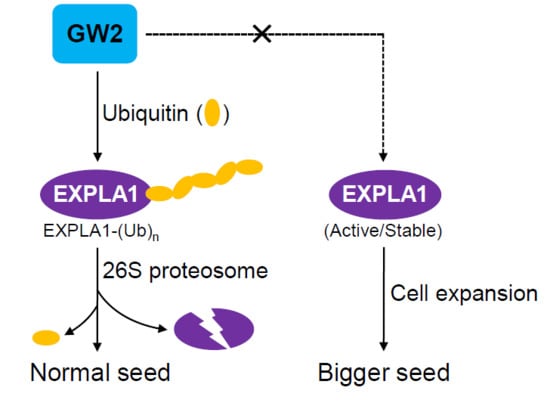
GW2 Functions as an E3 Ubiquitin Ligase for Rice Expansin-Like 1
Abstract
1. Introduction
2. Results
2.1. The Seeds of GW2 Mutants Have a Chalky Endosperm
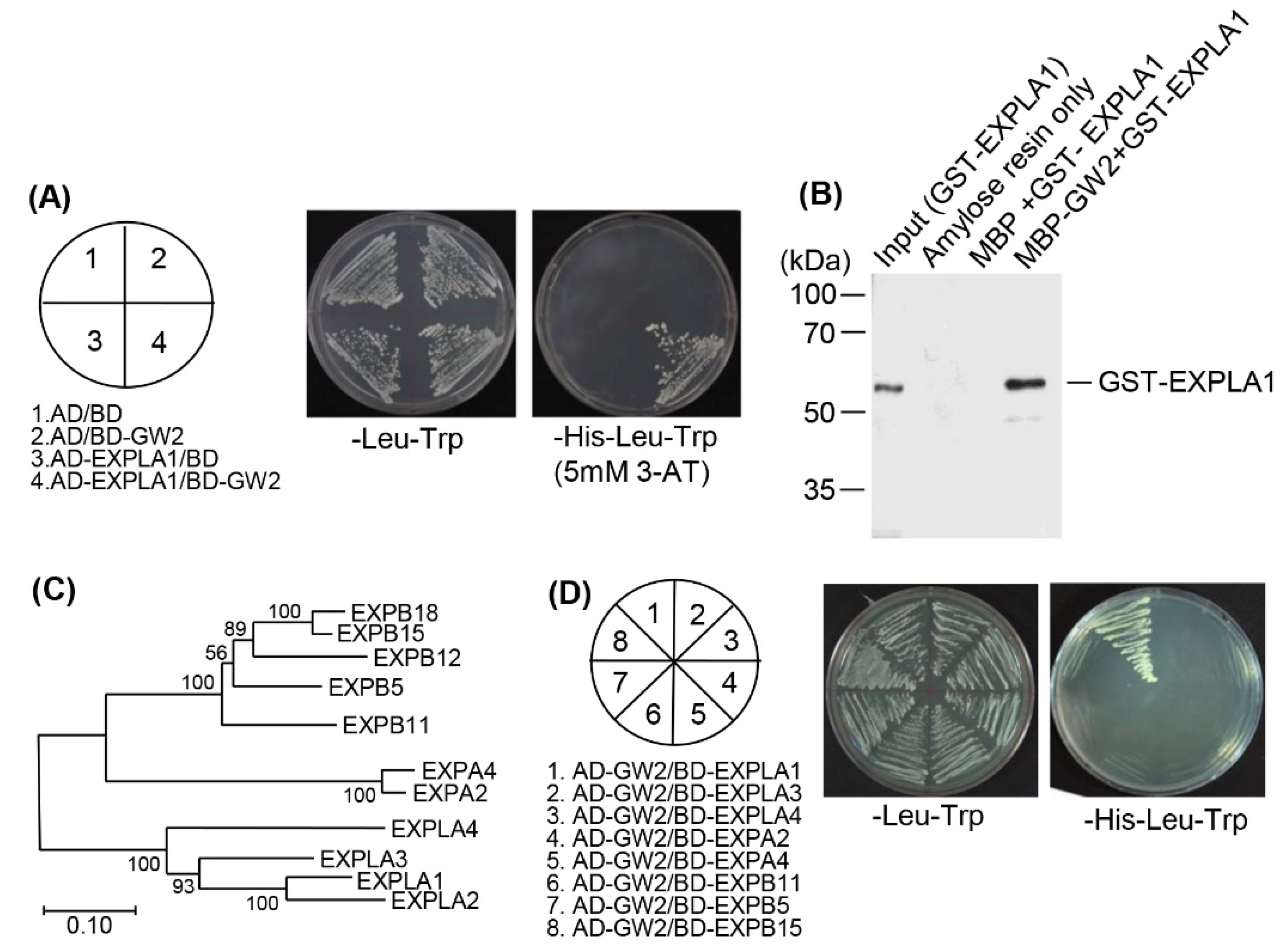
2.2. EXPLA1 Specifically Interacts with GW2
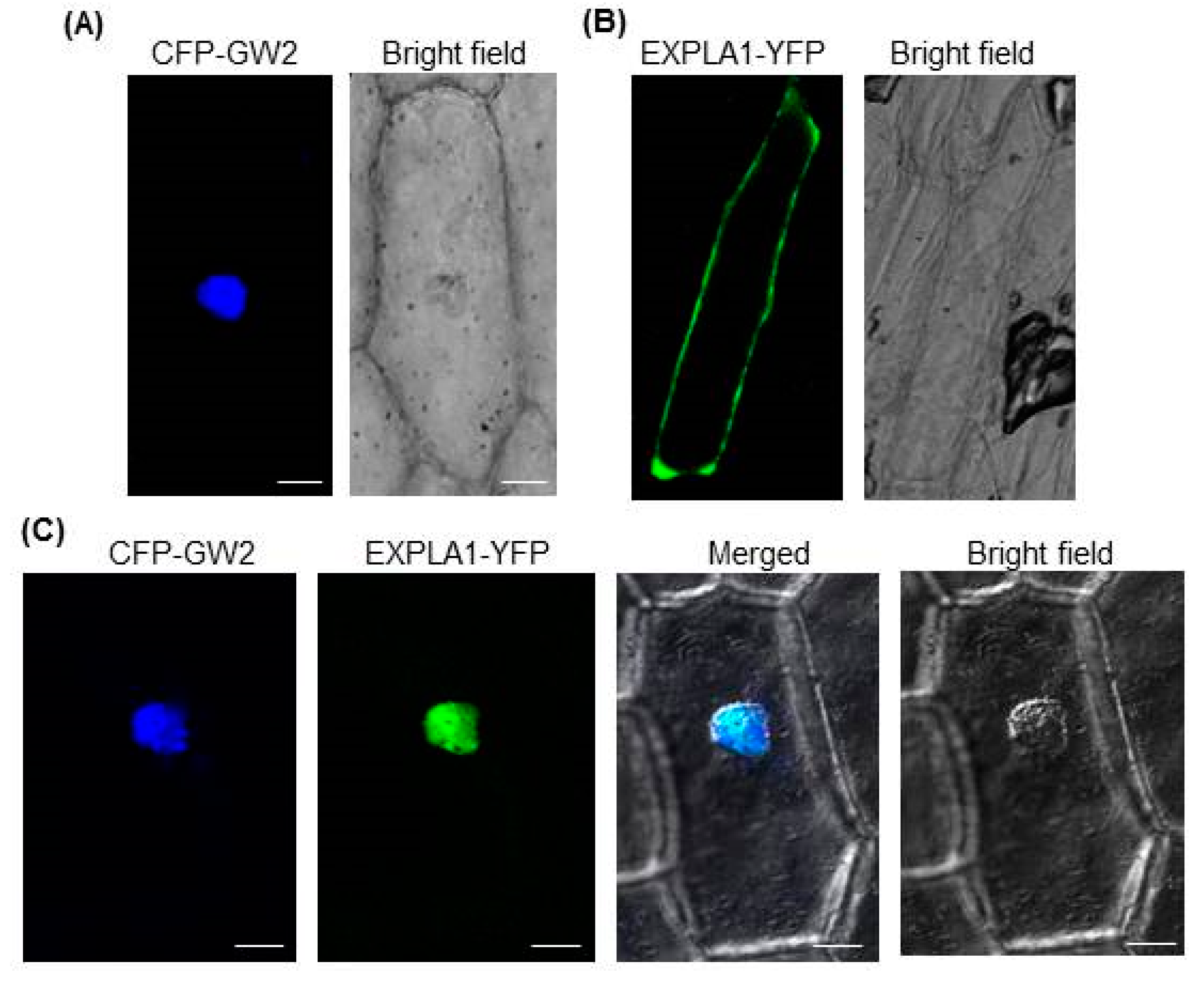
2.3. GW2 and EXPLA1 Colocalize to the Nucleus
2.4. EXPLA1 Is Ubiquitinated by GW2
2.5. GW2 Is Highly Expressed in Panicle and Anther
3. Discussion
4. Materials and Methods
4.1. Bacteria, Plant Materials, and Growth Conditions
4.2. Genotyping of Natural gw2 Mutant Oochikara
4.3. Yeast Two-Hybrid Screening
4.4. Yeast Two-Hybrid Assay
4.5. Construction of Recombinant Plasmids
4.6. Purification of Recombinant Proteins
4.7. In Vitro Pull-Down Assays
4.8. Subcellular Localization of GW2 and EXPLA1
4.9. In Vitro Ubiquitination Assay
4.10. Promoter Activity Analysis
4.11. Microscopic Analysis of gw2 Mutant Seeds
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 12, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Song, X.J.; Huang, W.; Shi, M.; Zhu, M.Z.; Lin, H.X. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat. Genet. 2007, 39, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Luo, L.; Yan, W.; Kovi, M.R.; Zhan, W.; Xing, Y. Genetic dissection of rice grain shape using a recombinant inbred line population derived from two contrasting parents and fine mapping a pleiotropic quantitative trait locus qGL7. BMC Genet. 2010, 11. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.F.; Xing, Y.Z.; Li, J.X.; Yu, S.B.; Xu, C.G.; Zhang, Q. Genetic bases of appearance quality of rice grains in Shanyou 63, an elite rice hybrid. Theor. Appl. Genet. 2000, 101, 823–829. [Google Scholar] [CrossRef]
- Su, Z.; Hao, C.; Wang, L.; Dong, Y.; Zhang, X. Identification and development of a functional marker of TaGW2 associated with grain weight in bread wheat (Triticum aestivum L.). Theor. Appl. Genet. 2011, 122, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, X.; Bai, G.; Warburton, M.L.; Mahuku, G.; Gore, M.; Dai, J.; Li, J.; Yan, J. Cloning and characterization of a putative GS3 ortholog involved in maize kernel development. Theor. Appl. Genet. 2010, 120, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Osato, Y.; Yokoyama, R.; Nishitani, K. A principal role for AtXTH18 in Arabidopsis thaliana root growth: A functional analysis using RNAi plants. J. Plant Res. 2006, 19, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Yum, H.; Kim, E.S.; Cho, H.; Gothandam, K.M.; Hyun, J.; Chung, Y.Y. BcXTH1, a Brassica campestris homologue of Arabidopsis XTH9, is associated with cell expansion. Planta 2006, 224, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Lu, S.M.; Zhang, J.F.; Liu, S.; Lu, Y.T. A xyloglucan endotransglucosylase/hydrolase involves in growth of primary root and alters the deposition of cellulose in Arabidopsis. Planta 2007, 226, 1547–1560. [Google Scholar] [CrossRef] [PubMed]
- Bashline, L.; Lei, L.; Li, S.; Gu, Y. Cell wall, cytoskeleton, and cell expansion in higher plants. Mol. Plant 2014, 7, 586–600. [Google Scholar] [CrossRef] [PubMed]
- Sampedro, J.; Cosgrove, D.J. The expansin superfamily. Genome Biol. 2005, 6, 242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kende, H.; Bradford, K.J.; Brummell, D.; Cho, H.T.; Cosgrove, D.J.; Fleming, A.J.; Voesenek, L.A.C.J. Nomenclature for members of the expansin superfamily of genes and proteins. Plant Mol. Biol. 2004, 55, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Marowa, P.; Ding, A.; Kong, Y. Expansins: Roles in plant growth and potential applications in crop improvement. Plant Cell Rep. 2016, 35, 949–965. [Google Scholar] [CrossRef] [PubMed]
- Ciechanover, A. The ubiquitin-proteasome proteolytic pathway. Cell 1994, 79, 13–21. [Google Scholar] [CrossRef]
- Vierstra, R.D. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat. Rev. Mol. Cell Biol. 2009, 10, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Bie, P.; Ciechanover, A. Ubiquitination of E3 ligases: Self-regulation of the ubiquitin system via proteolytic and non-proteolytic mechanisms. Cell Death Differ. 2011, 18, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Miura, K.; Aya, K.; Kitano, H.; Matsuoka, M. Genes offering the potential for designing yield-related traits in rice. Curr. Opin. Plant Biol. 2013, 16, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.T.; Kende, H. Tissue localization of expansins in deepwater rice. Plant J. 1998, 15, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Shomura, A.; Izawa, T.; Ebana, K.; Ebitani, T.; Kanegae, H.; Konishi, S.; Yano, M. Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 2008, 40, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Gu, S.; Wan, X.; Gao, H.; Guo, T.; Su, N.; Lei, C.; Zhang, X.; Cheng, Z.; Guo, X. Isolation and initial characterization of GW5, a major QTL associated with rice grain width and weight. Cell Res. 2008, 18, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Kai, N.T.; Jiang, H.; Kubo, T.; Sweeney, M.; Matsumoto, T.; Kanamori, H.; Padhukasahasram, B.; Bustamante, C.; Yoshimura, A.; Doi, K.; et al. Evolutionary history of GS3, a gene conferring grain length in rice. Genetics 2009, 182, 1323–1334. [Google Scholar]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q.; et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J. New genes and new biological roles for expansins. Curr. Opin. Plant Biol. 2000, 3, 73–78. [Google Scholar] [CrossRef]
- Strohmeier, M.; Hrmova, M.; Fischer, M.; Harvey, A.J.; Fincher, G.B.; Pleiss, J. Molecular modeling of family GH16 glycoside hydrolases: Potential roles for xyloglucan transglucosylases/hydrolases in cell wall modification in the Poaceae. Protein Sci. 2004, 13, 3200–3213. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Thiemann, A.; Schrag, T.A.; Melchinger, A.E.; Scholten, S.; Frisch, M. Dissecting grain yield pathways and their interactions with grain dry matter content by a two-step correlation approach with maize seedling transcriptome. BMC Plant Biol. 2010, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Lizana, X.C.; Riegel, R.; Gomez, L.D.; Herrera, J.; Isla, A.; McQueen-Mason, S.J.; Calderini, D.F. Expansins expression is associated with grain size dynamics in wheat (Triticum aestivum L.). J. Exp. Bot. 2010, 4, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Kwak, M.S.; Noh, S.A.; Oh, M.J.; Kim, Y.S.; Shin, J.S. Overexpression of sweetpotato expansin cDNA (IbEXP1) increases seed yield in Arabidopsis. Transgenic Res. 2014, 23, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.J.; Li, L.C.; Cho, H.T.; Hoffmann-Benning, S.; Moore, R.C.; Blecker, D. The growing world of expansins. Plant Cell Physiol. 2002, 43, 1436–1444. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, D. Biochemical Properties and Localization of the β-Expansin OsEXPB3 in Rice (Oryza sativa L.). Mol. Cells 2005, 20, 119–126. [Google Scholar] [PubMed]
- Li, J.; Chu, H.; Zhang, Y.; Mou, T.; Wu, C.; Zhang, Q.; Xu, J. The rice HGW gene encodes a ubiquitin-associated (UBA) domain protein that regulates heading date and grain weight. PLoS ONE 2012, 7, e34231. [Google Scholar] [CrossRef] [PubMed]
- Ngangkham, U.; Samantaray, S.; Yadav, M.K.; Kumar, A.; Chidambaranathan, P.; Katara, J.L. Effect of multiple allelic combinations of genes on regulating grain size in rice. PLoS ONE 2018, 13, e0190684. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef] [PubMed]


| Colony | Protein ID | Inserted Region | Full Length |
|---|---|---|---|
| 1 | Ubiquitin family protein, Os06g46770, (polyubiquitin) | 378–534aa | (534aa) |
| 2 | Ubiquitin family protein, Os06g46770, (polyubiquitin) | 204–534aa | (534aa) |
| 5 | Ubiquitin family protein, Os06g46770, (polyubiquitin) | 258–534aa | (534aa) |
| 8 | Ubiquitin-conjugating enzyme, Os01g60410 | 1–149aa | (149aa) |
| 9 | Ubiquitin family protein, Os02g06640, (polyubiquitin) | 28–121 | (458aa) |
| 11 | Ubiquitin family protein, Os02g06640, (polyubiquitin) | 316–458aa | (458aa) |
| 12 | Ubiquitin family protein, Os06g46770, (polyubiquitin) | 456–534aa | (534aa) |
| 13 | Ubiquitin family protein, Os02g06640, (polyubiquitin) | 279–458aa | (458aa) |
| 14 | Ubiquitin family protein, Os02g06640, (polyubiquitin) | 355–458aa | (458aa) |
| 15 | Ubiquitin family protein, Os06g46770, (polyubiquitin) | 454–534aa | (534aa) |
| 16 | Ubiquitin family protein, Os06g46770.3, (polyubiquitin) | 302–458aa | (458aa) |
| 18 | Ubiquitin family protein, Os02g06640, (polyubiquitin) | 259–458aa | (458aa) |
| 20 | Expansin precursor, Os03g04020, (EXPLA1) | 154–280aa | (280aa) |
| 21 | Ubiquitin family protein, Os06g46770.3, (polyubiquitin) | 354–458aa | (458aa) |
| 22 | Ubiquitin family protein, Os06g46770.3, (polyubiquitin) | 160–458aa | (458aa) |
| 24 | Polyphenol oxidase, Os04g53300 | 467–571aa | (1713aa) |
| 25 | Glycosyl hydrolase, Os06g46284 | 760–886aa | (886aa) |
| 26 | Ubiquitin-40S ribosomal protein, Os01g22490 | 1–156aa | (156aa) |
| 28 | S10/S20 domain containing ribosomal protein, Os06g04290 | 50–129aa | (129aa) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.S.; Kim, Y.J.; Markkandan, K.; Koo, Y.J.; Song, J.T.; Seo, H.S. GW2 Functions as an E3 Ubiquitin Ligase for Rice Expansin-Like 1. Int. J. Mol. Sci. 2018, 19, 1904. https://doi.org/10.3390/ijms19071904
Choi BS, Kim YJ, Markkandan K, Koo YJ, Song JT, Seo HS. GW2 Functions as an E3 Ubiquitin Ligase for Rice Expansin-Like 1. International Journal of Molecular Sciences. 2018; 19(7):1904. https://doi.org/10.3390/ijms19071904
Chicago/Turabian StyleChoi, Beom Seok, Yeon Jeong Kim, Kesavan Markkandan, Yeon Jong Koo, Jong Tae Song, and Hak Soo Seo. 2018. "GW2 Functions as an E3 Ubiquitin Ligase for Rice Expansin-Like 1" International Journal of Molecular Sciences 19, no. 7: 1904. https://doi.org/10.3390/ijms19071904
APA StyleChoi, B. S., Kim, Y. J., Markkandan, K., Koo, Y. J., Song, J. T., & Seo, H. S. (2018). GW2 Functions as an E3 Ubiquitin Ligase for Rice Expansin-Like 1. International Journal of Molecular Sciences, 19(7), 1904. https://doi.org/10.3390/ijms19071904