The Production of Human β-Glucocerebrosidase in Nicotiana benthamiana Root Culture
Abstract
:1. Introduction
2. Results
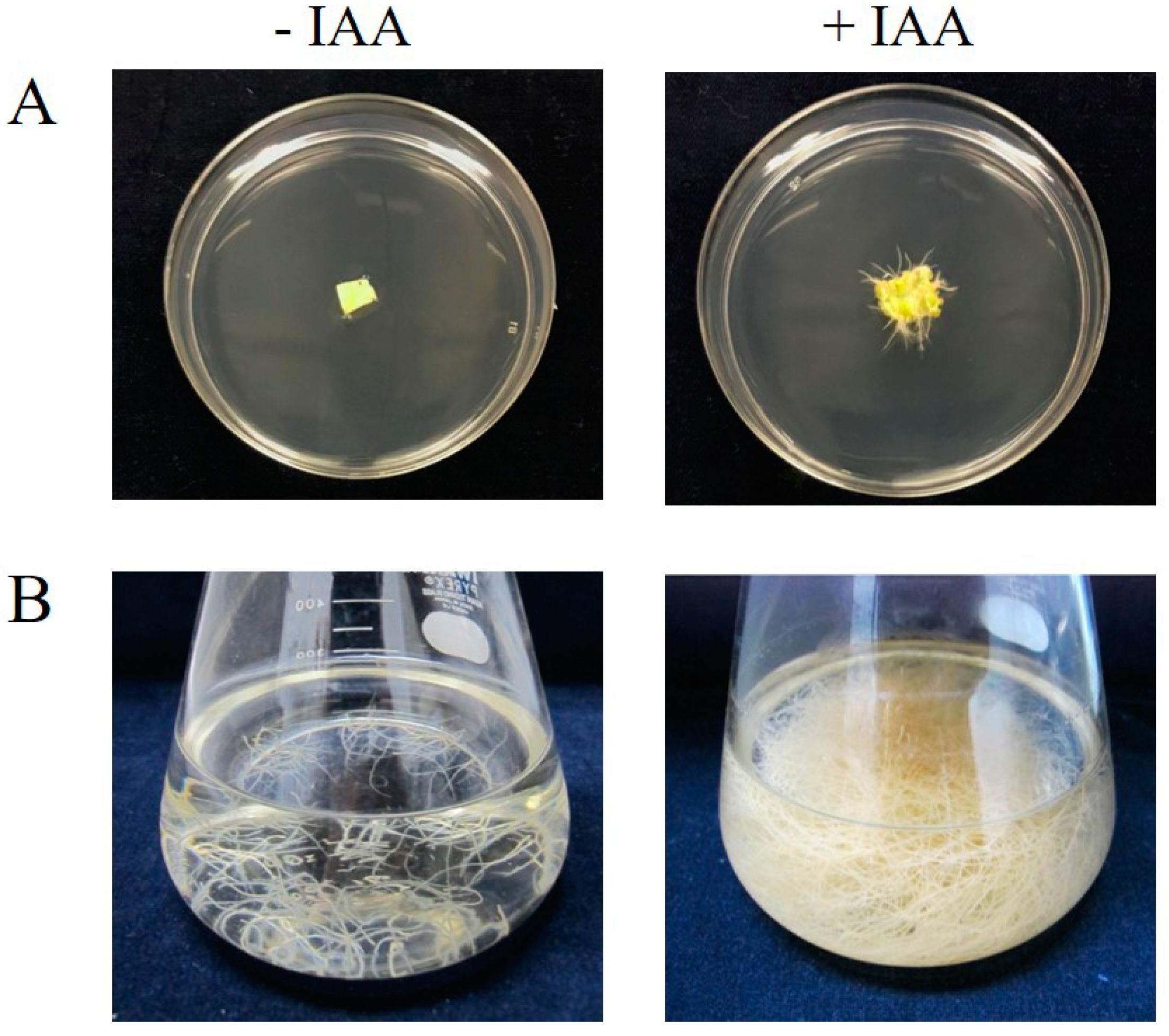
2.1. Root Generation from Leaves of a GCase-Producing N. benthamiana Plant
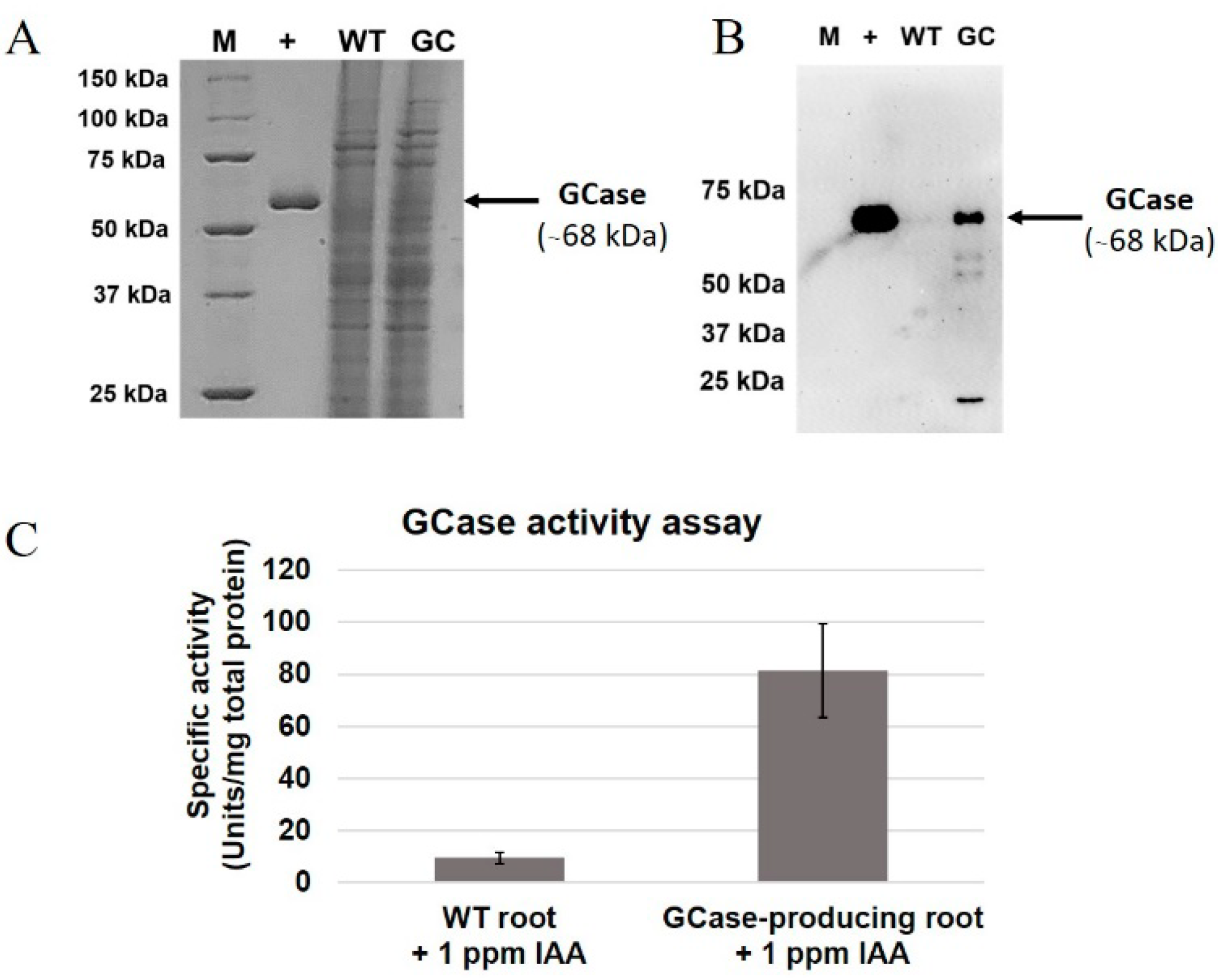
2.2. Production of Active GCases in Root Cultures
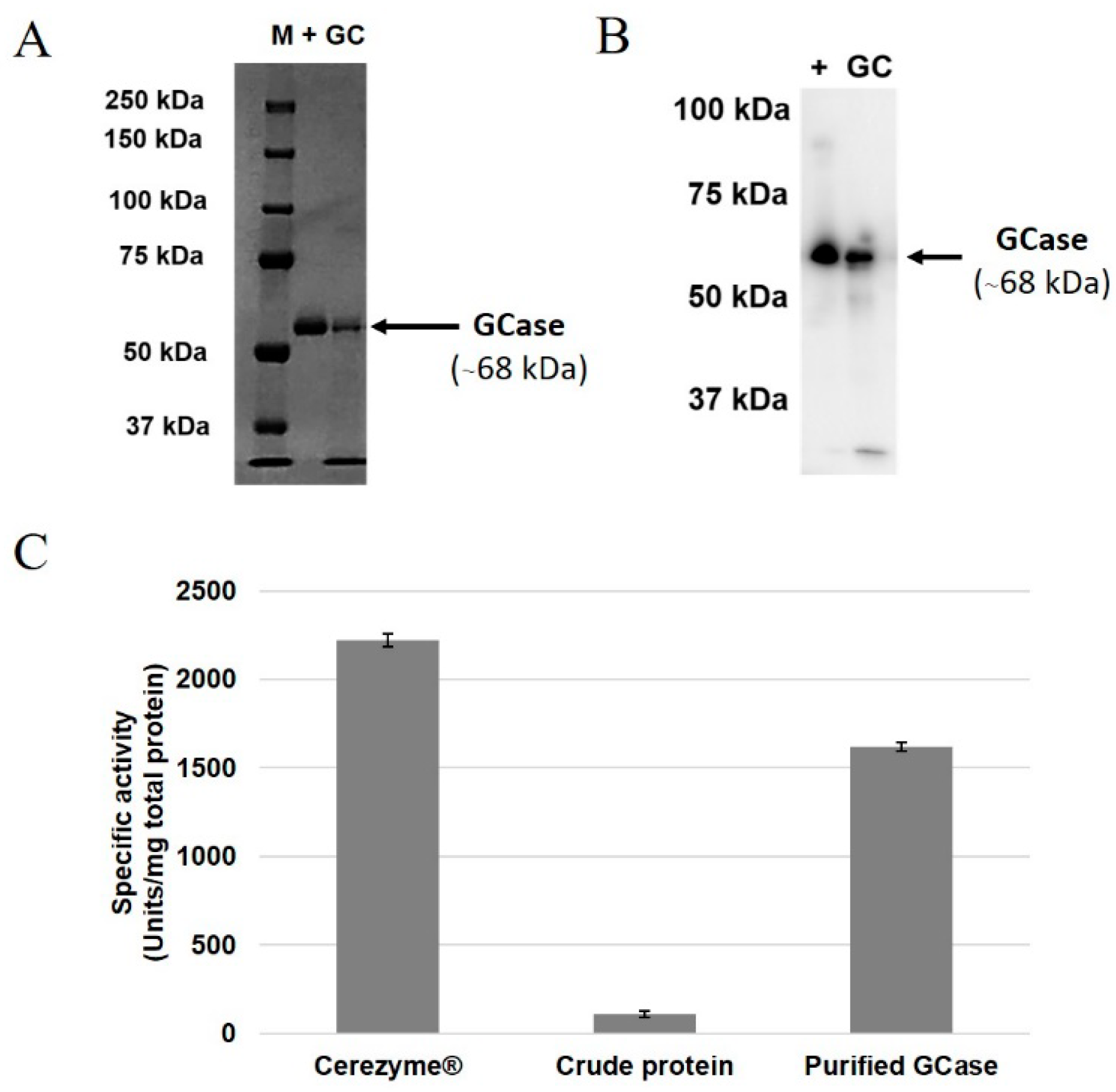
2.3. Purification of GCase from Root Culture
2.4. N-Glycan Analysis of Purified GCase
3. Discussion
4. Materials and Methods
4.1. Transgenic GCase-Producing Plants and Growth Condition
4.2. Root Generation from Leaves and Roots of Transgenic Plants
4.3. Root Culture Induction by Indole-3-Acetic Acid (IAA) in Liquid Media
4.4. Protein Extraction from Root Culture
4.5. GCase Enzymatic Assay
4.6. Western Blot Analysis
4.7. GCase Purification from Root Culture
4.8. N-Glycan Analysis
5. Conclusions
Author Contributions
Acknowledgment
Conflicts of Interest
Abbreviations
| IAA | Indole-3 acetic acid |
| Man | Mannose |
| GlcNAc | N-Acetylglucosamine |
| Xyl | Xylose |
| Fuc | Fucose |
| 4-MUGP | 4-Methylumbelliferyl β-d-glucopyranoside |
| 4-MU | 4-Methylumbelliferone |
References
- Hruska, K.S.; LaMarca, M.E.; Scott, C.R.; Sidransky, E. Gaucher disease: Mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum. Mutat. 2008, 29, 567–583. [Google Scholar] [CrossRef] [PubMed]
- Stirnemann, J.; Belmatoug, N.; Camou, F.; Serratrice, C.; Froissart, R.; Caillaud, C.; Levade, T.; Astudillo, L.; Serratrice, J.; Brassier, A.; et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017, 18, 441. [Google Scholar] [CrossRef] [PubMed]
- Nagral, A. Gaucher Disease. J. Clin. Exp. Hepatol. 2014, 4, 37–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grabowski, G.A. Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet 2008, 372, 1263–1271. [Google Scholar] [CrossRef]
- Goker-Alpan, O. Therapeutic approaches to bone pathology in Gaucher disease: Past, present and future. Mol. Genet. Metab. 2011, 104, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.N.; Shrivastava, N.; Padh, H. Production of heterologous proteins in plants: Strategies for optimal expression. Biotechnol. Adv. 2010, 28, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.M. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat. Biotechnol. 2004, 22, 1393. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, G.; Ren, X.; Herrler, G. Select what you need: A comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J. Biotechnol. 2007, 127, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Hollak, C.E.M.; vom Dahl, S.; Aerts, J.M.F.G.; Belmatoug, N.; Bembi, B.; Cohen, Y.; Collin-Histed, T.; Deegan, P.; van Dussen, L.; Giraldo, P.; et al. Force Majeure: Therapeutic measures in response to restricted supply of imiglucerase (Cerezyme) for patients with Gaucher disease. Blood Cells Mol. Dis. 2010, 44, 41–47. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Galpin, J.D.; Tropak, M.B.; Mahuran, D.; Haselhorst, T.; von Itzstein, M.; Kolarich, D.; Packer, N.H.; Miao, Y.; Jiang, L.; et al. Production of active human glucocerebrosidase in seeds of Arabidopsis thaliana complex-glycan-deficient (cgl) plants. Glycobiology 2012, 22, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Limkul, J.; Misaki, R.; Kato, K.; Fujiyama, K. The combination of plant translational enhancers and terminator increase the expression of human glucocerebrosidase in Nicotiana benthamiana plants. Plant Sci. 2015, 240, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.-J.; Kwon, J.-Y.; Choi, H.-Y.; Kang, S.-H.; Jung, H.-S.; Kim, D.-I. Production and Purification of Recombinant Glucocerebrosidase in Transgenic Rice Cell Suspension Cultures. Appl. Biochem. Biotechnol. 2017, 181, 1401–1415. [Google Scholar] [CrossRef] [PubMed]
- Goodin, M.M.; Zaitlin, D.; Naidu, R.A.; Lommel, S.A. Nicotiana benthamiana: Its History and Future as a Model for Plant–Pathogen Interactions. Mol. Plant Microbe Interact. 2008, 21, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Clemente, T. Nicotiana (Nicotiana tobaccum, Nicotiana benthamiana). In Agrobacterium Protocols; Wang, K., Ed.; Humana Press: Totowa, NJ, USA, 2006; pp. 143–154. [Google Scholar]
- Melina, A.T.; Ana Laura Wevar, O.; Paola, S.G.; Elizabeth, A. Hairy Roots, their Multiple Applications and Recent Patents. Recent Pat. Biotechnol. 2012, 6, 115–133. [Google Scholar]
- Huet, Y.; Ekouna, J.-P.E.; Caron, A.; Mezreb, K.; Boitel-Conti, M.; Guerineau, F. Production and secretion of a heterologous protein by turnip hairy roots with superiority over tobacco hairy roots. Biotechnol. Lett. 2014, 36, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Haissig, B.E. Influence of Indole-3-Acetic Acid on Adventitious Root Primordia of Brittle Willow. Planta 1970, 95, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-F.; Wei, J.-Y.; Chen, H.-W.; Liu, Y.-Y.; Lu, H.-Y.; Chou, J.-Y. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal. Behav. 2015, 10, e1048052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pilet, P.-E.; Saugy, M. Effect on root growth of endogenous and applied IAA and ABA: A critical reexamination. Plant Physiol. 1987, 83, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, S.; Zhang, B. Glycan analysis of therapeutic glycoproteins. mAbs 2016, 8, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Gomord, V.; Chamberlain, P.; Jefferis, R.; Faye, L. Biopharmaceutical production in plants: Problems, solutions and opportunities. Trends Biotechnol. 2005, 23, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Gomord, V.; Fitchette, A.C.; Menu-Bouaouiche, L.; Saint-Jore-Dupas, C.; Plasson, C.; Michaud, D.; Faye, L. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol. J. 2010, 8, 564–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rup, B.; Alon, S.; Amit-Cohen, B.-C.; Almon, E.B.; Chertkoff, R.; Tekoah, Y.; Rudd, P.M. Immunogenicity of glycans on biotherapeutic drugs produced in plant expression systems—The taliglucerase alfa story. PLoS ONE 2017, 12, e0186211. [Google Scholar] [CrossRef] [PubMed]
- Limkul, J.; Iizuka, S.; Sato, Y.; Misaki, R.; Ohashi, T.; Ohashi, T.; Fujiyama, K. The production of human glucocerebrosidase in glyco-engineered Nicotiana benthamiana plants. Plant Biotechnol. J. 2016, 14, 1682–1694. [Google Scholar] [CrossRef] [PubMed]
- Tekoah, Y.; Tzaban, S.; Kizhner, T.; Hainrichson, M.; Gantman, A.; Golembo, M.; Aviezer, D.; Shaaltiel, Y. Glycosylation and functionality of recombinant β-glucocerebrosidase from various production systems. Biosci. Rep. 2013, 33, e00071. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Sames, L.; Moore, A.; Ekins, S. Multifaceted roles of ultra-rare and rare disease patients/parents in drug discovery. Drug Discov. Today 2013, 18, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Sawkar, A.R.; Adamski-Werner, S.L.; Cheng, W.-C.; Wong, C.-H.; Beutler, E.; Zimmer, K.-P.; Kelly, J.W. Gaucher Disease-Associated Glucocerebrosidases Show Mutation-Dependent Chemical Chaperoning Profiles. Chem. Biol. 2005, 12, 1235–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankoke, H.; Buschmann, T.; Müller, C. Role of plant β-glucosidases in the dual defense system of iridoid glycosides and their hydrolyzing enzymes in Plantago lanceolata and Plantago major. Phytochemistry 2013, 94, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Akiyama, T.; Opassiri, R.; Kuaprasert, B.; Cairns, J.K. Structural and Enzymatic Characterization of Os3BGlu6, a Rice β-Glucosidase Hydrolyzing Hydrophobic Glycosides and (1→3)- and (1→2)-Linked Disaccharides. Plant Physiol. 2009, 151, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Moothoo, D.N.; Naismith, J.H. Concanavalin A distorts the β-GlcNAc-(1→2)-Man linkage of β-GlcNAc-(1→2)-α-Man-(1→3)-[β-GlcNAc-(1→2)-α-Man-(1→6)]-Man upon binding. Glycobiology 1998, 8, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Rayon, C.; Lerouge, P.; Faye, L. The protein N-glycosylation in plants. J. Exp. Bot. 1998, 49, 1463–1472. [Google Scholar] [CrossRef]
- Zimran, A.; Wajnrajch, M.; Hernandez, B.; Pastores, G.M. Taliglucerase alfa: Safety and efficacy across 6 clinical studies in adults and children with Gaucher disease. Orphanet J. Rare Dis. 2018, 13, 36. [Google Scholar] [CrossRef] [PubMed]
- Morelle, W.; Michalski, J.-C. Analysis of protein glycosylation by mass spectrometry. Nat. Protoc. 2007, 2, 1585. [Google Scholar] [CrossRef] [PubMed]
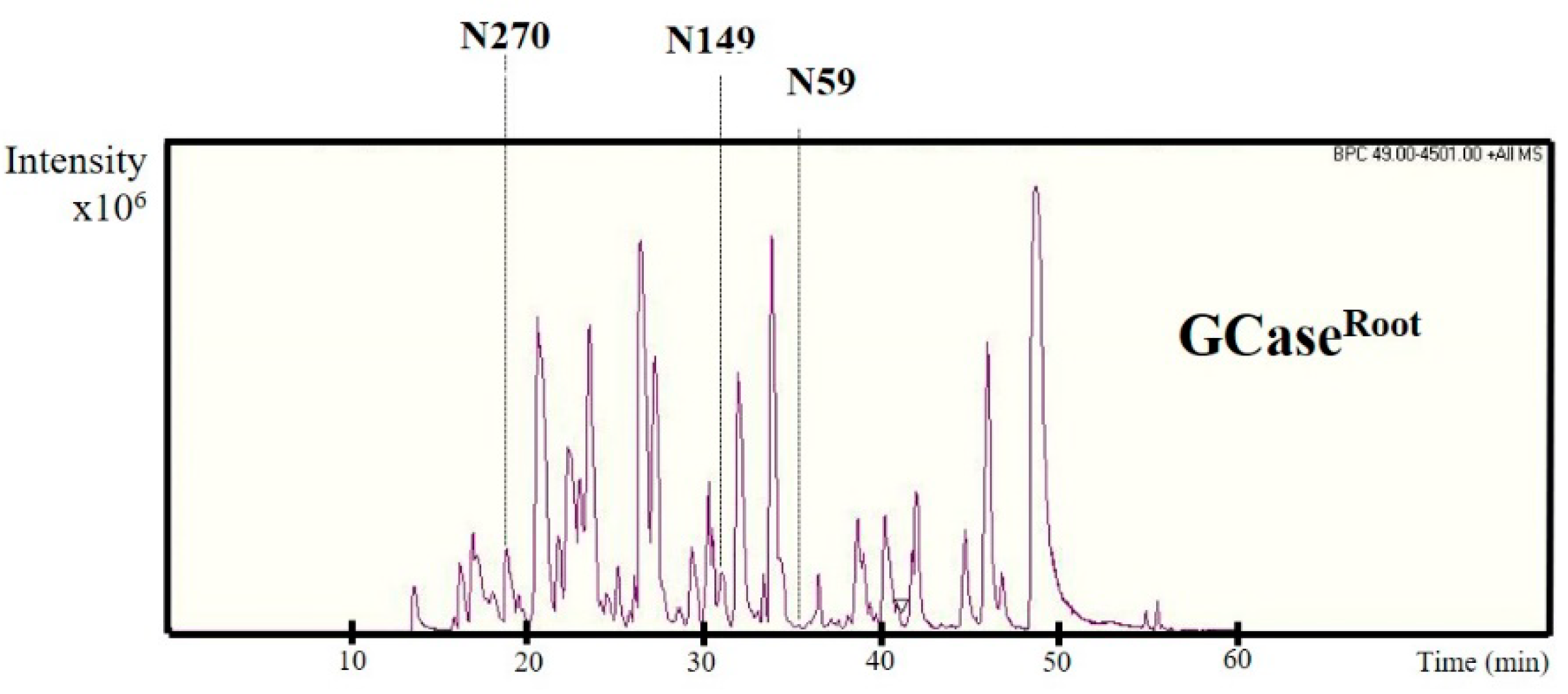
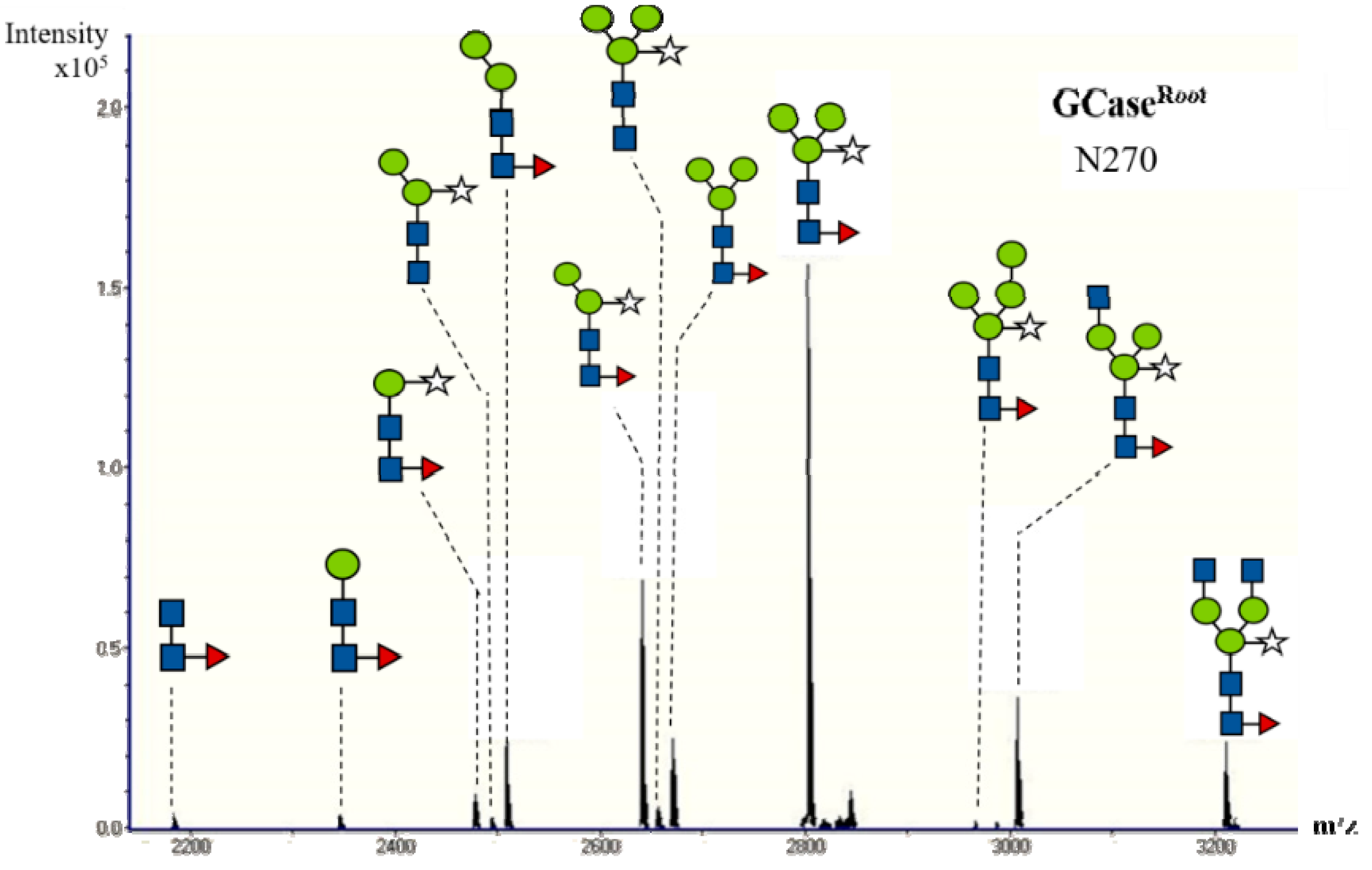
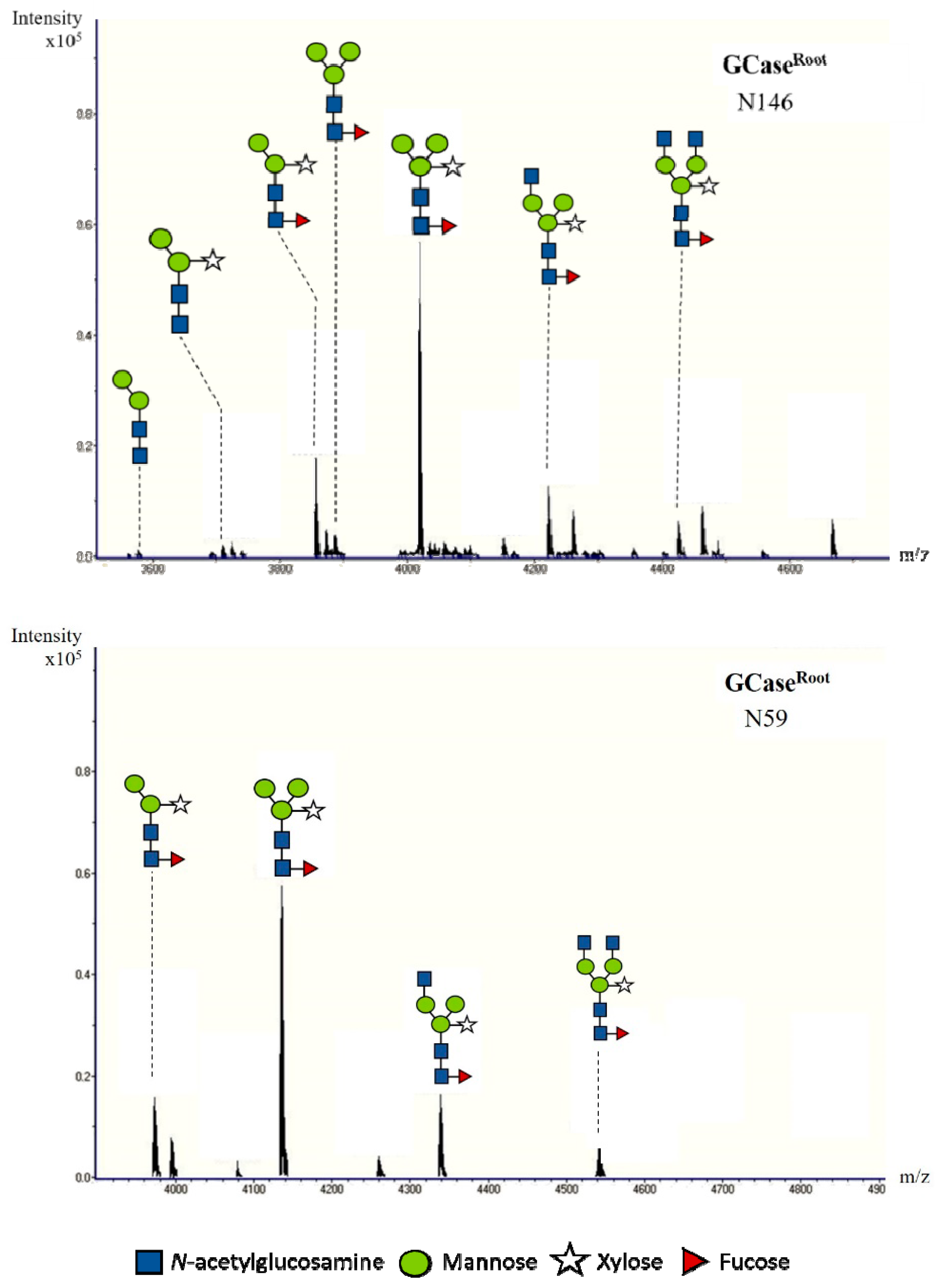
| Structure | Amount of Composition (%) | |||||
|---|---|---|---|---|---|---|
| N270 | N146 | N59 | ||||
| GCWT | GCRoot | GCWT | GCRoot | GCWT | GCRoot | |
| FucGlcNAc2 | - | 1.1 | - | - | - | - |
| ManFucGlcNAc2 | - | 1.0 | - | - | - | - |
| Man2FucGlcNAc2 | 6.7 | 6.6 | - | - | - | - |
| Man3FucGlcNAc2 | 9.2 | 6.9 | 4.6 | 3.7 | 4.0 | - |
| Man2GlcNAc2 | - | - | - | 1.0 | - | - |
| GlcNAc2Man3XylFucGlcNAc2 | 5.2 | 6.6 | - | 6.2 | 7.2 | 8.5 |
| GlcNAcMan3XylFucGlcNAc2 | 10.0 | 10.1 | 5.2 | 12.6 | 19.5 | 16.7 |
| Man4XylFucGlcNAc2 | - | 0.4 | - | - | - | - |
| Man3XylFucGlcNAc2 | 43.5 | 43.3 | 65.5 | 56.8 | 56.1 | 58.7 |
| Man3XylGlcNAc2 | 1.5 | 1.6 | 5.5 | - | 1.8 | - |
| Man2XylFucGlcNAc2 | 23.9 | 19 | 16.5 | 17.7 | 11.3 | 16.1 |
| Man2XylGlcNAc2 | - | 0.8 | 2.7 | 2.0 | - | - |
| ManXylFucGlcNAc2 | - | 2.6 | - | - | - | - |
| 100 | 100 | 100 | 100 | 100 | 100 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naphatsamon, U.; Ohashi, T.; Misaki, R.; Fujiyama, K. The Production of Human β-Glucocerebrosidase in Nicotiana benthamiana Root Culture. Int. J. Mol. Sci. 2018, 19, 1972. https://doi.org/10.3390/ijms19071972
Naphatsamon U, Ohashi T, Misaki R, Fujiyama K. The Production of Human β-Glucocerebrosidase in Nicotiana benthamiana Root Culture. International Journal of Molecular Sciences. 2018; 19(7):1972. https://doi.org/10.3390/ijms19071972
Chicago/Turabian StyleNaphatsamon, Uthailak, Takao Ohashi, Ryo Misaki, and Kazuhito Fujiyama. 2018. "The Production of Human β-Glucocerebrosidase in Nicotiana benthamiana Root Culture" International Journal of Molecular Sciences 19, no. 7: 1972. https://doi.org/10.3390/ijms19071972
APA StyleNaphatsamon, U., Ohashi, T., Misaki, R., & Fujiyama, K. (2018). The Production of Human β-Glucocerebrosidase in Nicotiana benthamiana Root Culture. International Journal of Molecular Sciences, 19(7), 1972. https://doi.org/10.3390/ijms19071972






