Liver X Receptors: A Possible Link between Lipid Disorders and Female Infertility
Abstract
:1. Introduction
2. Fertility Disorders and Abnormal Lipid Homeostasis
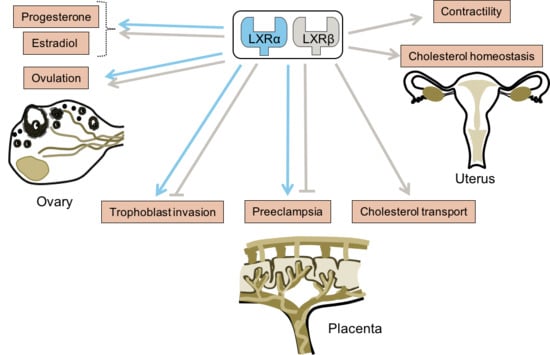
3. LXRs and Their Ligands in the Ovary
4. LXRs, Uterus, and Placenta
5. LXRs, Modulation of Estrogen Activity, and Breast Cancer
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1/G1 | ATP-binding cassetteA1/G1 |
| AMH | anti-Mullerian hormone |
| APO | apolipoprotein |
| COX | cyclooxygenase |
| Cyp11A1 | cytochrome P450 side-chain cleavage |
| Cyp19a1 | cytochrome P450 aromatase |
| ER | estrogen receptor |
| EST | estrogen sulfotransferase |
| FFMAS | follicular fluid meiosis-activating sterol |
| hCG | human chorionic gonadotropin |
| HDL | high density lipoprotein |
| Hsd3b | 3 β-hydroxysteroid dehydrogenase/∆5-4-isomerase |
| IL6 | interleukin 6 |
| iNOS | inducible nitric oxide synthase |
| JAK/STAT | janus kinase/signal transducer and activator of transcription protein |
| LDL | low density lipoprotein |
| LDLR | LDL receptor; LXR, liver X receptor |
| Lxr−/− mice | LXR-deficient mice |
| NR | nuclear receptor |
| OHSS | ovarian hyper stimulation syndrome |
| PCOS | polycystic ovary syndrome |
| PI3K | phosphoinositide 3-kinase |
| SCARB1 | scavenger receptor class B type |
| SREBP | sterol response element binding protein |
| SLiMs | selective liver X receptor modulators |
| StAR | steroidogenic acute regulatory protein |
| SULT2A1 | Sulfotransferase Family 2A Member 1 |
| VEGF | vascular endothelial growth factor |
| 27OHC | 27-hydroxycholesterol |
References
- Petraglia, F.; Serour, G.I.; Chapron, C. The changing prevalence of infertility. Int. J. Gynaecol. Obstet. 2013, 123, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, S.S.; Tannir, J.; Brannigan, R.E. The metabolic syndrome and male infertility. J. Androl. 2008, 29, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Norman, R.J.; Noakes, M.; Wu, R.; Davies, M.J.; Moran, L.; Wang, J.X. Improving reproductive performance in overweight/obese women with effective weight management. Hum. Reprod. Update 2004, 10, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Schisterman, E.F.; Mumford, S.L.; Browne, R.W.; Barr, D.B.; Chen, Z.; Louis, G.M.B. Lipid concentrations and couple fecundity: The LIFE study. J. Clin. Endocrinol. Metab. 2014, 99, 2786–2794. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.L.; Kim, S.; Chen, Z.; Sundaram, R.; Schisterman, E.F.; Buck Louis, G.M. The relationship between male BMI and waist circumference on semen quality: Data from the LIFE study. Hum. Reprod. 2014, 29, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Apridonidze, T.; Essah, P.A.; Iuorno, M.J.; Nestler, J.E. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2005, 90, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Maqdasy, S.; Baptissart, M.; Vega, A.; Baron, S.; Lobaccaro, J.-M.A.; Volle, D.H. Cholesterol and male fertility: What about orphans and adopted? Mol. Cell. Endocrinol. 2013, 368, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Pitteloud, N.; Hardin, M.; Dwyer, A.A.; Valassi, E.; Yialamas, M.; Elahi, D.; Hayes, F.J. Increasing insulin resistance is associated with a decrease in Leydig cell testosterone secretion in men. J. Clin. Endocrinol. Metab. 2005, 90, 2636–2641. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Christiansen-Weber, T.A.; Voland, J.R.; Wu, Y.; Ngo, K.; Roland, B.L.; Nguyen, S.; Peterson, P.A.; Fung-Leung, W.P. Functional loss of ABCA1 in mice causes severe placental malformation, aberrant lipid distribution, and kidney glomerulonephritis as well as high-density lipoprotein cholesterol deficiency. Am. J. Pathol. 2000, 157, 1017–1029. [Google Scholar] [CrossRef]
- Yesilaltay, A.; Dokshin, G.A.; Busso, D.; Wang, L.; Galiani, D.; Chavarria, T.; Vasile, E.; Quilaqueo, L.; Orellana, J.A.; Walzer, D.; et al. Excess cholesterol induces mouse egg activation and may cause female infertility. Proc. Natl. Acad. Sci. USA 2014, 111, E4972–E4980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummins, C.L.; Volle, D.H.; Zhang, Y.; McDonald, J.G.; Sion, B.; Lefrançois-Martinez, A.-M.; Caira, F.; Veyssière, G.; Mangelsdorf, D.J.; Lobaccaro, J.-M.A. Liver X receptors regulate adrenal cholesterol balance. J. Clin. Investig. 2006, 116, 1902–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, S.B.; Castrillo, A.; Laffitte, B.A.; Mangelsdorf, D.J.; Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003, 9, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Maqdasy, S.; El Hajjaji, F.-Z.; Baptissart, M.; Viennois, E.; Oumeddour, A.; Brugnon, F.; Trousson, A.; Tauveron, I.; Volle, D.; Lobaccaro, J.-M.A.; et al. Identification of the functions of Liver X Receptor β in Sertoli cells using a targeted expression-rescue model. Endocrinology 2015, 156, 4545–4557. [Google Scholar] [CrossRef] [PubMed]
- Mouzat, K.; Baron, S.; Marceau, G.; Caira, F.; Sapin, V.; Volle, D.H.; Lumbroso, S.; Lobaccaro, J.-M. Emerging roles for LXRs and LRH-1 in female reproduction. Mol. Cell. Endocrinol. 2013, 368, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Mouzat, K.; Volat, F.; Baron, S.; Alves, G.; Pommier, A.J.C.; Volle, D.H.; Marceau, G.; DeHaze, A.; Déchelotte, P.; Duggavathi, R.; et al. Absence of nuclear receptors for oxysterols liver X receptor induces ovarian hyperstimulation syndrome in mice. Endocrinology 2009, 150, 3369–3375. [Google Scholar] [CrossRef] [PubMed]
- Volle, D.H.; Mouzat, K.; Duggavathi, R.; Siddeek, B.; Déchelotte, P.; Sion, B.; Veyssière, G.; Benahmed, M.; Lobaccaro, J.-M.A. Multiple roles of the nuclear receptors for oxysterols liver X receptor to maintain male fertility. Mol. Endocrinol. Baltim. 2007, 21, 1014–1027. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.R.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef] [PubMed]
- Grøndahl, C.; Ottesen, J.L.; Lessl, M.; Faarup, P.; Murray, A.; Grønvald, F.C.; Hegele-Hartung, C.; Ahnfelt-Rønne, I. Meiosis-activating sterol promotes resumption of meiosis in mouse oocytes cultured in vitro in contrast to related oxysterols. Biol. Reprod. 1998, 58, 1297–1302. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, K.R.; Robertson, K.; Gustafsson, J.-A.; Andersen, C.Y. Reduced fertility and inability of oocytes to resume meiosis in mice deficient of the Lxr genes. Mol. Cell. Endocrinol. 2006, 256, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Sarfati, J.; Young, J.; Christin-Maitre, S. Obesity and female reproduction. Ann. Endocrinol. 2010, 71 (Suppl. 1), S49–S53. [Google Scholar] [CrossRef]
- Teede, H.J.; Meyer, C.; Hutchison, S.K.; Zoungas, S.; McGrath, B.P.; Moran, L.J. Endothelial function and insulin resistance in polycystic ovary syndrome: The effects of medical therapy. Fertil. Steril. 2010, 93, 184–191. [Google Scholar] [CrossRef] [PubMed]
- March, W.A.; Moore, V.M.; Willson, K.J.; Phillips, D.I.W.; Norman, R.J.; Davies, M.J. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum. Reprod. 2010, 25, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Woods, K.S.; Reyna, R.; Key, T.J.; Knochenhauer, E.S.; Yildiz, B.O. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 2004, 89, 2745–2749. [Google Scholar] [CrossRef] [PubMed]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, E.; Pavone, M.E.; Hirshfeld-Cytron, J.E. Metabolic syndrome and oocyte quality. Trends Endocrinol. Metab. 2011, 22, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, E.; de Pergola, G.; Rosania, R.; Loverro, G. Obesity as disruptor of the female fertility. Reprod. Biol. Endocrinol. 2018, 16, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merhi, Z. Advanced glycation end products and their relevance in female reproduction. Hum. Reprod. 2014, 29, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ramlau-Hansen, C.H.; Thulstrup, A.M.; Nohr, E.A.; Bonde, J.P.; Sørensen, T.I.A.; Olsen, J. Subfecundity in overweight and obese couples. Hum. Reprod. 2007, 22, 1634–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracia, C.R.; Freeman, E.W.; Sammel, M.D.; Lin, H.; Nelson, D.B. The relationship between obesity and race on inhibin B during the menopause transition. Menopause 2005, 12, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.W.; Gracia, C.R.; Sammel, M.D.; Lin, H.; Lim, L.C.-L.; Strauss, J.F. Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil. Steril. 2007, 87, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Minge, C.E.; Bennett, B.D.; Norman, R.J.; Robker, R.L. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reverses the adverse effects of diet-induced obesity on oocyte quality. Endocrinology 2008, 149, 2646–2656. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, E.S.; Schoeller, E.L.; Marquard, K.L.; Louden, E.D.; Schaffer, J.E.; Moley, K.H. Diet-induced obesity model: Abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology 2010, 151, 4039–4046. [Google Scholar] [CrossRef] [PubMed]
- Igosheva, N.; Abramov, A.Y.; Poston, L.; Eckert, J.J.; Fleming, T.P.; Duchen, M.R.; McConnell, J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS ONE 2010, 5, e10074. [Google Scholar] [CrossRef] [PubMed]
- Trigatti, B.; Rayburn, H.; Viñals, M.; Braun, A.; Miettinen, H.; Penman, M.; Hertz, M.; Schrenzel, M.; Amigo, L.; Rigotti, A.; et al. Influence of the high density lipoprotein receptor SR-BI on reproductive and cardiovascular pathophysiology. Proc. Natl. Acad. Sci. USA 1999, 96, 9322–9327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Dai, P.; Cheng, D.; Zhang, L.; Chen, Z.; Meng, X.; Zhang, F.; Han, X.; Liu, J.; Pan, J.; et al. Obesity occurring in apolipoprotein E-knockout mice has mild effects on fertility. Reproduction 2014, 147, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, V.Y.; Kane, J.P.; Ishida, B.Y.; Bloom, M.S.; Browne, R.W. High-density lipoprotein metabolism and the human embryo. Hum. Reprod. Update 2010, 16, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Mahley, R.W.; Rall, S.C. Apolipoprotein E: Far more than a lipid transport protein. Annu. Rev. Genom. Hum. Genet. 2000, 1, 507–537. [Google Scholar] [CrossRef] [PubMed]
- Corbo, R.M.; Ulizzi, L.; Scacchi, R.; Martínez-Labarga, C.; De Stefano, G.F. Apolipoprotein E polymorphism and fertility: A study in pre-industrial populations. Mol. Hum. Reprod. 2004, 10, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Janowski, B.A.; Grogan, M.J.; Jones, S.A.; Wisely, G.B.; Kliewer, S.A.; Corey, E.J.; Mangelsdorf, D.J. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA 1999, 96, 266–271. [Google Scholar] [CrossRef] [PubMed]
- De Medina, P.; Paillasse, M.R.; Segala, G.; Voisin, M.; Mhamdi, L.; Dalenc, F.; Lacroix-Triki, M.; Filleron, T.; Pont, F.; Saati, T.A.; et al. Dendrogenin A arises from cholesterol and histamine metabolism and shows cell differentiation and anti-tumour properties. Nat. Commun. 2013, 4, 1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirot, M.; Silvente-Poirot, S. The tumor-suppressor cholesterol metabolite, dendrogenin A, is a new class of LXR modulator activating lethal autophagy in cancers. Biochem. Pharmacol. 2018, 153, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Drouineaud, V.; Sagot, P.; Garrido, C.; Logette, E.; Deckert, V.; Gambert, P.; Jimenez, C.; Staels, B.; Lagrost, L.; Masson, D. Inhibition of progesterone production in human luteinized granulosa cells treated with LXR agonists. Mol. Hum. Reprod. 2007, 13, 373–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardsen, L.; Wiersma, A.; Baltsen, M.; Byskov, A.G.; Andersen, C.Y. Regulation of spontaneous and induced resumption of meiosis in mouse oocytes by different intracellular pathways. J. Reprod. Fertil. 2000, 120, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Grøndahl, C. Oocyte maturation. Basic and clinical aspects of in vitro maturation (IVM) with special emphasis of the role of FF-MAS. Dan. Med. Bull. 2008, 55, 1–16. [Google Scholar] [PubMed]
- Marín Bivens, C.L.; Lindenthal, B.; O’Brien, M.J.; Wigglesworth, K.; Blume, T.; Grøndahl, C.; Eppig, J.J. A synthetic analogue of meiosis-activating sterol (FF-MAS) is a potent agonist promoting meiotic maturation and preimplantation development of mouse oocytes maturing in vitro. Hum. Reprod. 2004, 19, 2340–2344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Hernández-Ledezma, J.J.; Hutchison, S.M.; Bogan, R.L. The liver X receptors and sterol regulatory element binding proteins alter progesterone secretion and are regulated by human chorionic gonadotropin in human luteinized granulosa cells. Mol. Cell. Endocrinol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Artini, P.G.; Monti, M.; Fasciani, A.; Battaglia, C.; D’Ambrogio, G.; Genazzani, A.R. Vascular endothelial growth factor, interleukin-6 and interleukin-2 in serum and follicular fluid of patients with ovarian hyperstimulation syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 101, 169–174. [Google Scholar] [CrossRef]
- Bar-Joseph, H.; Ben-Ami, I.; Ron-El, R.; Shalgi, R.; Chuderland, D. Pigment epithelium-derived factor regulation by human chorionic gonadotropin in granulosa cells. Reproduction 2016, 151, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.D.; Chen, H.F.; Lu, H.F.; Chen, S.U.; Ho, H.N.; Yang, Y.S. Value of serum and follicular fluid cytokine profile in the prediction of moderate to severe ovarian hyperstimulation syndrome. Hum. Reprod. 2000, 15, 1037–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuderland, D.; Ben-Ami, I.; Kaplan-Kraicer, R.; Grossman, H.; Ron-El, R.; Shalgi, R. The role of pigment epithelium-derived factor in the pathophysiology and treatment of ovarian hyperstimulation syndrome in mice. J. Clin. Endocrinol. Metab. 2013, 98, E258–E266. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.; Chuderland, D.; Ron-El, R.; Shalgi, R.; Ben-Ami, I. GnRH Agonist Triggering Modulates PEDF to VEGF Ratio Inversely to hCG in Granulosa Cells. J. Clin. Endocrinol. Metab. 2015, 100, E1428–E1436. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.-H.; Chou, C.-H.; Chen, M.-W.; Rose-John, S.; Kuo, M.-L.; Chen, S.-U.; Yang, Y.-S. The role of IL-6 trans-signaling in vascular leakage: Implications for ovarian hyperstimulation syndrome in a murine model. J. Clin. Endocrinol. Metab. 2013, 98, E472–E484. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, A.; Albert, C.; Mercader, A.; Bonilla-Musoles, F.; Remohí, J.; Simón, C. The pathogenesis of ovarian hyperstimulation syndrome: In vivo studies investigating the role of interleukin-1beta, interleukin-6, and vascular endothelial growth factor. Fertil. Steril. 1999, 71, 482–489. [Google Scholar] [CrossRef]
- Landis, M.S.; Patel, H.V.; Capone, J.P. Oxysterol activators of liver X receptor and 9-cis-retinoic acid promote sequential steps in the synthesis and secretion of tumor necrosis factor-alpha from human monocytes. J. Biol. Chem. 2002, 277, 4713–4721. [Google Scholar] [CrossRef] [PubMed]
- Walczak, R.; Joseph, S.B.; Laffitte, B.A.; Castrillo, A.; Pei, L.; Tontonoz, P. Transcription of the vascular endothelial growth factor gene in macrophages is regulated by liver X receptors. J. Biol. Chem. 2004, 279, 9905–9911. [Google Scholar] [CrossRef] [PubMed]
- Beyer, C.; Huang, J.; Beer, J.; Zhang, Y.; Palumbo-Zerr, K.; Zerr, P.; Distler, A.; Dees, C.; Maier, C.; Munoz, L.; et al. Activation of liver X receptors inhibits experimental fibrosis by interfering with interleukin-6 release from macrophages. Ann. Rheum. Dis. 2015, 74, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Noghero, A.; Perino, A.; Seano, G.; Saglio, E.; Lo Sasso, G.; Veglio, F.; Primo, L.; Hirsch, E.; Bussolino, F.; Morello, F. Liver X receptor activation reduces angiogenesis by impairing lipid raft localization and signaling of vascular endothelial growth factor receptor-2. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Nunomura, S.; Okayama, Y.; Matsumoto, K.; Hashimoto, N.; Endo-Umeda, K.; Terui, T.; Makishima, M.; Ra, C. Activation of LXRs using the synthetic agonist GW3965 represses the production of pro-inflammatory cytokines by murine mast cells. Allergol. Int. 2015, 64 (Suppl. 1), 11–17. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, K.R.; Andersen, M.L.; Schantz, A.L. Obesity and pregnancy. Acta Obstet. Gynecol. Scand. 2004, 83, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Sebire, N.J.; Jolly, M.; Harris, J.P.; Wadsworth, J.; Joffe, M.; Beard, R.W.; Regan, L.; Robinson, S. Maternal obesity and pregnancy outcome: A study of 287,213 pregnancies in London. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Bricker, L.; Wray, S.; Quenby, S. Poor uterine contractility in obese women. Int. J. Obstet. Gynaecol. 2007, 114, 343–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barau, G.; Robillard, P.-Y.; Hulsey, T.C.; Dedecker, F.; Laffite, A.; Gérardin, P.; Kauffmann, E. Linear association between maternal pre-pregnancy body mass index and risk of caesarean section in term deliveries. Int. J. Obstet. Gynaecol. 2006, 113, 1173–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doherty, D.A.; Magann, E.F.; Francis, J.; Morrison, J.C.; Newnham, J.P. Pre-pregnancy body mass index and pregnancy outcomes. Int. J. Gynaecol. Obstet. 2006, 95, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Kabiru, W.; Raynor, B.D. Obstetric outcomes associated with increase in BMI category during pregnancy. Am. J. Obstet. Gynecol. 2004, 191, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Zhong, Y.; Kang, P.; Lau, F.; Blackburn, M.L.; Chen, J.-R.; Borengasser, S.J.; Ronis, M.J.J.; Badger, T.M. Maternal obesity promotes a proinflammatory signature in rat uterus and blastocyst. Endocrinology 2011, 152, 4158–4170. [Google Scholar] [CrossRef] [PubMed]
- Garris, D.R.; Garris, B.L. Cytolipotoxicity-induced involution of the female reproductive tract following expression of obese (ob/ob) and diabetes (db/db) genotype mutations: Progressive, hyperlipidemic transformation into adipocytic tissues. Reprod. Toxicol. 2004, 18, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.; Babiychuk, E.B.; Noble, K.; Draeger, A.; Wray, S. Increased cholesterol decreases uterine activity: Functional effects of cholesterol alteration in pregnant rat myometrium. Am. J. Physiol. Cell Physiol. 2005, 288, C982–C988. [Google Scholar] [CrossRef] [PubMed]
- Noble, K.; Zhang, J.; Wray, S. Lipid rafts, the sarcoplasmic reticulum and uterine calcium signalling: An integrated approach. J. Physiol. 2006, 570, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Mouzat, K.; Prod’homme, M.; Volle, D.H.; Sion, B.; Déchelotte, P.; Gauthier, K.; Vanacker, J.-M.; Lobaccaro, J.-M.A. Oxysterol nuclear receptor LXRbeta regulates cholesterol homeostasis and contractile function in mouse uterus. J. Biol. Chem. 2007, 282, 4693–4701. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.A. The endocrine function of human placenta: An overview. Reprod. Biomed. Online 2016, 32, 14–43. [Google Scholar] [CrossRef] [PubMed]
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Wojdyla, D.; Say, L.; Gülmezoglu, A.M.; Van Look, P.F.A. WHO analysis of causes of maternal death: A systematic review. Lancet 2006, 367, 1066–1074. [Google Scholar] [CrossRef]
- Myatt, L. Role of placenta in preeclampsia. Endocrine 2002, 19, 103–111. [Google Scholar] [CrossRef]
- Myatt, L.; Webster, R.P. Vascular biology of preeclampsia. J. Thromb. Haemost. 2009, 7, 375–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar] [CrossRef] [PubMed]
- Pavan, L.; Hermouet, A.; Tsatsaris, V.; Thérond, P.; Sawamura, T.; Evain-Brion, D.; Fournier, T. Lipids from oxidized low-density lipoprotein modulate human trophoblast invasion: Involvement of nuclear liver X receptors. Endocrinology 2004, 145, 4583–4591. [Google Scholar] [CrossRef] [PubMed]
- Marceau, G.; Volle, D.H.; Gallot, D.; Mangelsdorf, D.J.; Sapin, V.; Lobaccaro, J.-M.A. Placental expression of the nuclear receptors for oxysterols LXRalpha and LXRbeta during mouse and human development. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2005, 283, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Plösch, T.; Gellhaus, A.; van Straten, E.M.E.; Wolf, N.; Huijkman, N.C.A.; Schmidt, M.; Dunk, C.E.; Kuipers, F.; Winterhager, E. The liver X receptor (LXR) and its target gene ABCA1 are regulated upon low oxygen in human trophoblast cells: A reason for alterations in preeclampsia? Placenta 2010, 31, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Schroepfer, G.J. Oxysterols: Modulators of cholesterol metabolism and other processes. Physiol. Rev. 2000, 80, 361–554. [Google Scholar] [CrossRef] [PubMed]
- Woollett, L.A. Review: Transport of maternal cholesterol to the fetal circulation. Placenta 2011, 32 (Suppl. 2), S218–S221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fournier, T.; Handschuh, K.; Tsatsaris, V.; Guibourdenche, J.; Evain-Brion, D. Role of nuclear receptors and their ligands in human trophoblast invasion. J. Reprod. Immunol. 2008, 77, 161–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aye, I.L.M.H.; Waddell, B.J.; Mark, P.J.; Keelan, J.A. Oxysterols inhibit differentiation and fusion of term primary trophoblasts by activating liver X receptors. Placenta 2011, 32, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Weedon-Fekjær, M.S.; Johnsen, G.M.; Anthonisen, E.H.; Sugulle, M.; Nebb, H.I.; Duttaroy, A.K.; Staff, A.C. Expression of liver X receptors in pregnancies complicated by preeclampsia. Placenta 2010, 31, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Henry-Berger, J.; Mouzat, K.; Baron, S.; Bernabeu, C.; Marceau, G.; Saru, J.-P.; Sapin, V.; Lobaccaro, J.-M.A.; Caira, F. Endoglin (CD105) expression is regulated by the liver X receptor alpha (NR1H3) in human trophoblast cell line JAR. Biol. Reprod. 2008, 78, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Venkatesha, S.; Toporsian, M.; Lam, C.; Hanai, J.; Mammoto, T.; Kim, Y.M.; Bdolah, Y.; Lim, K.-H.; Yuan, H.-T.; Libermann, T.A.; et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 2006, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Mouzat, K.; Mercier, E.; Polge, A.; Evrard, A.; Baron, S.; Balducchi, J.-P.; Brouillet, J.-P.; Lumbroso, S.; Gris, J.-C. A common polymorphism in NR1H2 (LXRbeta) is associated with preeclampsia. BMC Med. Genet. 2011, 12, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, H.J.; Mangu, P.B.; Somerfield, M.R.; Schrag, D.; Samson, D.; Holt, L.; Zelman, D.; Ajani, J.A. American Society of Clinical Oncology American Society of Clinical Oncology clinical practice guideline update on the use of chemotherapy sensitivity and resistance assays. J. Clin. Oncol. 2011, 29, 3328–3330. [Google Scholar] [CrossRef] [PubMed]
- Obiorah, I.; Jordan, V.C. Progress in endocrine approaches to the treatment and prevention of breast cancer. Maturitas 2011, 70, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Kaaks, R.; Vainio, H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002, 3, 565–574. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Berrington de González, A.; Freedman, N.D.; Huxley, R.; Mok, Y.; Jee, S.H.; Samet, J.M. Total cholesterol and cancer risk in a large prospective study in Korea. J. Clin. Oncol. 2011, 29, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Ferraroni, M.; Gerber, M.; Decarli, A.; Richardson, S.; Marubini, E.; Crastes de Paulet, P.; Crastes de Paulet, A.; Pujol, H. HDL-cholesterol and breast cancer: A joint study in northern Italy and southern France. Int. J. Epidemiol. 1993, 22, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Roberts, D.L.; Dive, C. Obesity and cancer: Pathophysiological and biological mechanisms. Arch. Physiol. Biochem. 2008, 114, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Vedin, L.-L.; Lewandowski, S.A.; Parini, P.; Gustafsson, J.-A.; Steffensen, K.R. The oxysterol receptor LXR inhibits proliferation of human breast cancer cells. Carcinogenesis 2009, 30, 575–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, W.C. Biochemistry and reproductive endocrinology of estrogen sulfotransferase. Ann. N. Y. Acad. Sci. 2001, 948, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kramer, P.R.; Wray, S. 17-Beta-estradiol regulates expression of genes that function in macrophage activation and cholesterol homeostasis. J. Steroid Biochem. Mol. Biol. 2002, 81, 203–216. [Google Scholar] [CrossRef]
- Lundholm, L.; Movérare, S.; Steffensen, K.R.; Nilsson, M.; Otsuki, M.; Ohlsson, C.; Gustafsson, J.-A.; Dahlman-Wright, K. Gene expression profiling identifies liver X receptor alpha as an estrogen-regulated gene in mouse adipose tissue. J. Mol. Endocrinol. 2004, 32, 879–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.-I.; Komatsu, Y.; Murayama, A.; Steffensen, K.R.; Nakagawa, Y.; Nakajima, Y.; Suzuki, M.; Oie, S.; Parini, P.; Vedin, L.-L.; et al. Estrogen receptor ligands ameliorate fatty liver through a nonclassical estrogen receptor/Liver X receptor pathway in mice. Hepatol. Baltim. 2014, 59, 1791–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Eon, T.M.; Souza, S.C.; Aronovitz, M.; Obin, M.S.; Fried, S.K.; Greenberg, A.S. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J. Biol. Chem. 2005, 280, 35983–35991. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Vu, T.; Vedin, L.-L.; Liu, K.; Jonsson, P.; Lin, J.Z.; Candelaria, N.R.; Candelaria, L.P.; Addanki, S.; Williams, C.; Gustafsson, J.-Å.; et al. Liver × receptor ligands disrupt breast cancer cell proliferation through an E2F-mediated mechanism. Breast Cancer Res. 2013, 15, R51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Roz, A.; Bard, J.-M.; Valin, S.; Huvelin, J.-M.; Nazih, H. Macrophage apolipoprotein E and proliferation of MCF-7 breast cancer cells: Role of LXR. Anticancer Res. 2013, 33, 3783–3789. [Google Scholar] [PubMed]
- El Roz, A.; Bard, J.-M.; Huvelin, J.-M.; Nazih, H. LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: Relation to proliferation and apoptosis. Anticancer Res. 2012, 32, 3007–3013. [Google Scholar] [PubMed]
- El Roz, A.; Bard, J.M.; Huvelin, J.M.; Nazih, H. The anti-proliferative and pro-apoptotic effects of the trans9, trans11 conjugated linoleic acid isomer on MCF-7 breast cancer cells are associated with LXR activation. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Guo, P.; Zhai, Y.; Zhou, J.; Uppal, H.; Jarzynka, M.J.; Song, W.-C.; Cheng, S.-Y.; Xie, W. Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol. Endocrinol. Baltim. 2007, 21, 1781–1790. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.S.; Paniccia, A.; Russo, V.; Steffensen, K.R. LXR Inhibits Proliferation of Human Breast Cancer Cells through the PI3K-Akt Pathway. Nucl. Recept. Res. 2015, 2, 101154. [Google Scholar] [CrossRef]
- Nelson, E.R.; Wardell, S.E.; Jasper, J.S.; Park, S.; Suchindran, S.; Howe, M.K.; Carver, N.J.; Pillai, R.V.; Sullivan, P.M.; Sondhi, V.; et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 2013, 342, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Warner, M.; Gustafsson, J.-A. On estrogen, cholesterol metabolism, and breast cancer. N. Engl. J. Med. 2014, 370, 572–573. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dallel, S.; Tauveron, I.; Brugnon, F.; Baron, S.; Lobaccaro, J.M.A.; Maqdasy, S. Liver X Receptors: A Possible Link between Lipid Disorders and Female Infertility. Int. J. Mol. Sci. 2018, 19, 2177. https://doi.org/10.3390/ijms19082177
Dallel S, Tauveron I, Brugnon F, Baron S, Lobaccaro JMA, Maqdasy S. Liver X Receptors: A Possible Link between Lipid Disorders and Female Infertility. International Journal of Molecular Sciences. 2018; 19(8):2177. https://doi.org/10.3390/ijms19082177
Chicago/Turabian StyleDallel, Sarah, Igor Tauveron, Florence Brugnon, Silvère Baron, Jean Marc A. Lobaccaro, and Salwan Maqdasy. 2018. "Liver X Receptors: A Possible Link between Lipid Disorders and Female Infertility" International Journal of Molecular Sciences 19, no. 8: 2177. https://doi.org/10.3390/ijms19082177