Radioprotective Effects of Dermatan Sulfate in a Preclinical Model of Oral Mucositis—Targeting Inflammation, Hypoxia and Junction Proteins without Stimulating Proliferation
Abstract
:1. Introduction
2. Results
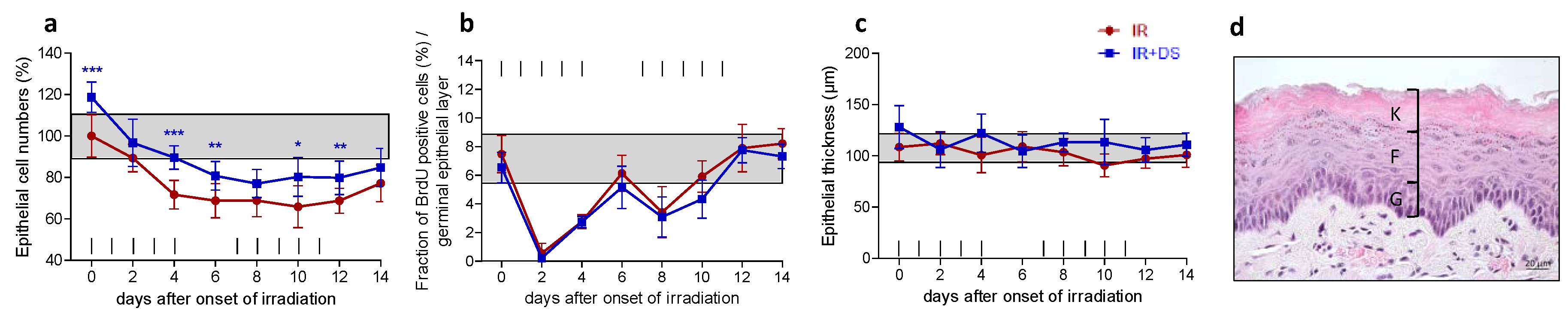
2.1. Epithelial Morphology and Proliferation
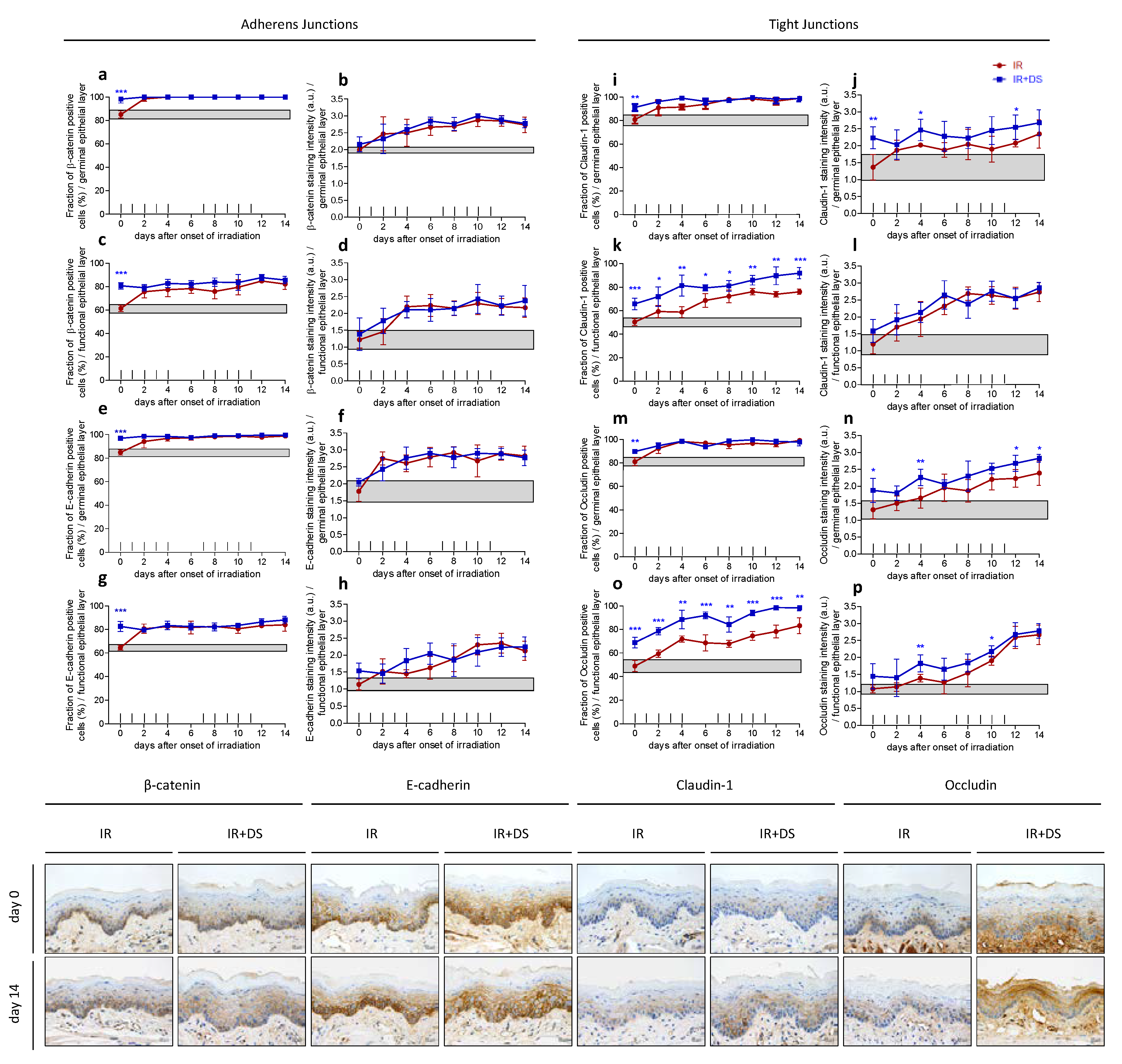
2.2. Epithelial Cell Junctions
2.2.1. Adherens Junctions—β-Catenin and e-Cadherin
2.2.2. Tight Junctions—Claudin-1 and Occludin
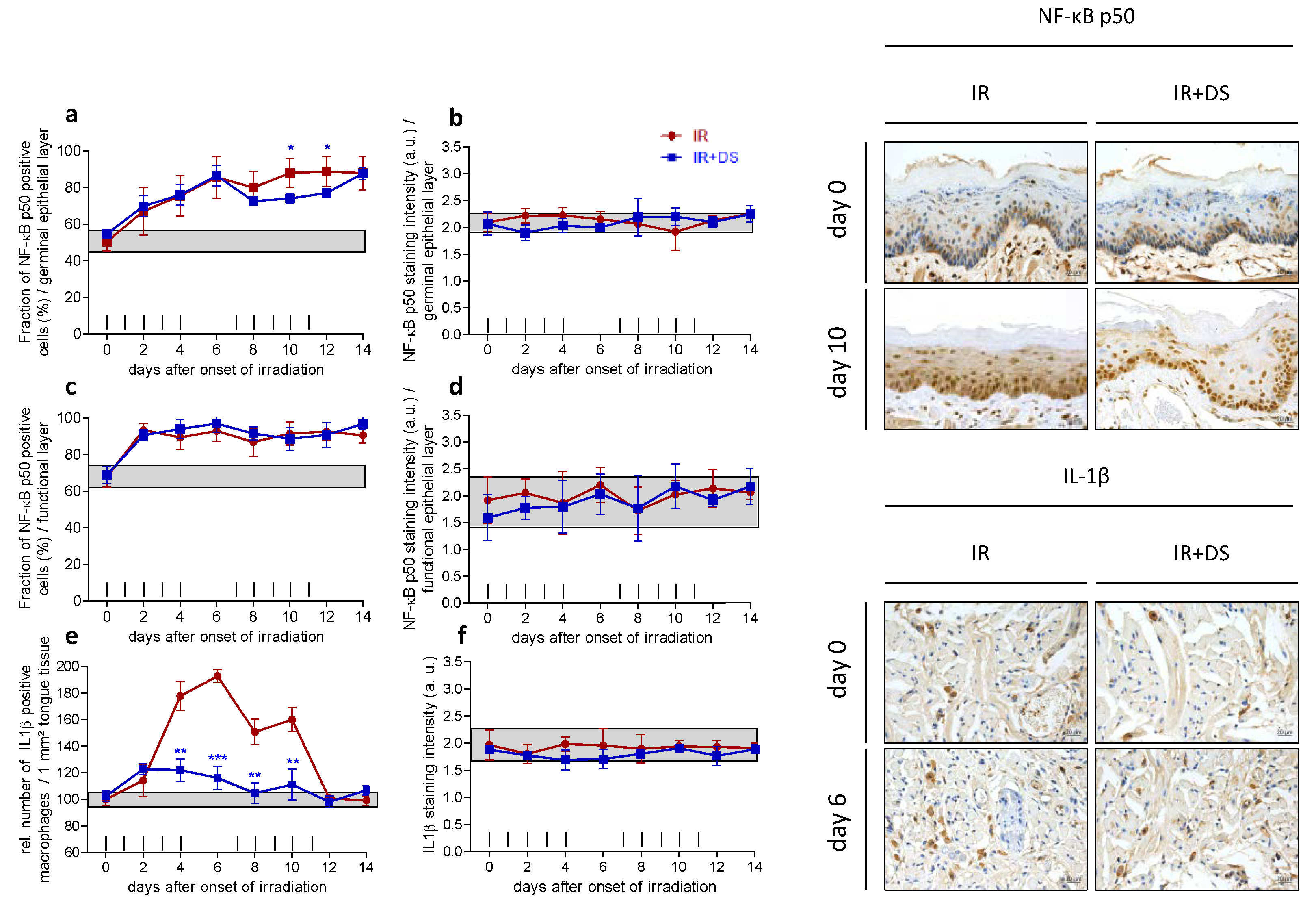
2.3. Inflammation
2.3.1. Epithelial NF-κB Expression
2.3.2. IL-1β Positive Macrophages
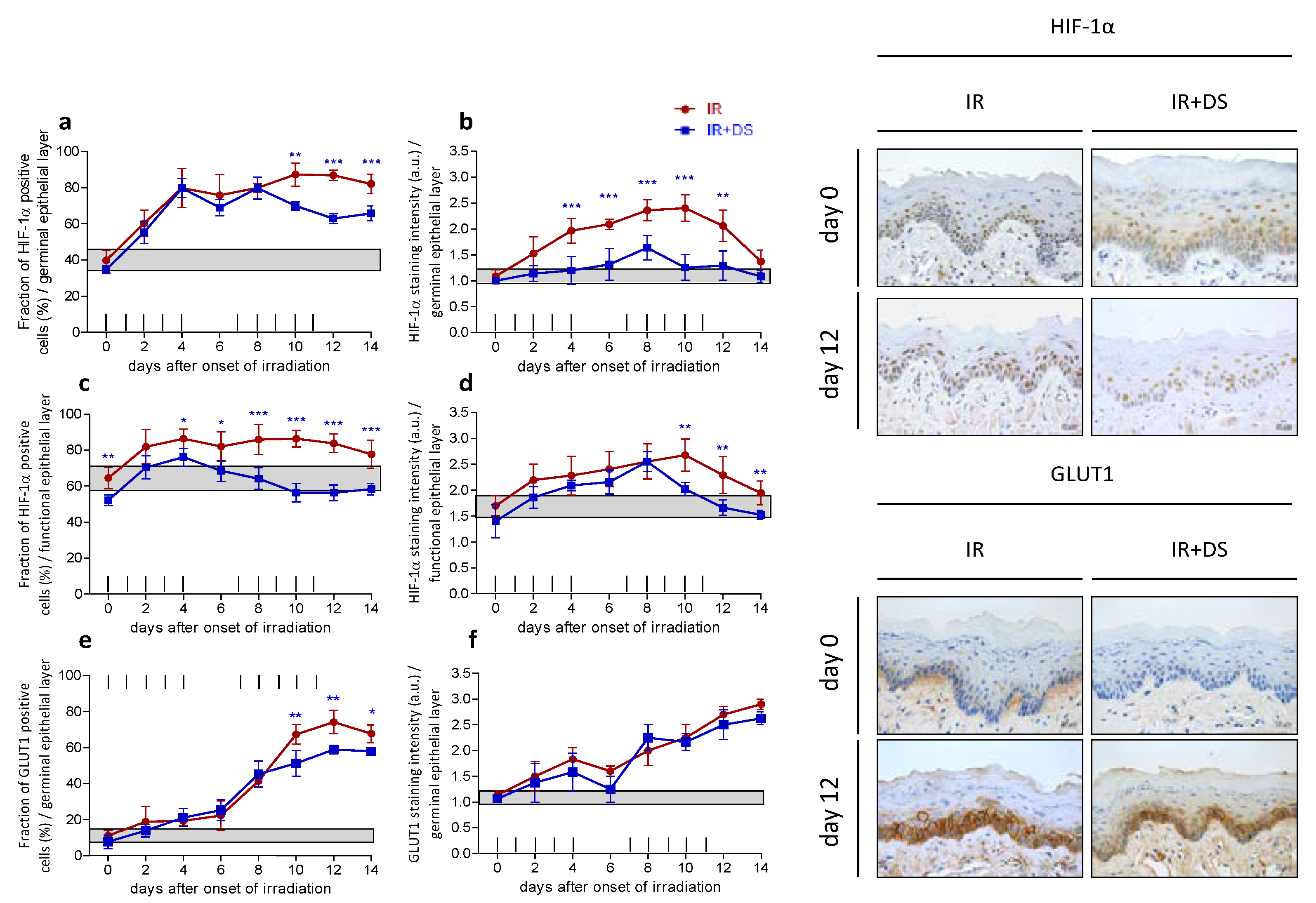
2.4. Hypoxia
2.4.1. HIF-1α
2.4.2. GLUT1
3. Discussion
4. Material and Methods
4.1. Animals and Housing
4.2. Irradiation
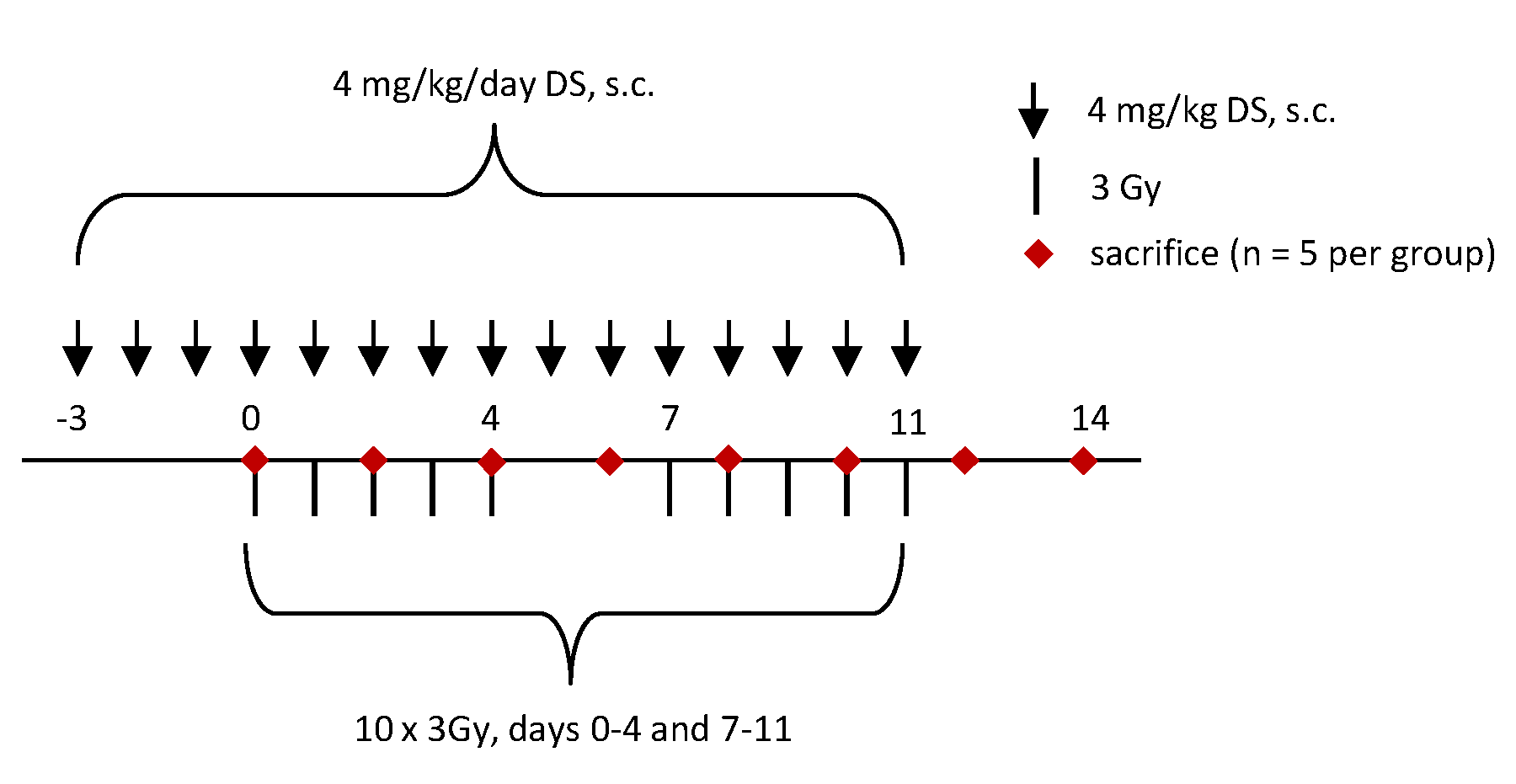
4.3. Experimental Protocol
4.4. Immunohistochemistry
4.5. Histological Analyses
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Compliance with Ethical Guidelines
Acknowledgments
Conflicts of Interest
References
- Elting, L.S.; Keefe, D.M.; Sonis, S.T.; Garden, A.S.; Spijkervet, F.K.L.; Barasch, A.; Tishler, R.B.; Canty, T.P.; Kudrimoti, M.K.; Vera-Llonch, M.; et al. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: Demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 2008, 113, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Groeger, S.; Meyle, J. Epithelial barrier and oral bacterial infection. Periodontology 2015, 69, 46–67. [Google Scholar] [CrossRef] [PubMed]
- Laheij, A.M.G.A.; de Soet, J.J.; von dem Borne, P.A.; Kuijper, E.J.; Kraneveld, E.A.; van Loveren, C.; Raber-Durlacher, J.E. Oral bacteria and yeasts in relationship to oral ulcerations in hematopoietic stem cell transplant recipients. Support. Care Cancer 2012, 20, 3231–3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moslemi, D.; Mohammadi, A.; Taheri, M. Management of chemo/radiation-induced oral mucositis in patients with head and neck cancer: A review of the current literature. Radiother. Oncol. 2016, 120, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, C.I.; Murphy, B.A.; Russi, E.G. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: Clinical practical recommendations. Crit. Rev. Oncol. Hematol. 2015, 99, 100–103. [Google Scholar] [CrossRef]
- Stiff, P. Mucositis associated with stem cell transplantation: Current status and innovative approaches to management. Bone Marrow Transpl. 2001, 27, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Shui, C.; Khan, W.; Benson, W.; Lacey, D.; Knox, S. Effects of keratinocyte growth factor on the proliferation and radiation survival of human squamous cell carcinoma cell lines in vitro and in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 177–187. [Google Scholar] [CrossRef]
- Hille, A.; Grüger, S.; Christiansen, H.; Wolff, H.A.; Volkmer, B.; Lehmann, J.; Dörr, W.; Rave-Fränk, M. Effect of tumour-cell-derived or recombinant keratinocyte growth factor (KGF) on proliferation and radioresponse of human epithelial tumour cells (HNSCC) and normal keratinocytes in vitro. Radiat. Environ. Biophys. 2010, 49, 261–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, M.E.; Hinz, T.K.; Kono, S.A.; Singleton, K.R.; Bichon, B.; Ware, K.E.; Marek, L.; Frederick, B.A.; Raben, D.; Heasley, L.E. Fibroblast growth factor receptors are components of autocrine signaling networks in head and neck squamous cell carcinoma cells. Clin. Cancer Res. 2011, 17, 5016–5025. [Google Scholar] [CrossRef] [PubMed]
- Vadhan-Raj, S.; Goldberg, J.D.; Perales, M.-A.; Berger, D.P.; Brink, M.R.M. Clinical applications of palifermin: Amelioration of oral mucositis and other potential indications. J. Cell. Mol. Med. 2013, 17, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Cinausero, M.; Aprile, G.; Ermacora, P.; Basile, D.; Vitale, M.G.; Fanotto, V.; Parisi, G.; Calvetti, L.; Sonis, S.T. New frontiers in the pathobiology and treatment of cancer regimen-related mucosal injury. Front. Pharmacol. 2017, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Frings, K.; Kuess, P.; Dörr, W. Protective effects of systemic dermatan sulfate treatment in a preclinical model of radiation-induced oral mucositis. Strahlenther. Onkol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugahara, K.; Özbek, S. Evolution of glycosaminoglycans: Comparative biochemical study. Commun. Integr. Biol. 2011, 4, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Trowbridge, J.M.; Rudisill, J.A.; Ron, D.; Gallo, R.L. Dermatan sulfate binds and potentiates activity of keratinocyte growth factor (FGF-7)*. J. Biol. Chem. 2002, 277, 42815–42820. [Google Scholar] [CrossRef] [PubMed]
- Belvedere, R.; Bizzarro, V.; Parente, L.; Petrella, F. The pharmaceutical Device Prisma ® skin promotes in vitro angiogenesis through endothelial to mesenchymal transition during skin wound healing. Int. J. Mol. Sci. 2017, 18, 1614. [Google Scholar] [CrossRef] [PubMed]
- Cofrancesco, E.; Boschetti, C.; Gianese, F.; Cortellaro, M. Dermatan sulfate for the treatment of disseminated intravascular coagulation (DIC) in acute leukaemia: A randomised heparin-controlled pilot study. Thromb. Res. 1994, 74, 65–75. [Google Scholar] [CrossRef]
- Gabella, P.; Ordine, O.; Ordine, O.; Vitale, C.; Berutti, S.; Bagnis, C. Dermatan sulfate: An alternative to unfractionated heparin for anticoagulation in hemodialysis patients. J. Nephrol. 2013, 26, 158–163. [Google Scholar] [CrossRef]
- Agholme, M.B.; Karlsson-sjo, J. Pretherapeutic plasma pro- and anti- inflammatory mediators are related to high risk of oral mucositis in pediatric patients with acute leukemia: A prospective cohort study. PLoS ONE 2013, 8, e64918. [Google Scholar] [CrossRef]
- Gruber, S.; Hamedinger, D.; Bozsaky, E.; Schmidt, M.; Wolfram, K.; Haagen, J.; Habelt, B.; Puttrich, M.; Dörr, W. Local hypoxia in oral mucosa (mouse) during daily fractionated irradiation—Effect of pentoxifylline. Radiother. Oncol. 2015, 116, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.; Bozsaky, E.; Roitinger, E.; Schwarz, K.; Schmidt, M.; Dörr, W. Early inflammatory changes in radiation-induced oral mucositis Effect of pentoxifylline in a mouse model. Strahlenther. Onkol. 2017, 193, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Raber-Durlacher, J.; von Bültzingslöwen, I.; Logan, R.; Bowen, J.; Rahman, A.; Everaus, H. Systematic review of cytokines and growth factors for the management of oral mucositis in cancer patients. Support. Care Cancer 2013, 21, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M.K. Nuclear factor-κB (NF-κB) and cyclooxygenase-2 (COX-2) expression in the oral mucosa following cancer chemotherapy. Oral Oncol. 2007, 43, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hong, J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.-J.; Guo, J.-B.; Jiang, H.-W.; Zhu, S.X.; Li, C.Y.; Cheng, B.; Chen, Y.; Wang, H.Y. Spatio-temporal localization of HIF-1α and COX-2 during irradiation-induced oral mucositis in a rat model system. Int. J. Radiat. Biol. 2008, 84, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2016, 138, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.; Zheng, G. e-Cadherin/β-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011, 2011, 567305. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-cell junctions organize structural and signaling networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar] [CrossRef] [PubMed]
- Dörr, W. Three A’s of repopulation during fractionated irradiation of squamous epithelia: Asymmetry loss, Acceleration of stem-cell divisions and Abortive divisions. Int. J. Radiat. Biol. 1997, 72, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.A. Inflammatory cytokines and mucosal injury. J. Natl. Cancer Inst. Monogr. 2001, 26–30. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11694562 (accessed on 19 September 2014). [CrossRef]
- Xanthinaki, A.; Nicolatou-galitis, O. Apoptotic and inflammation markers in oral mucositis in head and neck cancer patients receiving radiotherapy: Preliminary report. Support. Care Cancer 2008, 16, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.A.; Trowbridge, J.M.; Taylor, K.R.; Morhenn, V.B.; Gallo, R.L. Dermatan sulfate proteoglycan and glycosaminoglycan synthesis is induced in fibroblasts by transfer to a three-dimensional extracellular environment. J. Biol. Chem. 2004, 279, 48640–48646. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Tamaki, H.; Fukui, S. Detection of oligosaccharide ligands for Hepatocyte growth factor/Scatter factor (HGF/SF), Keratinocyte growth factor (KGF/FGF-7), RANTES and Heparin cofactor II by neoglycolipid microarrays of glycosaminoglycan-derived oligosaccharide fragments. Glycoconj. J. 2006, 23, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Translocation, B.; Wang, Z.; Li, R.; Tan, J.; Peng, L.; Wang, P. Syndecan-1 acts in synergy with tight junction through STAT3 signaling to maintain intestinal mucosal barrier and prevent. Inflamm. Bowel Dis. 2015, 21, 1894–1907. [Google Scholar] [CrossRef]
- Kozlowski, E.O.; Pavao, M.S.; Borsig, L. Ascidian dermatan sulfates attenuate metastasis, inflammation and thrombosis by inhibition of P-selectin. J. Thromb. Haemost. 2011, 9, 1807–1815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casu, B.; Guerrini, M.; Torri, G. Structural and conformational aspects of the anticoagulant and antithrombotic activity of heparin and dermatan sulfate. Curr. Pharm. Des. 2004, 10, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Pavão, M.S.G.; Aiello, K.R.M.; Werneck, C.C.; Silva, L.C.F.; Valente, A.P.; Mulloy, B.; Colwell, N.S.; Tollefsen, D.M.; Mourão, P.A. Highly sulfated dermatan sulfates from ascidians: Structure versus anticoagulant activity of these glycosaminoglycans. J. Biol. Chem. 1998, 273, 27848–27857. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xia, T.; Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 2015, 64, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Yang, B.; Xu, Y.; Jiang, L.; Tsui, C.; Liang, X. FK506 suppresses hypoxia-induced inflammation and protects tight junction function via the CaN-NFATc1 signaling pathway in retinal microvascular epithelial cells. Mol. Med. Rep. 2017, 16, 6974–6980. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, S.; Arnold, M.; Cini, N.; Gernedl, V.; Hetzendorfer, S.; Kowald, L.-M.; Kuess, P.; Mayer, J.; Morava, S.; Pfaffinger, S.; et al. Radioprotective Effects of Dermatan Sulfate in a Preclinical Model of Oral Mucositis—Targeting Inflammation, Hypoxia and Junction Proteins without Stimulating Proliferation. Int. J. Mol. Sci. 2018, 19, 1684. https://doi.org/10.3390/ijms19061684
Gruber S, Arnold M, Cini N, Gernedl V, Hetzendorfer S, Kowald L-M, Kuess P, Mayer J, Morava S, Pfaffinger S, et al. Radioprotective Effects of Dermatan Sulfate in a Preclinical Model of Oral Mucositis—Targeting Inflammation, Hypoxia and Junction Proteins without Stimulating Proliferation. International Journal of Molecular Sciences. 2018; 19(6):1684. https://doi.org/10.3390/ijms19061684
Chicago/Turabian StyleGruber, Sylvia, Marlene Arnold, Nilsu Cini, Victoria Gernedl, Sabine Hetzendorfer, Lisa-Marie Kowald, Peter Kuess, Julia Mayer, Susanne Morava, Stephanie Pfaffinger, and et al. 2018. "Radioprotective Effects of Dermatan Sulfate in a Preclinical Model of Oral Mucositis—Targeting Inflammation, Hypoxia and Junction Proteins without Stimulating Proliferation" International Journal of Molecular Sciences 19, no. 6: 1684. https://doi.org/10.3390/ijms19061684



