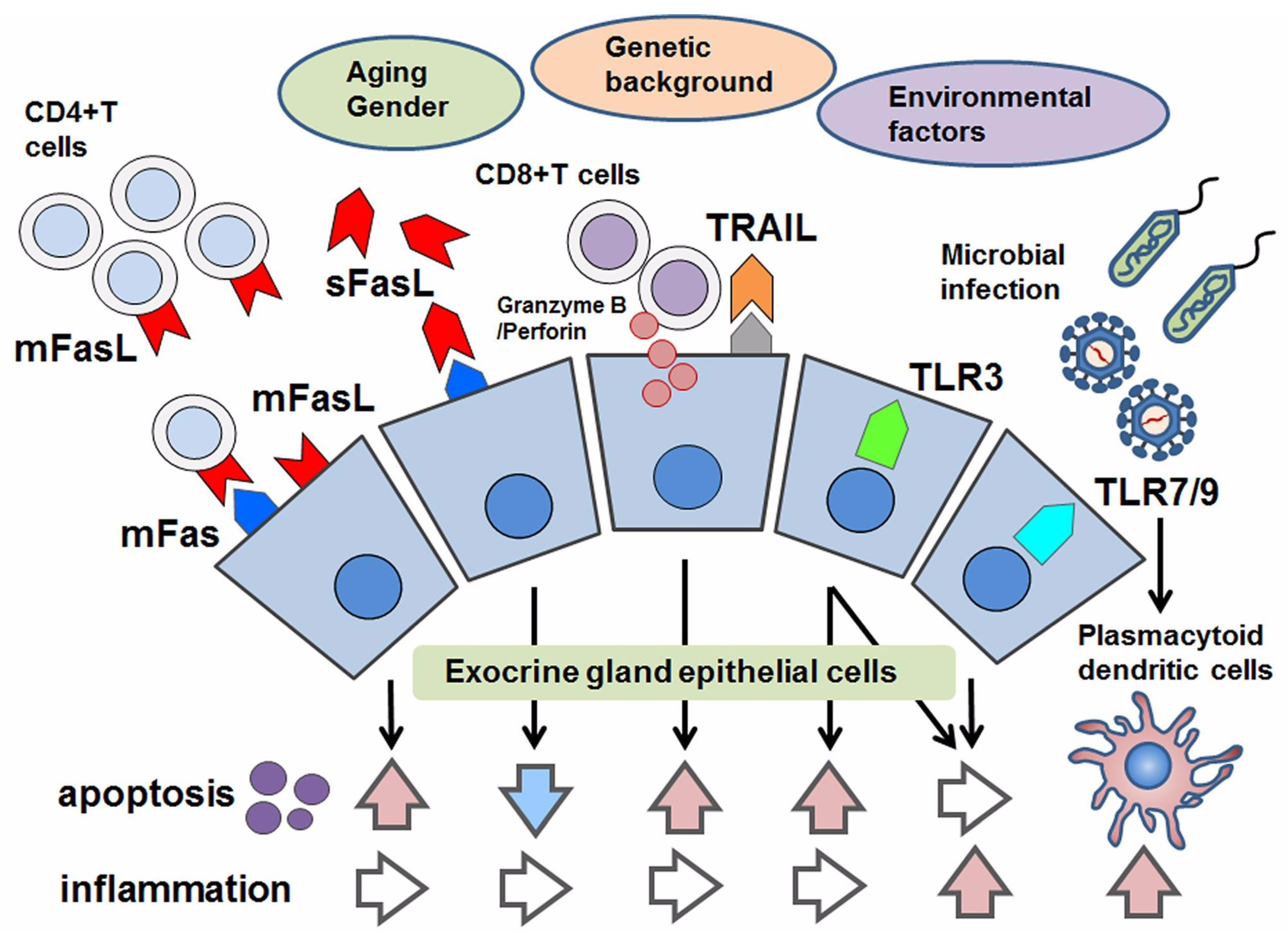
:1. Introduction
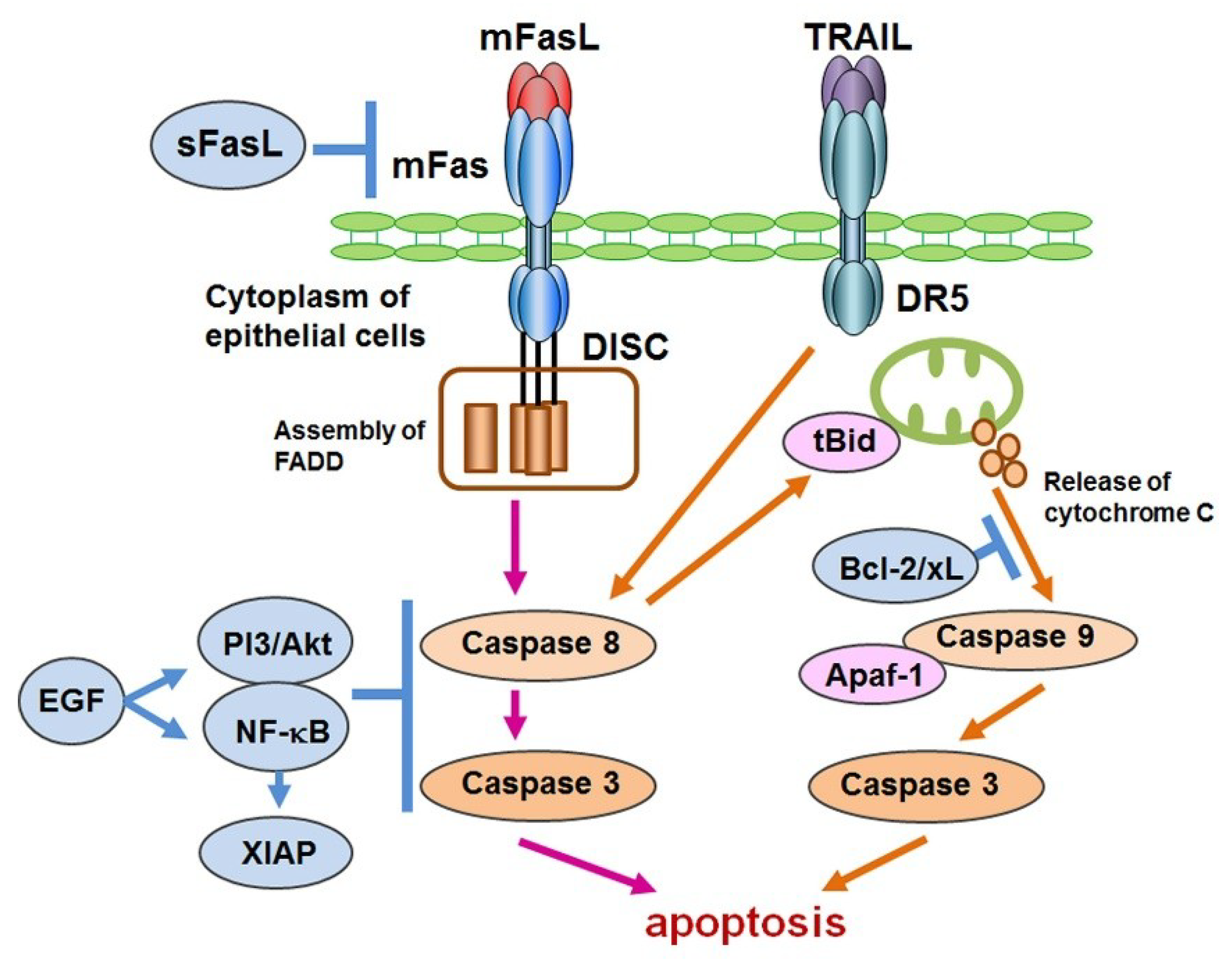
2. Fas and Protease-Mediated Apoptosis in Sjögren’s Syndrome
2.1. Membrane-Bound Fas and Apoptosis in Individuals with SS
2.2. Membrane-Bound Fas and Apoptosis in a Murine Model of SS
3. Soluble Fas/FasL and Apoptosis in Sjögren’s Syndrome
Cytotoxic Granules and Apoptosis in SS
4. TRAIL-Mediated Apoptosis in Sjögren’s Syndrome
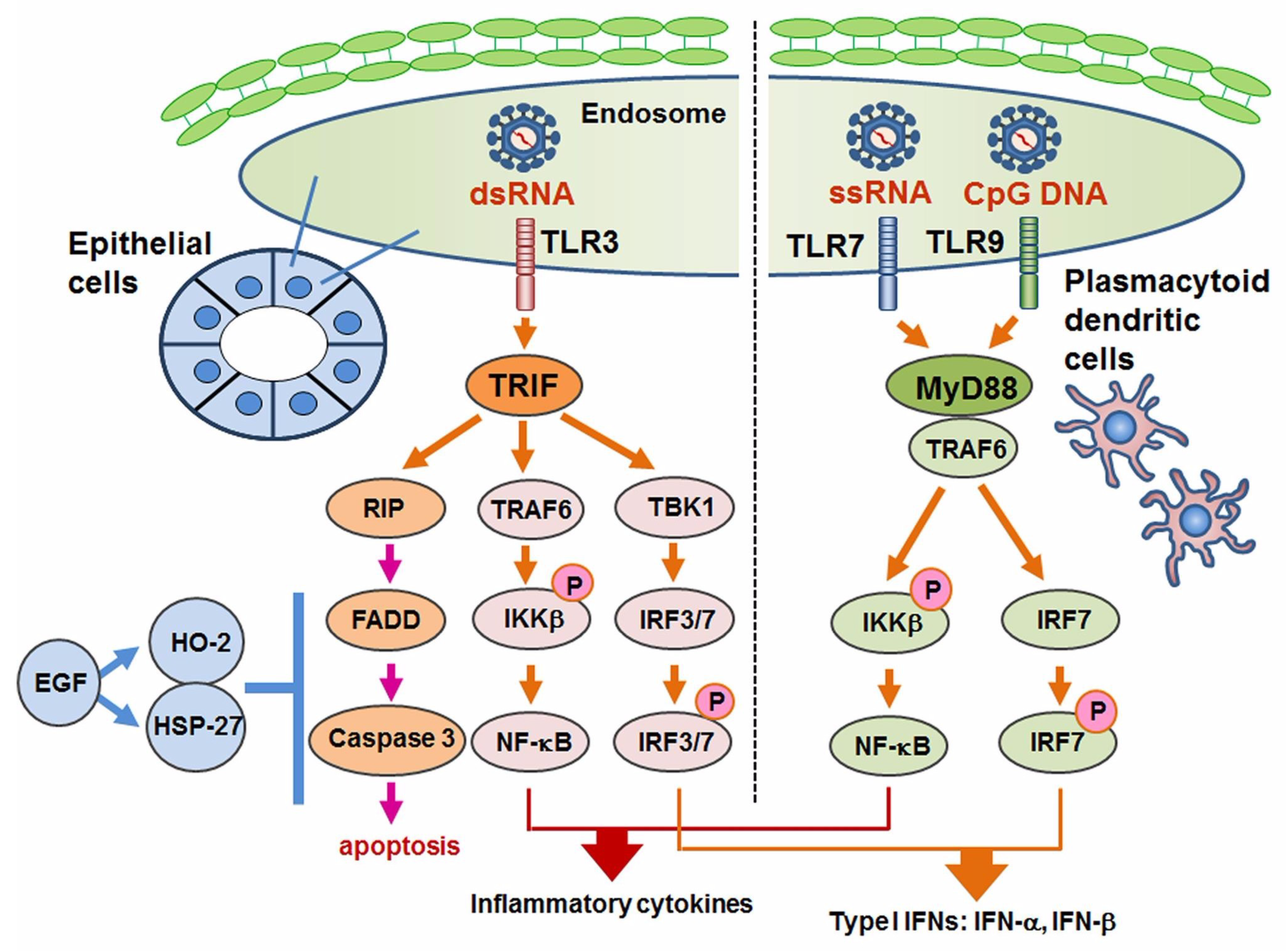
5. The Involvement of TLRs in Sjögren’s Syndrome
5.1. TLRs and Autoimmune Diseases
5.2. TLRs in Sjögren’s Syndrome
5.2.1. TLR1–4 in SS
5.2.2. TLR7–9 in Sjögren’s Syndrome and SLE
6. TLR3-Mediated Apoptosis in Sjögren’s Syndrome
7. Control of Apoptosis in Sjögren’s Syndrome
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mariette, X.; Criswell, L.A. Primary Sjögren’s syndrome. N. Engl. J. Med. 2018, 378, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kawakami, A.; Eguchi, K. Mechanisms of autoantibody production and the relationship between autoantibodies and the clinical manifestations in Sjögren’s syndrome. Transl. Res. 2006, 148, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Alani, H.; Henty, J.R.; Thompson, N.L.; Jury, E.; Ciurtin, C. Systematic review and meta-analysis of the epidemiology of polyautoimmunity in Sjögren’s syndrome (secondary Sjögren’s syndrome) focusing on autoimmune rheumatic diseases. Scand. J. Rheumatol. 2018, 47, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Vivino, F.B. Sjogren’s syndrome: Clinical aspects. Clin. Immunol. 2017, 182, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Ferro, F.; Marcucci, E.; Orlandi, M.; Baldini, C.; Bartoloni-Bocci, E. One year in review 2017: Primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2017, 35, 179–191. [Google Scholar] [PubMed]
- Adler, S.; Körner, M.; Förger, F.; Huscher, D.; Caversaccio, M.D.; Villiger, P.M. Evaluation of histologic, serologic, and clinical changes in response to abatacept treatment of primary Sjögren’s syndrome: A pilot study. Arthritis Care Res. 2013, 65, 1862–1868. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, H.; Matsumoto, I.; Hagiwara, S.; Hirota, T.; Takahashi, H.; Ebe, H.; Yokosawa, M.; Yagishita, M.; Takahashi, H.; Kurata, I.; et al. Effectiveness of abatacept for patients with Sjögren’s syndrome associated with rheumatoid arthritis. An open label, multicenter, one-year, prospective study: ROSE (Rheumatoid Arthritis with Orencia Trial toward Sjögren’s syndrome Endocrinopathy) trial. Mod. Rheumatol. 2016, 6, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.J.; Everett, C.C.; O’Dwyer, J.L.; Emery, P.; Pitzalis, C.; Ng, W.F.; Pease, C.T.; Price, E.J.; Sutcliffe, N.; Gendi, N.S.T.; et al. Randomized controlled trial of rituximab and cost-effectiveness analysis in treating fatigue and oral dryness in primary Sjögren’s syndrome. Arthritis Rheumatol. 2017, 69, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Quartuccio, L.; Salvin, S.; Corazza, L.; Gandolfo, S.; Fabris, M.; De Vita, S. Efficacy of belimumab and targeting of rheumatoid factor-positive B-cell expansion in Sjögren’s syndrome: Follow-up after the end of the phase II open-label BELISS study. Clin. Exp. Rheumatol. 2016, 34, 311–314. [Google Scholar] [PubMed]
- Taylor, K.E.; Wong, Q.; Levine, D.M.; McHugh, C.; Laurie, C.; Doheny, K.; Lam, M.Y.; Baer, A.N.; Challacombe, S.; Lanfranchi, H.; et al. Genome-wide association analysis reveals genetic heterogeneity of Sjögren’s syndrome according to ancestry. Arthritis Rheumatol. 2017, 69, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, K.; Chen, H.; Sun, F.; Xu, J.; Wu, Z.; Li, P.; Zhang, L.; Du, Y.; Luan, H.; et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjögren’s syndrome at 7q11.23. Nat. Genet. 2013, 45, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Karabiyik, A.; Peck, A.B.; Nguyen, C.Q. The important role of T cells and receptor expression in Sjögren’s syndrome. Scand. J. Immunol. 2013, 78, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Nocturne, G.; Mariette, X. Advances in understanding the pathogenesis of primary Sjögren’s syndrome. Nat. Rev. Rheumatol. 2013, 9, 544–556. [Google Scholar] [CrossRef] [PubMed]
- Verstappen, G.M.; Corneth, O.B.J.; Bootsma, H.; Kroese, F.G.M. Th17 cells in primary Sjögren’s syndrome: Pathogenicity and plasticity. J. Autoimmun. 2018, 87, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Barone, F.; Bombardieri, M.; Manzo, A.; Blades, M.C.; Morgan, P.R.; Challacombe, S.J.; Valesini, G.; Pitzalis, C. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjögren’s syndrome. Arthritis Rheum. 2005, 52, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Bombardieri, M.; Barone, F.; Lucchesi, D.; Nayar, S.; Van Den Berg, W.B.; Proctor, G.; Buckley, C.D.; Pitzalis, C. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J. Immunol. 2012, 189, 3767–3776. [Google Scholar] [CrossRef] [PubMed]
- Cornec, D.; Devauchelle-Pensec, V.; Tobón, G.J.; Pers, J.O.; Jousse-Joulin, S.; Saraux, A. B cells in Sjögren’s syndrome: From pathophysiology to diagnosis and treatment. J. Autoimmun. 2012, 39, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Armitage, R.J.; Fanslow, W.C.; Strockbine, L.; Sato, T.A.; Clifford, K.N.; Macduff, B.M.; Anderson, D.M.; Gimpel, S.D.; Davis-Smith, T.; Maliszewski, C.R. Molecular and biological characterization of a murine ligand for CD40. Nature 1992, 357, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Splawski, J.B.; Fu, S.M.; Lipsky, P.E. Immunoregulatory role of CD40 in human B cell differentiation. J. Immunol. 1993, 150, 1276–1285. [Google Scholar] [PubMed]
- Fox, R.I. The salivary gland epithelial cell in Sjogren’s Syndrome: What are the steps involved in wounding or killing their secretory function? J. Rheumatol. 2012, 39, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, P.; Fietta, P. Apoptosis and Sjögren syndrome. Semin. Arthritis Rheum. 2003, 33, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Hemmi, H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol. Lett. 2003, 85, 85–95. [Google Scholar] [CrossRef]
- Gilliet, M.; Cao, W.; Liu, Y.J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Santana-de Anda, K.; Gómez-Martín, D.; Soto-Solís, R.; Alcocer-Varela, J. Plasmacytoid dendritic cells: Key players in viral infections and autoimmune diseases. Semin. Arthritis Rheum. 2013, 43, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, W.J.; Offermann, M.K. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J. Immunol. 2005, 174, 4942–4952. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wei, H.; Tian, Z. Poly I:C enhances cycloheximide-induced apoptosis of tumor cells through TLR3 pathway. BMC Cancer 2008, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Yonehara, S.; Ishii, A.; Yonehara, M.; Mizushima, S.; Sameshima, M.; Hase, A.; Seto, Y.; Nagata, S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell 1991, 66, 233–243. [Google Scholar] [CrossRef]
- Watanabe-Fukunaga, R.; Brannan, C.I.; Itoh, N.; Yonehara, S.; Copeland, N.G.; Jenkins, N.A.; Nagata, S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J. Immunol. 1992, 148, 1274–1279. [Google Scholar] [PubMed]
- Yonehara, S.; Nishimura, Y.; Kishil, S.; Yonehara, M.; Takazawa, K.; Tamatani, T.; Ishii, A. Involvement of apoptosis antigen Fas in clonal deletion of human thymocytes. Int. Immunol. 1994, 6, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, T.; Golstein, P.; Nagata, S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75, 1169–1178. [Google Scholar] [CrossRef]
- Takahashi, T.; Tanaka, M.; Brannan, C.I.; Jenkins, N.A.; Copeland, N.G.; Suda, T.; Nagata, S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 1994, 76, 969–976. [Google Scholar] [CrossRef]
- Nagata, S.; Suda, T. Fas and Fas ligand: Lpr and gld mutations. Immunol. Today 1995, 16, 39–43. [Google Scholar] [CrossRef]
- Strasser, A.; Jost, P.J.; Nagata, S. The many roles of FAS receptor signaling in the immune system. Immunity 2009, 30, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, Y.; Arimori, K.; Yoshida, M.; Horiki, T.; Hoshina, Y.; Morita, K.; Uchiyama, M.; Shimizu, H.; Moriuchi, J.; Takaya, M. Abnormal expression of apoptosis-related antigens, Fas and bcl-2, on circulating T-lymphocyte subsets in primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 1995, 13, 307–313. [Google Scholar] [PubMed]
- Kong, L.; Ogawa, N.; Nakabayashi, T.; Liu, G.T.; D’Souza, E.; McGuff, H.S.; Guerrero, D.; Talal, N.; Dang, H. Fas and Fas ligand expression in the salivary glands of patients with primary Sjögren’s syndrome. Arthritis Rheum. 1997, 40, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Koji, T.; Tominaga, M.; Kawakami, A.; Migita, K.; Kawabe, Y.; Nakamura, T.; Shirabe, S.; Eguchi, K. Apoptosis in labial salivary glands from Sjögren’s syndrome (SS) patients: Comparison with human T lymphotropic virus-I (HTLV-I)-seronegative and -seropositive SS patients. Clin. Exp. Immunol. 1998, 114, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, R.; Umemiya, K.; Kagami, M.; Tomioka, H.; Tanabe, E.; Sugiyama, T.; Sueishi, M.; Nakajima, A.; Azuma, M.; Okumura, K.; et al. Glandular and extraglandular expression of the Fas-Fas ligand and apoptosis in patients with Sjögren’s syndrome. Clin. Exp. Rheumatol. 1998, 16, 561–568. [Google Scholar] [PubMed]
- Nakamura, H.; Kawakami, A.; Iwamoto, N.; Ida, H.; Koji, T.; Eguchi, K. Rapid and significant induction of TRAIL-mediated type II cells in apoptosis of primary salivary epithelial cells in primary Sjögren’s syndrome. Apoptosis 2008, 13, 1322–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Yang, L.; Liu, Y.; Tang, A.; Li, X.; Zhang, J.; Yang, Z. LY294002 and Rapamycin promote coxsackievirus-induced cytopathic effect and apoptosis via inhibition of PI3K/AKT/mTOR signaling pathway. Mol. Cell. Biochem. 2014, 385, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Ogawa, N.; Sugai, S. Novel role of CD40 in Fas-dependent apoptosis of cultured salivary epithelial cells from patients with Sjögren’s syndrome. Arthritis Rheum. 2005, 52, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yanagawa, T.; Yoshida, H.; Azuma, M.; Nishida, T.; Yura, Y.; Sato, M. Expression of vasoactive intestinal polypeptide and amylase in a human parotid gland adenocarcinoma cell line in culture. J. Natl. Cancer Inst. 1987, 79, 1025–1037. [Google Scholar] [PubMed]
- Miyazaki, K.; Takeda, N.; Ishimaru, N.; Omotehara, F.; Arakaki, R.; Hayashi, Y. Analysis of in vivo role of α-fodrin autoantigen in primary Sjogren’s syndrome. Am. J. Pathol. 2005, 167, 1051–1059. [Google Scholar] [CrossRef]
- Haneji, N.; Nakamura, T.; Takio, K.; Yanagi, K.; Higashiyama, H.; Saito, I.; Noji, S.; Sugino, H.; Hayashi, Y. Identification of alpha-fodrin as a candidate autoantigen in primary Sjögren’s syndrome. Science 1997, 276, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, K.; Ishimaru, N.; Haneji, N.; Saegusa, K.; Saito, I.; Hayashi, Y. Anti-120-kDa alpha-fodrin immune response with Th1-cytokine profile in the NOD mouse model of Sjögren’s syndrome. Eur. J. Immunol. 1998, 28, 3336–3345. [Google Scholar] [CrossRef]
- Watanabe, T.; Tsuchida, T.; Kanda, N.; Mori, K.; Hayashi, Y.; Tamaki, K. Anti-α-fodrin antibodies in Sjögren syndrome and lupus erythematosus. Arch. Dermatol. 1999, 135, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Maeno, N.; Takei, S.; Imanaka, H.; Oda, H.; Yanagi, K.; Hayashi, Y.; Miyata, K. Anti-α-fodrin antibodies in Sjögren’s syndrome in children. J. Rheumatol. 2001, 28, 860–864. [Google Scholar] [PubMed]
- Tsuzaka, K.; Matsumoto, Y.; Sasaki, Y.; Abe, T.; Tsubota, K.; Takeuchi, T. Down-regulation of Fas-ligand mRNA in Sjögren’s syndrome patients with enlarged exocrine glands. Autoimmunity 2007, 40, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Haruta, K.; Shimuta, M.; Inoue, H.; Sakurai, H.; Yamada, K.; Ishimaru, N.; Higashiyama, H.; Sumida, T.; Ishida, H.; et al. Fas ligand-mediated exocrinopathy resembling Sjögren’s syndrome in mice transgenic for IL-10. J. Immunol. 1999, 162, 2488–2494. [Google Scholar] [PubMed]
- Ishimaru, N.; Saegusa, K.; Yanagi, K.; Haneji, N.; Saito, I.; Hayashi, Y. Estrogen deficiency accelerates autoimmune exocrinopathy in murine Sjögren’s syndrome through fas-mediated apoptosis. Am. J. Pathol. 1999, 155, 173–181. [Google Scholar] [CrossRef]
- Ishimaru, N.; Yoneda, T.; Saegusa, K.; Yanagi, K.; Haneji, N.; Moriyama, K.; Saito, I.; Hayashi, Y. Severe destructive autoimmune lesions with aging in murine Sjögren’s syndrome through Fas-mediated apoptosis. Am. J. Pathol. 2000, 156, 1557–1564. [Google Scholar] [CrossRef]
- Yamada, A.; Arakaki, R.; Saito, M.; Kudo, Y.; Ishimaru, N. Dual role of Fas/FasL-mediated signal in peripheral immune tolerance. Front. Immunol. 2017, 8, 403. [Google Scholar] [CrossRef] [PubMed]
- Tsubota, K.; Fujita, H.; Tadano, K.; Takeuchi, T.; Murakami, T.; Saito, I.; Hayashi, Y. Improvement of lacrimal function by topical application of CyA in murine models of Sjögren’s syndrome. Investig. Ophthalmol. Vis. Sci. 2001, 42, 101–110. [Google Scholar]
- Kong, L.; Robinson, C.P.; Peck, A.B.; Vela-Roch, N.; Sakata, K.M.; Dang, H.; Talal, N.; Humphreys-Beher, M.G. Inappropriate apoptosis of salivary and lacrimal gland epithelium of immunodeficient NOD-scid mice. Clin. Exp. Rheumatol. 1998, 16, 675–681. [Google Scholar] [PubMed]
- Wu, G.; Wu, N.; Li, T.; Lu, W.; Yu, G. Total glucosides of peony ameliorates Sjögren’s syndrome by affecting Th1/Th2 cytokine balance. Exp. Ther. Med. 2016, 11, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhou, T.; Liu, C.; Shapiro, J.P.; Brauer, M.J.; Kiefer, M.C.; Barr, P.J.; Mountz, J.D. Protection from Fas-mediated apoptosis by a soluble form of the Fas molecule. Science 1994, 263, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Suda, T.; Takahashi, T.; Nagata, S. Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J. 1995, 14, 1129–1135. [Google Scholar] [PubMed]
- Nozawa, K.; Kayagaki, N.; Tokano, Y.; Yagita, H.; Okumura, K.; Hasimoto, H. Soluble Fas (APO-1, CD95) and soluble Fas ligand in rheumatic diseases. Arthritis Rheum. 1997, 40, 1126–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, H.; Kawakami, A.; Izumi, M.; Nakashima, T.; Takagi, Y.; Ida, H.; Nakamura, T.; Nakamura, T.; Eguchi, K. Detection of the soluble form of Fas ligand (sFasL) and sFas in the saliva from patients with Sjögren’s syndrome. Clin. Exp. Rheumatol. 2005, 23, 915. [Google Scholar] [PubMed]
- Rubin, P.; Holt, J.F. Secretory sialography in diseases of the major salivary glands. Am. J. Roentgenol. Radium. Ther. Nucl. Med. 1957, 77, 575–598. [Google Scholar] [PubMed]
- Luo, J.; Wang, Y.; Yu, B.; Qian, H.; He, Y.; Shi, G. A potential of sFasL in preventing gland injury in Sjogren’s syndrome. Biomed. Res. Int. 2017, 2017, 5981432–5981538. [Google Scholar] [CrossRef] [PubMed]
- Ishimaru, N.; Yanagi, K.; Ogawa, K.; Suda, T.; Saito, I.; Hayashi, Y. Possible role of organ-specific autoantigen for Fas ligand-mediated activation-induced cell death in murine Sjögren’s syndrome. J. Immunol. 2001, 167, 6031–6037. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Talavera, B.A.; Palafox-Sánchez, C.A.; Muñoz-Valle, J.F.; Orozco-Barocio, G.; Navarro-Hernández, R.E.; Vázquez-Del Mercado, M.; García de la Torre, I.; Oregon-Romero, E. FAS–670A>G promoter polymorphism is associated with soluble Fas levels in primary Sjögren’s syndrome. Genet. Mol. Res. 2014, 13, 4831–4838. [Google Scholar] [CrossRef] [PubMed]
- Jenne, D.E.; Tschopp, J. Granzymes, a family of serine proteases released from granules of cytolytic T lymphocytes upon T cell receptor stimulation. Immunol. Rev. 1988, 103, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Heusel, J.W.; Wesselschmidt, R.L.; Shresta, S.; Russell, J.H.; Ley, T.J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 1994, 76, 977–987. [Google Scholar] [CrossRef]
- Huang, M.; Ida, H.; Kamachi, M.; Iwanaga, N.; Izumi, Y.; Tanaka, F.; Aratake, K.; Arima, K.; Tamai, M.; Hida, A.; et al. Detection of apoptosis-specific autoantibodies directed against granzyme B-induced cleavage fragments of the SS-B (La) autoantigen in sera from patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2005, 142, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Lindner, S.; Dahlke, K.; Sontheimer, K.; Hagn, M.; Kaltenmeier, C.; Barth, T.F.; Beyer, T.; Reister, F.; Fabricius, D.; Lotfi, R.; et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T. cells. Cancer Res. 2013, 73, 2468–2479. [Google Scholar] [CrossRef] [PubMed]
- Papp, G.; Gyimesi, E.; Szabó, K.; Zöld, É.; Zeher, M. Increased IL-21 expression induces granzyme B in peripheral CD5(+) B cells as a potential counter-regulatory effect in primary Sjögren’s syndrome. Mediators Inflamm. 2016, 2016, 4328372–4328380. [Google Scholar] [CrossRef] [PubMed]
- Lowin, B.; Hahne, M.; Mattmann, C.; Tschopp, J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 1994, 370, 650–652. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Persechini, P.M.; Young, J.D. Perforin and lymphocyte-mediated cytolysis. Immunol. Rev. 1995, 146, 145–175. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, T.; Fujita, H.; Tsubota, K.; Saito, K.; Tsuzaka, K.; Abe, T.; Takeuchi, T. Preferential localization of CD8+ alpha E beta 7+ T cells around acinar epithelial cells with apoptosis in patients with Sjögren’s syndrome. J. Immunol. 1999, 163, 2226–2235. [Google Scholar] [PubMed]
- Wiley, S.R.; Schooley, K.; Smolak, P.J.; Din, W.S.; Huang, C.P.; Nicholl, J.K.; Sutherland, G.R.; Smith, T.D.; Rauch, C.; Smith, C.A.; et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity 1995, 3, 673–682. [Google Scholar] [CrossRef]
- Pan, G.; O’Rourke, K.; Chinnaiyan, A.M.; Gentz, R.; Ebner, R.; Ni, J.; Dixit, V.M. The receptor for the cytotoxic ligand TRAIL. Science 1997, 276, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.P.; Marsters, S.A.; Pitti, R.M.; Gurney, A.; Skubatch, M.; Baldwin, D.; Ramakrishnan, L.; Gray, C.L.; Baker, K.; Wood, W.I.; et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science 1997, 277, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, R.; Umemiya, K.; Kagami, M.; Tomioka, H.; Tanabe, E.; Sugiyama, T.; Sueishi, M.; Kayagaki, N.; Yagita, H.; Okumura, K. Expression of TNF-related apoptosis inducing ligand (TRAIL) on infiltrating cells and of TRAIL receptors on salivary glands in patients with Sjögren’s syndrome. Clin. Exp. Rheumatol. 2002, 20, 791–798. [Google Scholar] [PubMed]
- Chen, W.S.; Lin, K.C.; Chen, C.H.; Liao, H.T.; Wang, H.P.; Li, W.Y.; Lee, H.T.; Tsai, C.Y.; Chou, C.T. Autoantibody and biopsy grading are associated with expression of ICAM-1, MMP-3, and TRAIL in salivary gland mononuclear cells of Chinese patients with Sjogren’s syndrome. J. Rheumatol. 2009, 36, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Schuster, I.S.; Wikstrom, M.E.; Brizard, G.; Coudert, J.D.; Estcourt, M.J.; Manzur, M.; O’Reilly, L.A.; Smyth, M.J.; Trapani, J.A.; Hill, G.R.; et al. TRAIL+ NK cells control CD4+ T cell responses during chronic viral infection to limit autoimmunity. Immunity 2014, 41, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Kell, A.M.; Gale, M.J. RIG-I in RNA virus recognition. Virology 2015, 479–480, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Griebel, T.; Maekawa, T.; Parker, J.E. NOD-like receptor cooperativity in effector-triggered immunity. Trends Immunol. 2014, 35, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Nicolas, E.; Michaut, L.; Reichhart, J.M.; Hoffmann, J.A. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 1996, 86, 973–983. [Google Scholar] [CrossRef]
- Satoh, T.; Akira, S. Toll-Like receptor signaling and its inducible proteins. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Pelka, K.; Shibata, T.; Miyake, K.; Latz, E. Nucleic acid-sensing TLRs and autoimmunity: Novel insights from structural and cell biology. Immunol. Rev. 2016, 269, 60–75. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Wei, T. Structure modeling of Toll-like receptors. Methods Mol. Biol. 2014, 1169, 45–53. [Google Scholar] [PubMed]
- Abdollahi-Roodsaz, S.; Joosten, L.A.; Koenders, M.I.; Devesa, I.; Roelofs, M.F.; Radstake, T.R.; Heuvelmans-Jacobs, M.; Akira, S.; Nicklin, M.J.; Ribeiro-Dias, F.; et al. Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J. Clin. Investig. 2008, 118, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahi-Roodsaz, S.; Koenders, M.I.; Walgreen, B.; Bolscher, J.; Helsen, M.M.; Van den Bersselaar, L.A.; Van Lent, P.L.; Van de Loo, F.A.; Van den Berg, W.B. Toll-like receptor 2 controls acute immune complex-driven arthritis in mice by regulating the inhibitory Fcγ receptor IIB. Arthritis Rheum. 2013, 65, 2583–2593. [Google Scholar] [PubMed]
- Chamberlain, N.D.; Kim, S.J.; Vila, O.M.; Volin, M.V.; Volkov, S.; Pope, R.M.; Arami, S.; Mandelin, A.M., II; Shahrara, S. Ligation of TLR7 by rheumatoid arthritis synovial fluid single strand RNA induces transcription of TNFα in monocytes. Ann. Rheum. Dis. 2013, 72, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Patole, P.S.; Pawar, R.D.; Lech, M.; Zecher, D.; Schmidt, H.; Segerer, S.; Ellwart, A.; Henger, A.; Kretzler, M.; Anders, H.J. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Fas(lpr) mice. Nephrol. Dial. Transplant. 2006, 21, 3062–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, S.R.; Shupe, J.; Nickerson, K.; Kashgarian, M.; Flavell, R.A.; Shlomchik, M.J. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 2006, 25, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, K.M.; Christensen, S.R.; Shupe, J.; Kashgarian, M.; Kim, D.; Elkon, K.; Shlomchik, M.J. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J. Immunol. 2010, 184, 1840–1848. [Google Scholar] [CrossRef] [PubMed]
- Mande, P.; Zirak, B.; Ko, W.C.; Taravati, K.; Bride, K.L.; Brodeur, T.Y.; Deng, A.; Dresser, K.; Jiang, Z.; Ettinger, R.; et al. Fas ligand promotes an inducible TLR-dependent model of cutaneous lupus-like inflammation. J. Clin. Investig. 2018, 128, 2966–2978. [Google Scholar] [CrossRef] [PubMed]
- Derkow, K.; Bauer, J.M.; Hecker, M.; Paap, B.K.; Thamilarasan, M.; Koczan, D.; Schott, E.; Deuschle, K.; Bellmann-Strobl, J.; Paul, F.; et al. Multiple sclerosis: Modulation of toll-like receptor (TLR) expression by interferon-β includes upregulation of TLR7 in plasmacytoid dendritic cells. PLoS ONE 2013, 8, e70626. [Google Scholar] [CrossRef] [PubMed]
- Spachidou, M.P.; Bourazopoulou, E.; Maratheftis, C.I.; Kapsogeorgou, E.K.; Moutsopoulos, H.M.; Tzioufas, A.G.; Manoussakis, M.N. Expression of functional Toll-like receptors by salivary gland epithelial cells: Increased mRNA expression in cells derived from patients with primary Sjögren’s syndrome. Clin. Exp. Immunol. 2007, 147, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Nakashima, K.; Tamai, M.; Nakamura, H.; Iwanaga, N.; Fujikawa, K.; Aramaki, T.; Arima, K.; Iwamoto, N.; Ichinose, K.; et al. Toll-like receptor in salivary glands from patients with Sjögren’s syndrome: Functional analysis by human salivary gland cell line. J. Rheumatol. 2007, 34, 1019–1026. [Google Scholar] [PubMed]
- Ittah, M.; Miceli-Richard, C.; Gottenberg, J.E.; Sellam, J.; Eid, P.; Lebon, P.; Pallier, C.; Lepajolec, C.; Mariette, X. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur. J. Immunol. 2008, 38, 1058–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, S.K.; Cho, M.L.; Her, Y.M.; Oh, H.J.; Park, M.K.; Lee, S.Y.; Woo, Y.J.; Ju, J.H.; Park, K.S.; Kim, H.Y.; et al. TLR2 ligation induces the production of IL-23/IL-17 via IL-6, STAT3 and NF-kB pathway in patients with primary Sjogren’s syndrome. Arthritis Res. Ther. 2012, 14, R64. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S.K.; Lee, J.; Yu, D.; Kang, K.Y.; Cho, M.L.; Kim, H.R.; Ju, J.H.; Lee, S.H.; Park, S.H.; Kim, H.Y. A pathogenetic role for IL-21 in primary Sjögren syndrome. Nat. Rev. Rheumatol. 2015, 11, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Miyake, K.; Kaisho, T. Homeostatic inflammation in innate immunity. Curr. Opin. Immunol. 2014, 30, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Heil, F.; Hemmi, H.; Hochrein, H.; Ampenberger, F.; Kirschning, C.; Akira, S.; Lipford, G.; Wagner, H.; Bauer, S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 2004, 303, 1526–1529. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.M.; Alexopoulou, L.; Sato, A.; Karow, M.; Adams, N.C.; Gale, N.W.; Iwasaki, A.; Flavell, R.A. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 2004, 101, 5598–5603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Yi, G.; Chen, A.; Bhardwaj, K.; Tragesser, B.J.; Valverde, R.A.; Zlotnick, A.; Mukhopadhyay, S.; Ranjith-Kumar, C.T.; Kao, C.C. Viral double-strand RNA-binding proteins can enhance innate immune signaling by toll-like receptor 3. PLoS ONE 2011, 6, e25837. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Wang, Y.H.; Liu, Y.J. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin. Immunopathol. 2005, 26, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Haas, F.; Yamauchi, K.; Murat, M.; Bernasconi, M.; Yamanaka, N.; Speck, R.F.; Nadal, D. Activation of NF-κB via endosomal Toll-like receptor 7 (TLR7) or TLR9 suppresses murine herpesvirus 68 reactivation. J. Virol. 2014, 88, 10002–10012. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Zhang, Z.; Yu, C.; Yang, C. Expression of Toll-like receptors 7, 8, and 9 in primary Sjögren’s syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Higgs, R.; Lazzari, E.; Wynne, C.; Gabhann, N.J.; Espinosa, A.; Wahren-Herlenius, M.; Jefferies, C.A. Self protection from anti-viral responses — Ro52 promotes degradation of the transcription factor IRF7 downstream of the viral Toll-Like receptors. PLoS ONE 2010, 5, e11776. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, M.; Hansen, T.; Nordal, H.H.; Brun, J.G.; Jonsson, R.; Appel, S. Expression of Toll-like receptor -7 and -9 in B cell subsets from patients with primary Sjögren’s syndrome. PLoS ONE 2015, 10, e0120383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsen, M.; Jonsson, R.; Brun, J.G.; Appel, S.; Hansen, T. TLR-7 and -9 stimulation of peripheral blood B cells indicate altered TLR Signalling in primary Sjögren’s syndrome patients by increased secretion of cytokines. Scand. J. Immunol. 2015, 82, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Maria, N.I.; Steenwijk, E.C.; IJpma, A.S.; Van Helden-Meeuwsen, C.G.; Vogelsang, P.; Beumer, W.; Brkic, Z.; Van Daele, P.L.; Van Hagen, P.M.; Van der Spek, P.J.; et al. Contrasting expression pattern of RNA-sensing receptors TLR7, RIG-I and MDA5 in interferon-positive and interferon-negative patients with primary Sjögren’s syndrome. Ann. Rheum. Dis. 2017, 76, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Sagalovskiy, I.; Guo, Q.; Nezos, A.; Kapsogeorgou, E.K.; Lu, P.; Zhou, L.J.; Kirou, K.A.; Seshan, S.V.; Moutsopoulos, H.M.; et al. Expression of long interspersed nuclear element 1 retroelements and induction of type i interferon in patients with systemic autoimmune disease. Arthritis Rheumatol. 2016, 68, 2686–2696. [Google Scholar] [CrossRef] [PubMed]
- Kiripolsky, J.; McCabe, L.G.; Gaile, D.P.; Kramer, J.M. Myd88 is required for disease development in a primary Sjögren’s syndrome mouse model. J. Leukoc. Biol. 2017, 102, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Khvalevsky, E.; Rivkin, L.; Rachmilewitz, J.; Galun, E.; Giladi, H. TLR3 signaling in a hepatoma cell line is skewed towards apoptosis. J. Cell. Biochem. 2007, 100, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Sugimoto, K.; Shiraki, K.; Tanaka, J.; Beppu, T.; Fuke, H.; Yamamoto, N.; Masuya, M.; Horie, R.; Uchida, K.; et al. Dual topology of functional Toll-like receptor 3 expression in human hepatocellular carcinoma: Differential signaling mechanisms of TLR3-induced NF-κB activation and apoptosis. Int. J. Oncol. 2008, 33, 929–936. [Google Scholar] [PubMed]
- Shen, P.; Jiang, T.; Lu, H.; Han, H.; Luo, R. Combination of Poly I:C and arsenic trioxide triggers apoptosis synergistically via activation of TLR3 and mitochondrial pathways in hepatocellular carcinoma cells. Cell Biol. Int. 2011, 35, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, L.; Zhu, Y.; Zhang, Y.; He, S.; Qin, J.; Tang, X.; Zhou, J.; Wei, Y. Double-stranded RNA-induced TLR3 activation inhibits angiogenesis and triggers apoptosis of human hepatocellular carcinoma cells. Oncol. Rep. 2012, 27, 396–402. [Google Scholar] [PubMed]
- Manoussakis, M.N.; Spachidou, M.P.; Maratheftis, C.I. Salivary epithelial cells from Sjogren’s syndrome patients are highly sensitive to anoikis induced by TLR-3 ligation. J. Autoimmun. 2010, 35, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Horai, Y.; Suzuki, T.; Okada, A.; Ichinose, K.; Yamasaki, S.; Koji, T.; Kawakami, A. TLR3-mediated apoptosis and activation of phosphorylated Akt in the salivary gland epithelial cells of primary Sjögren’s syndrome patients. Rheumatol. Int. 2013, 33, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Polihronis, M.; Tapinos, N.I.; Theocharis, S.E.; Economou, A.; Kittas, C.; Moutsopoulos, H.M. Modes of epithelial cell death and repair in Sjögren’s syndrome (SS). Clin. Exp. Immunol. 1998, 114, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, M.; Skarstein, K.; Bolstad, A.I.; Johannessen, A.C.; Jonsson, R. Fas-induced apoptosis is a rare event in Sjögren’s syndrome. Lab. Investig. 2001, 81, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kawakami, A.; Tominaga, M.; Migita, K.; Kawabe, Y.; Nakamura, T.; Eguchi, K. Expression of CD40/CD40 ligand and Bcl-2 family proteins in labial salivary glands of patients with Sjögren’s syndrome. Lab. Investig. 1999, 79, 261–269. [Google Scholar] [PubMed]
- Nakamura, H.; Kawakami, A.; Yamasaki, S.; Kawabe, Y.; Nakamura, T.; Eguchi, K. Expression of mitogen activated protein kinases in labial salivary glands of patients with Sjögren’s syndrome. Ann. Rheum. Dis. 1999, 58, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kawakami, A.; Yamasaki, S.; Nakashima, T.; Kamachi, M.; Migita, K.; Kawabe, Y.; Nakamura, T.; Koji, T.; Hayashi, Y.; et al. Expression and function of X chromosome-linked inhibitor of apoptosis protein in Sjögren’s syndrome. Lab. Investig. 2000, 80, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Saito, I.; Shimuta, M.; Terauchi, K.; Tsubota, K.; Yodoi, J.; Miyasaka, N. Increased expression of human thioredoxin/adult T cell leukemia-derived factor in Sjögren’s syndrome. Arthritis Rheum. 1996, 39, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, C.; Kawano, S.; Tsuji, G.; Hatachi, S.; Jikimoto, T.; Sugiyama, D.; Kasagi, S.; Komori, T.; Nakamura, H.; Yodoi, J.; et al. Thioredoxin may exert a protective effect against tissue damage caused by oxidative stress in salivary glands of patients with Sjögren’s syndrome. J. Rheumatol. 2007, 34, 2035–2043. [Google Scholar] [PubMed]
- Gorgoulis, V.; Giatromanolaki, A.; Iliopoulos, A.; Kanavaros, P.; Aninos, D.; Ioakeimidis, D.; Kontomerkos, T.; Karameris, A. EGF and EGF-r immunoexpression in Sjögren’s syndrome secondary to rheumatoid arthritis. Correlation with EBV expression? Clin. Exp. Rheumatol. 1993, 11, 623–627. [Google Scholar] [PubMed]
- Koski, H.; Konttinen, Y.T.; Hietanen, J.; Tervo, T.; Malmström, M. Epidermal growth factor, transforming growth factor-α, and epidermal growth factor receptor in labial salivary glands in Sjögren’s syndrome. J. Rheumatol. 1997, 24, 1930–1935. [Google Scholar] [PubMed]
- Tsubota, K.; Goto, E.; Fujita, H.; Ono, M.; Inoue, H.; Saito, I.; Shimmura, S. Treatment of dry eye by autologous serum application in Sjögren’s syndrome. Br. J. Ophthalmol. 1999, 83, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Jones, D.; Ji, Z.; Afonso, A.; Monroy, D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999, 19, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Azuma, N.; Katada, Y.; Kitano, S.; Sekiguchi, M.; Kitano, M.; Nishioka, A.; Hashimoto, N.; Matsui, K.; Iwasaki, T.; Sano, H. Correlation between salivary epidermal growth factor levels and refractory intraoral manifestations in patients with Sjögren’s syndrome. Mod. Rheumatol. 2014, 24, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Azuma, N.; Katada, Y.; Kitano, S.; Sekiguchi, M.; Kitano, M.; Nishioka, A.; Hashimoto, N.; Matsui, K.; Iwasaki, T.; Sano, H. Rapid decrease in salivary epidermal growth factor levels in patients with Sjögren’s syndrome: A 3-year follow-up study. Mod. Rheumatol. 2015, 25, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Azuma, N.; Katada, Y.; Sano, H. Deterioration in saliva quality in patients with Sjögren’s syndrome: Impact of decrease in salivary epidermal growth factor on the severity of intraoral manifestations. Inflamm. Regen. 2018, 38, 6. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kawakami, A.; Ida, H.; Koji, T.; Eguchi, K. EGF activates PI3K-Akt and NF-κB via distinct pathways in salivary epithelial cells in Sjögren’s syndrome. Rheumatol. Int. 2007, 28, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kawakami, A.; Ida, H.; Koji, T.; Eguchi, K. Epidermal growth factor inhibits Fas-mediated apoptosis in salivary epithelial cells of patients with primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2007, 25, 831–837. [Google Scholar] [PubMed]
- Yang, L.; Wang, Y.; Xing, R.; Bai, L.; Li, C.; Li, Z.; Liu, X. Mimotope mimicking epidermal growth factor receptor alleviates mononuclear cell infiltration in exocrine glands induced by muscarinic acetylcholine 3 receptor. Clin. Immunol. 2016, 163, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, M.; Wakamatsu, E.; Tsuboi, H.; Nakamura, Y.; Hayashi, T.; Matsui, M.; Goto, D.; Ito, S.; Matsumoto, I.; Sumida, T. Pathogenic role of immune response to M3 muscarinic acetylcholine receptor in Sjögren’s syndrome-like sialoadenitis. J. Autoimmun. 2010, 35, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Tsuboi, H.; Segawa, S.; Asashima, H.; Iizuka-Koga, M.; Hirota, T.; Takahashi, H.; Kondo, Y.; Matsui, M.; Matsumoto, I.; et al. RORγt antagonist suppresses M3 muscarinic acetylcholine receptor-induced Sjögren’s syndrome-like sialadenitis. Clin. Exp. Immunol. 2017, 187, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Horai, Y.; Nakamura, H.; Nakashima, Y.; Hayashi, T.; Kawakami, A. Analysis of the downstream mediators of toll-like receptor 3-induced apoptosis in labial salivary glands in patients with Sjögren’s syndrome. Mod. Rheumatol. 2016, 26, 99–104. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, H.; Horai, Y.; Shimizu, T.; Kawakami, A. Modulation of Apoptosis by Cytotoxic Mediators and Cell-Survival Molecules in Sjögren’s Syndrome. Int. J. Mol. Sci. 2018, 19, 2369. https://doi.org/10.3390/ijms19082369
Nakamura H, Horai Y, Shimizu T, Kawakami A. Modulation of Apoptosis by Cytotoxic Mediators and Cell-Survival Molecules in Sjögren’s Syndrome. International Journal of Molecular Sciences. 2018; 19(8):2369. https://doi.org/10.3390/ijms19082369
Chicago/Turabian StyleNakamura, Hideki, Yoshiro Horai, Toshimasa Shimizu, and Atsushi Kawakami. 2018. "Modulation of Apoptosis by Cytotoxic Mediators and Cell-Survival Molecules in Sjögren’s Syndrome" International Journal of Molecular Sciences 19, no. 8: 2369. https://doi.org/10.3390/ijms19082369





