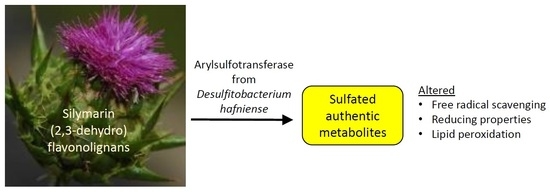
Sulfated Metabolites of Flavonolignans and 2,3-Dehydroflavonolignans: Preparation and Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sulfation of Silymarin Flavonolignans by Aryl Sulfotransferase from Rat Liver (AST IV)
2.2. Sulfation of Silymarin Flavonolignans with Aryl Sulfotransferase from Desulfitobacterium hafniense
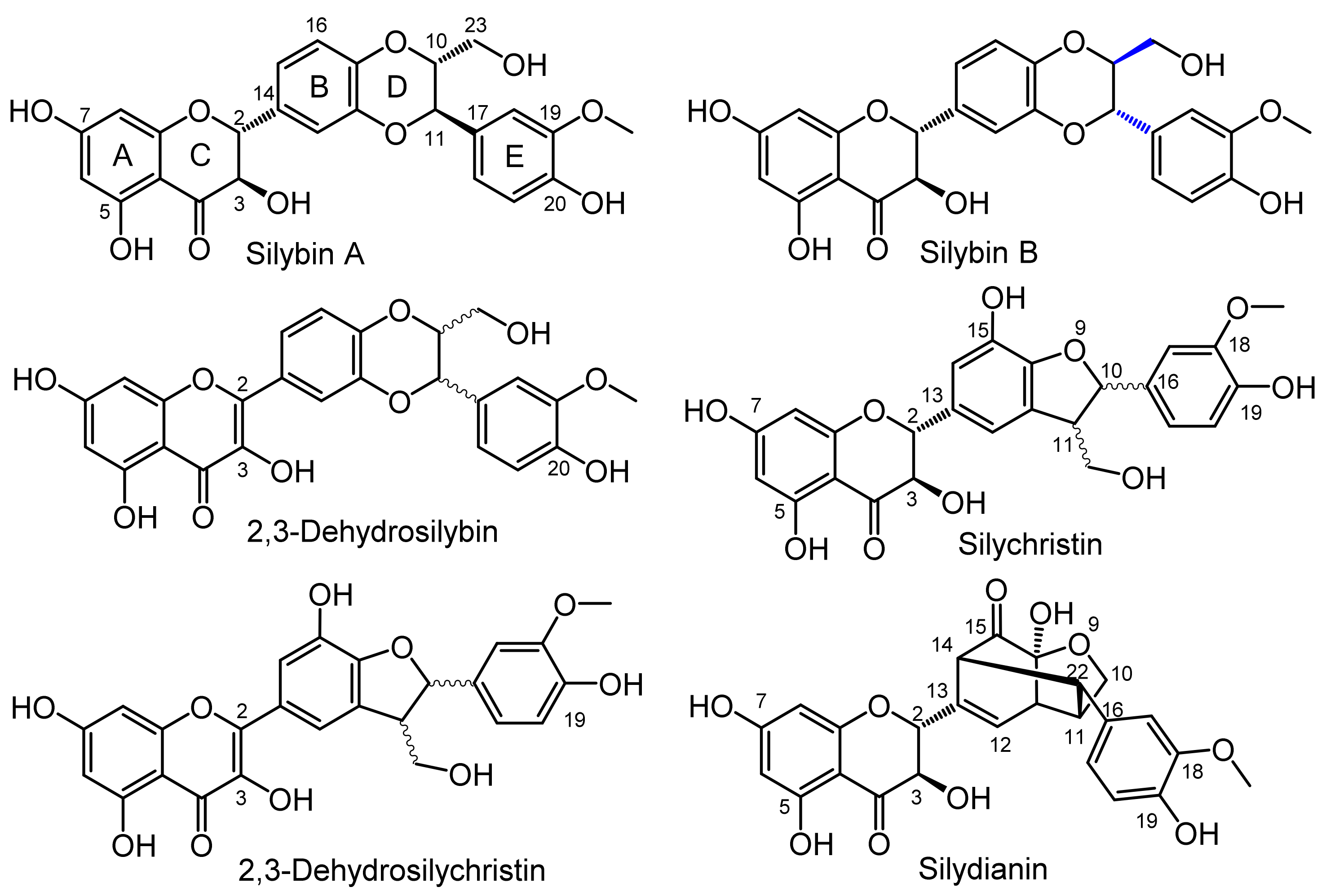
2.3. Analytical Characteristics and Structure Elucidation of the Sulfated Metabolites
2.4. The Effect of Sulfation on Radical Scavenging and Anti-Lipoperoxidant Activity of the Flavonolignans
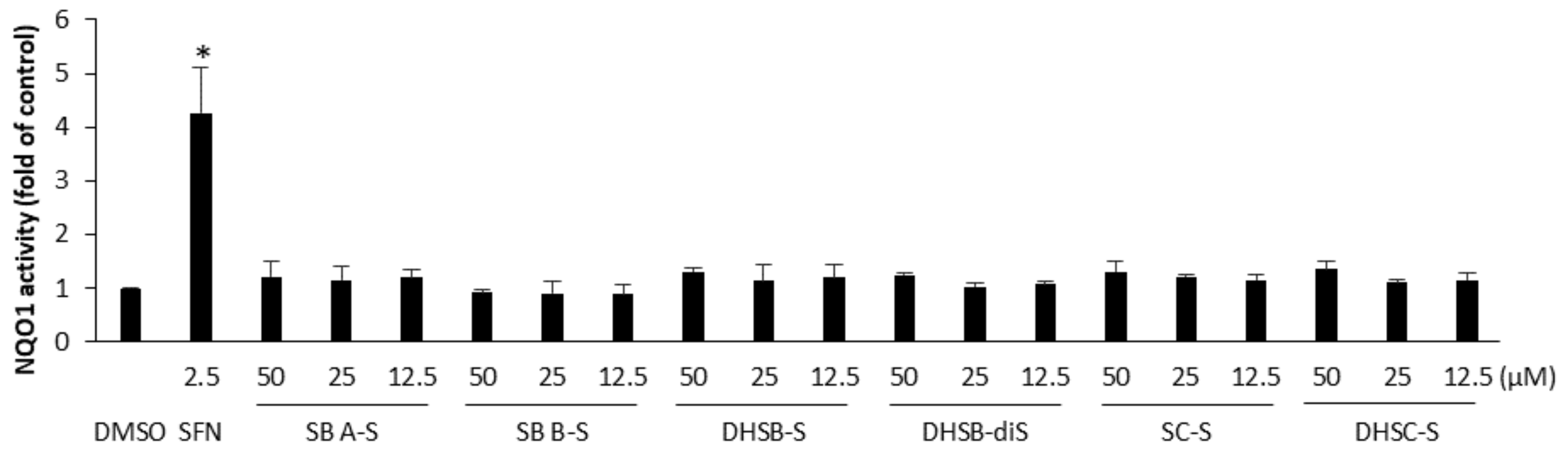
2.5. The Effect of the Sulfated Metabolites on the Nrf2 Pathway
3. Materials and Methods
3.1. General Methods
3.1.1. NMR
3.1.2. Mass Spectrometry (MS)
3.1.3. Analytical HPLC-PDA
3.2. Preparation of the Flavonolignans
3.3. Sulfation of the Flavonolignans by Aryl Sulfotransferase from Rat Liver (AST IV)
3.4. Preparation of Sulfated Flavonolignans by Aryl Sulfotransferase from Desulfitobacterium hafniense (AST DH)
3.4.1. Silybin
3.4.2. 2,3-Dehydrosilybin
3.4.3. Silychristin
3.4.4. Silydianin
3.5. Radical Scavenging and Anti-Lipoperoxidant Activities
3.6. Determination of Log p Values
3.7. Determination of NAD(P)H/Quinone Oxidoreductase 1 (NQO1) Activity in Hepa1c1c7 Cells
4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| AST | aryl sulfotransferase |
| AST DH | aryl sulfotransferase from Desulfitobacterium hafniense |
| AST IV | aryl sulfotransferase from rat liver |
| CE | vitamin C equivalents |
| COSY | correlation spectroscopy |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| DMPD | N,N-dimethyl-p-phenylenediamine |
| DMAP | 4-dimethylaminopyridine |
| DMSO | dimethylsulfoxide |
| FCR | Folin–Ciocalteu reagent |
| FRAP | ferric reducing antioxidant power |
| GAE | gallic acid equivalents |
| HMBC | heteronuclear multiple-bond correlation spectroscopy |
| HSQC | heteronuclear single-quantum correlation spectroscopy |
| IC50 | the concentration of the tested compound that inhibited the reaction by 50% |
| Lpx | lipid peroxidation |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NQO1 | NAD(P)H/quinone oxidoreductase 1 |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PBS | phosphate-buffered saline |
| p-NP | p-nitrophenol |
| p-NPS | p-nitrophenyl sulfate |
| PDA | photodiode array |
References
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Gažák, R.; Walterová, D.; Křen, V. Silybin and silymarin-new and emerging applications in medicine. Curr. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, D.; Vavříková, E.; Cvak, L.; Křen, V. Chemistry of silybin. Nat. Prod. Rep. 2014, 31, 1138–1157. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Seligmann, O.; Seitz, M.; Abraham, D.; Sonnenbichler, J. Silydianin and silychristin, 2 isomeric silymarins from Silybum marianum L. Gaertn (milk thistle). Z. Naturforsch. B 1976, 31, 876–884. [Google Scholar] [CrossRef]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojovic, M.; Popovic-Bijelic, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic. Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, D.; Buchta, M.; Holečková, V.; Sedlák, D.; Valentová, K.; Cvačka, J.; Bednárová, L.; Křenková, A.; Kuzma, M.; Škuta, C.; et al. Silychristin: Skeletal alterations and biological activities. J. Nat. Prod. 2016, 79, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Křenek, K.; Marhol, P.; Peikerová, Ž.; Křen, V.; Biedermann, D. Preparatory separation of the silymarin flavonolignans by Sephadex LH-20 gel. Food Res. Int. 2014, 65, 115–120. [Google Scholar] [CrossRef]
- Egea, J.; Fabregat, I.; Frapart, Y.M.; Ghezzi, P.; Görlach, A.; Kietzmann, T.; Kubaichuk, K.; Knaus, U.G.; Lopez, M.G.; Olaso-Gonzalez, G.; et al. European contribution to the study of ROS: A summary of the findings and prospects for the future from the COST action BM1203 (EU-ROS). Redox Biol. 2017, 13, 94–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Dev. Ther. 2016, 10, 23–42. [Google Scholar] [CrossRef]
- Křen, V.; Marhol, P.; Purchartová, K.; Gabrielová, E.; Modrianský, M. Biotransformation of silybin and its congeners. Curr. Drug Metab. 2013, 14, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Theodosiou, E.; Purchartová, K.; Stamatis, H.; Kolisis, F.; Křen, V. Bioavailability of silymarin flavonolignans: Drug formulations and biotransformation. Phytochem. Rev. 2014, 13, 1–18. [Google Scholar] [CrossRef]
- Miranda, S.R.; Lee, J.K.; Brouwer, K.L.R.; Wen, Z.M.; Smith, P.C.; Hawke, R.L. Hepatic metabolism and biliary excretion of silymarin flavonolignans in isolated perfused rat livers: Role of multidrug resistance-associated protein 2 (abcc2). Drug Metab. Dispos. 2008, 36, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Kr̆en, V.; Ulrichová, J.; Kosina, P.; Stevenson, D.; Sedmera, P.; Přikrylová, V.; Halada, P.; Šimánek, V. Chemoenzymatic preparation of silybin β-glucuronides and their biological evaluation. Drug Metab. Dispos. 2000, 28, 1513–1517. [Google Scholar] [PubMed]
- Marhol, P.; Bednář, P.; Kolářová, P.; Večeřa, R.; Ulrichová, J.; Tesařová, E.; Vavříková, E.; Kuzma, M.; Křen, V. Pharmacokinetics of pure silybin diastereoisomers and identification of their metabolites in rat plasma. J. Funct. Food. 2015, 14, 570–580. [Google Scholar] [CrossRef]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentová, K.; Williamson, G.; Santos, C.N. Bioavailability of quercetin in humans with a focus on interindividual variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef]
- Gonzalez-Sarrias, A.; Garcia-Villalba, R.; Romo-Vaquero, M.; Alasalvar, C.; Orem, A.; Zafrilla, P.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial. Mol. Nutr. Food Res. 2017, 61, ARTN 1600830. [Google Scholar] [CrossRef] [PubMed]
- Marhol, P.; Hartog, A.F.; van der Horst, M.A.; Wever, R.; Purchartová, K.; Fuksová, K.; Kuzma, M.; Cvačka, J.; Křen, V. Preparation of silybin and isosilybin sulfates by sulfotransferase from Desulfitobacterium hafniense. J. Mol. Catal. B-Enzym. 2013, 89, 24–27. [Google Scholar] [CrossRef]
- Purchartová, K.; Engels, L.; Marhol, P.; Šulc, M.; Kuzma, M.; Slámová, K.; Elling, L.; Křen, V. Enzymatic preparation of silybin phase II metabolites: Sulfation using aryl sulfotransferase from rat liver. Appl. Microbiol. Biot. 2013, 97, 10391–10398. [Google Scholar] [CrossRef] [PubMed]
- Purchartová, K.; Valentová, K.; Pelantová, H.; Marhol, P.; Cvačka, J.; Havlíček, L.; Křenkova, A.; Vavříková, E.; Biedermann, D.; Chambers, C.S.; et al. Prokaryotic and eukaryotic aryl sulfotransferases: Sulfation of quercetin and its derivatives. ChemCatChem 2015, 7, 3152–3162. [Google Scholar] [CrossRef]
- Trouillas, P.; Marsal, P.; Svobodová, A.; Vostálová, J.; Gažák, R.; Hrbáč, J.; Sedmera, P.; Křen, V.; Lazzaroni, R.; Duroux, J.-L.; et al. Mechanism of the antioxidant action of silybin and 2,3-dehydrosilybin flavonolignans: A joint experimental and theoretical study. J. Phys. Chem. A 2008, 112, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Gažák, R.; Trouillas, P.; Biedermann, D.; Fuksová, K.; Marhol, P.; Kuzma, M.; Křen, V. Base-catalyzed oxidation of silybin and isosilybin into 2,3-dehydro derivatives. Tetrahedron Lett. 2013, 54, 315–317. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Káňová, K.; Di Meo, F.; Pelantová, H.; Chambers, C.; Rydlová, L.; Petrásková, L.; Křenková, A.; Cvačka, J.; Trouillas, P. Chemoenzymatic preparation and biophysical properties of sulfated quercetin metabolites. Int. J. Mol. Sci. 2017, 18, 2231. [Google Scholar] [CrossRef] [PubMed]
- Roubalová, L.; Purchartová, K.; Papoušková, B.; Vacek, J.; Křen, V.; Ulrichová, J.; Vrba, J. Sulfation modulates the cell uptake, antiradical activity and biological effects of flavonoids in vitro: An examination of quercetin, isoquercitrin and taxifolin. Bioorg. Med. Chem. 2015, 23, 5402–5409. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, A.; Asres, K.; El-Fiky, F.K. Structure–radical scavenging activity relationships of flavonoids. Phytochemistry 2006, 67, 2058–2070. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.S.; Holečková, V.; Petrásková, L.; Biedermann, D.; Valentová, K.; Buchta, M.; Křen, V. The silymarin composition… and why does it matter??? Food Res. Int. 2017, 100, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Roubalová, L.; Dinkova-Kostova, A.T.; Biedermann, D.; Křen, V.; Ulrichová, J.; Vrba, J. Flavonolignan 2,3-dehydrosilydianin activates Nrf2 and upregulates NAD(P)H:quinone oxidoreductase 1 in Hepa1c1c7 cells. Fitoterapia 2017, 119, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Gažák, R.; Marhol, P.; Purchartová, K.; Monti, D.; Biedermann, D.; Riva, S.; Cvak, L.; Křen, V. Large-scale separation of silybin diastereoisomers using lipases. Process Biochem. 2010, 45, 1657–1663. [Google Scholar] [CrossRef]
- Džubák, P.; Hajdúch, M.; Gažák, R.; Svobodová, A.; Psotová, J.; Walterová, D.; Sedmera, P.; Křen, V. New derivatives of silybin and 2,3-dehydrosilybin and their cytotoxic and P-glycoprotein modulatory activity. Bioorg. Med. Chem. 2006, 14, 3793–3810. [Google Scholar] [CrossRef] [PubMed]
- Van der Horst, M.A.; van Lieshout, J.F.T.; Bury, A.; Hartog, A.F.; Wever, R. Sulfation of various alcoholic groups by an arylsulfate sulfotransferase from Desulfitobacterium hafniense and synthesis of estradiol sulfate. Adv. Synth. Catal. 2012, 354, 3501–3508. [Google Scholar] [CrossRef]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; RiceEvans, C.A. Factors influencing the antioxidant activity determined by the ABTS+ radical cation assay. Free Radic. Res. 1997, 26, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.; Pravadali-Cekic, S.; Dennis, G.R.; Bashir, R.; Mahon, P.J.; Shalliker, R.A. Ferric reducing antioxidant potential (FRAP) of antioxidants using reaction flow chromatography. Anal. Chim. Acta 2017, 967, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymol; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Joyeux, M.; Lobstein, A.; Anton, R.; Mortier, F. Comparative antilipoperoxidant, antinecrotic and scavenging properties of terpenes and biflavones from Ginkgo and some flavonoids. Planta Med. 1995, 61, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Heřmánková-Vavříková, E.; Křenková, A.; Petrásková, L.; Chambers, C.; Zápal, J.; Kuzma, M.; Valentová, K.; Křen, V. Synthesis and antiradical activity of isoquercitrin esters with aromatic acids and their homologues. Int. J. Mol. Sci. 2017, 18, 1074. [Google Scholar] [CrossRef] [PubMed]
- Vavříková, E.; Křen, V.; Jezova-Kalachova, L.; Biler, M.; Chantemargue, B.; Pyszková, M.; Riva, S.; Kuzma, M.; Valentová, K.; Ulrichová, J. Novel flavonolignan hybrid antioxidants: From enzymatic preparation to molecular rationalization. Eur. J. Med. Chem. 2017, 127, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Vavříková, E.; Langschwager, F.; Jezova-Kalachova, L.; Křenková, A.; Mikulová, B.; Kuzma, M.; Křen, V.; Valentová, K. Isoquercitrin esters with mono- or dicarboxylic acids: Enzymatic preparation and properties. Int. J. Mol. Sci. 2016, 17, 899. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Dinkova-Kostova, A.T.; Stephenson, K.K.; Talalay, P. The “Prochaska” microtiter plate bioassay for inducers of NQO1. In Methods in Enzymol; Academic Press: Cambridge, MA, USA, 2004; Volume 382, pp. 243–258. [Google Scholar]
- Vrba, J.; Papoušková, B.; Roubalová, L.; Zatloukalová, M.; Biedermann, D.; Křen, V.; Valentová, K.; Ulrichová, J.; Vacek, J. Metabolism of flavonolignans in human hepatocytes. J. Pharm. Biomed. Anal. 2018, 152, 94–101. [Google Scholar] [CrossRef] [PubMed]



| Substance | Formation of p-NP | Isolated Product(s) |
|---|---|---|
| Silybin A/B | + a | Silybin B 20-O-sulfate b |
| Silybin A | – c | − c |
| Silybin B | + a | Silybin B 20-O-sulfate |
| Silychristin | + a | – c |
| Silydianin | + a | – c |
| Compound | Purity [%] * | λmax [nm] † | HRMS-ESI m/z | RT [min] * | Isolated Yield | |
|---|---|---|---|---|---|---|
| [mg/ 100 mg] | [mol%] | |||||
| Silybin A-20-O-sulfate | 99 | 204/285 | 561.07037 | 3.835 | 65.5 | 56 |
| Silybin B 20-O-sulfate | 99.9 | 203/285 | 561.07068 | 3.840 | 46.5 | 40 |
| 2,3-Dehydrosilybin-20-O-sulfate | 95 | 254/368 | 559.05454 | 6.418 | 11 | 10 |
| 2,3-Dehydrosilybin-7,20-O-disulfate | 99 | 255/371 | 639.01115 | 3.439 | 15.5 | 12 |
| Silychristin-19-O-sulfate | 99.5 | 208/288/335 | 561.07092 | 5.422 | 37.5 | 32 |
| 2,3-Dehydrosilychristin-19-O-sulfate | 96 | 256/373 | 559.05526 | 6.756 | 1.1 (54) $ | 0.9.(55) $ |
| Silydianin-19-O-sulfate | ND ‡ | ND ‡ | ND ‡ | ND ‡ | 55 | 58 |
| Compound | DPPH a IC50 [μM] | ABTS+ b [CE] | FCR c [GAE] | DMPD+ d [CE] | FRAP e [Fe2+] |
|---|---|---|---|---|---|
| Silybin A | 490 ± 21 f | 1.01 ± 0.03 i | 0.33 ± 0.02 k | 0.96 ± 0.01 n | 0.06 ± 0.00 |
| Silybin A 20-O-sulfate | >2500 | 0.02 ± 0.00 | 0.08 ± 0.01 | 0.98 ± 0.01 n | 0.01 ± 0.00 p |
| Silybin B | 546 ± 17 f | 0.97 ± 0.05 i | 0.36 ± 0.03 k | 1.09 ± 0.01 n | 0.04 ± 0.00 q |
| Silybin B 20-O-sulfate | >2500 | 0.04 ± 0.00 | 0.16 ± 0.02 | 0.99 ± 0.01 n | 0.01 ± 0.00 p |
| 2,3-Dehydrosilybin | 13.3 ± 0.6 g | 0.77 ± 0.01 j | 1.51 ± 0.09 l | 1.02 ± 0.02 n | 0.81 ± 0.02 |
| 2,3-Dehydrosilybin-20-O-sulfate | 14.1 ± 0.5 g | 0.71 ± 0.03 j | 1.03 ± 0.01 m | 0.97 ± 0.02 n | 1.46 ± 0.03 r |
| 2,3-Dehydrosilybin-7,20-di-O-sulfate | 106 ± 4 | 0.55 ± 0.03 | 0.95 ± 0.01 m | 1.90 ± 0.10 | 1.61 ± 0.04 |
| Silychristin | 37.1 ± 3.1 | 1.50 ± 0.09 | 1.13 ± 0.23 m | 1.50 ± 0.02o | 0.64 ± 0.06 |
| Silychristin-19-O-sulfate | 587 ± 11 f | 0.62 ± 0.03 k | 0.80 ± 0.11 m | 1.56 ± 0.08o | 0.05 ± 0.01 q |
| 2,3-Dehydrosilychristin | 8.6 ± 0.8 h | 0.93 ± 0.03 l | 1.58 ± 0.04 l | 0.97 ± 0.02 n | 0.28 ± 0.01 |
| 2,3-Dehydro-silychristin-19-O-sulfate | 7.9 ± 0.3 h | 0.62 ± 0.02 n | 1.44 ± 0.01 l | 1.02 ± 0.04 n | 1.44 ± 0.04 r |
| Trolox | 2.9 ± 0.1 | ND | 0.33 ± 0.00 k | ND | ND |
| Compound | Lpx a IC50 [μM] | Log p | |
|---|---|---|---|
| Exp b | Pred c | ||
| Silybin A | 48.6 ± 0.4 | 1.52 | 1.47 |
| Silybin A 20-O-sulfate | >1000 | −1.65 | −2.03 |
| Silybin B | 68.6 ± 2.2 | 2.17 | 1.47 |
| Silybin B 20-O-sulfate | >1000 | −2.34 | −2.03 |
| 2,3-Dehydrosilybin | 10.4 ± 0.4 | >3 e | 2.44 |
| 2,3-Dehydrosilybin-20-O-sulfate | 13.7 ± 0.4 d | −2.16 | −1.06 |
| 2,3-Dehydrosilybin-7,20-di-O-sulfate | 90.7 ± 3.3 | <−3 f | −1.65 |
| Silychristin | 17.9 ± 0.7 | 1.47 | 1.26 |
| Silychristin-19-O-sulfate | 134 ± 2 | −2.25 | −2.23 |
| 2,3-Dehydrosilychristin | 14.6 ± 0.4 d | >3 e | 2.24 |
| 2,3-Dehydro-silychristin-19-O-sulfate | 12.0 ± 1.8 d | −2.24 | −1.26 |
| Trolox | 32.7 ± 2.4 | ND | 1.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentová, K.; Purchartová, K.; Rydlová, L.; Roubalová, L.; Biedermann, D.; Petrásková, L.; Křenková, A.; Pelantová, H.; Holečková-Moravcová, V.; Tesařová, E.; et al. Sulfated Metabolites of Flavonolignans and 2,3-Dehydroflavonolignans: Preparation and Properties. Int. J. Mol. Sci. 2018, 19, 2349. https://doi.org/10.3390/ijms19082349
Valentová K, Purchartová K, Rydlová L, Roubalová L, Biedermann D, Petrásková L, Křenková A, Pelantová H, Holečková-Moravcová V, Tesařová E, et al. Sulfated Metabolites of Flavonolignans and 2,3-Dehydroflavonolignans: Preparation and Properties. International Journal of Molecular Sciences. 2018; 19(8):2349. https://doi.org/10.3390/ijms19082349
Chicago/Turabian StyleValentová, Kateřina, Kateřina Purchartová, Lenka Rydlová, Lenka Roubalová, David Biedermann, Lucie Petrásková, Alena Křenková, Helena Pelantová, Veronika Holečková-Moravcová, Eva Tesařová, and et al. 2018. "Sulfated Metabolites of Flavonolignans and 2,3-Dehydroflavonolignans: Preparation and Properties" International Journal of Molecular Sciences 19, no. 8: 2349. https://doi.org/10.3390/ijms19082349