Comparative Analysis of Zearalenone Effects on Thyroid Receptor Alpha (TRα) and Beta (TRβ) Expression in Rat Primary Cerebellar Cell Cultures
Abstract
:1. Introduction
2. Results
2.1. Expression of Thyroid Hormone Receptor Alpha (TRα)
2.1.1. TRα mRNA Expression
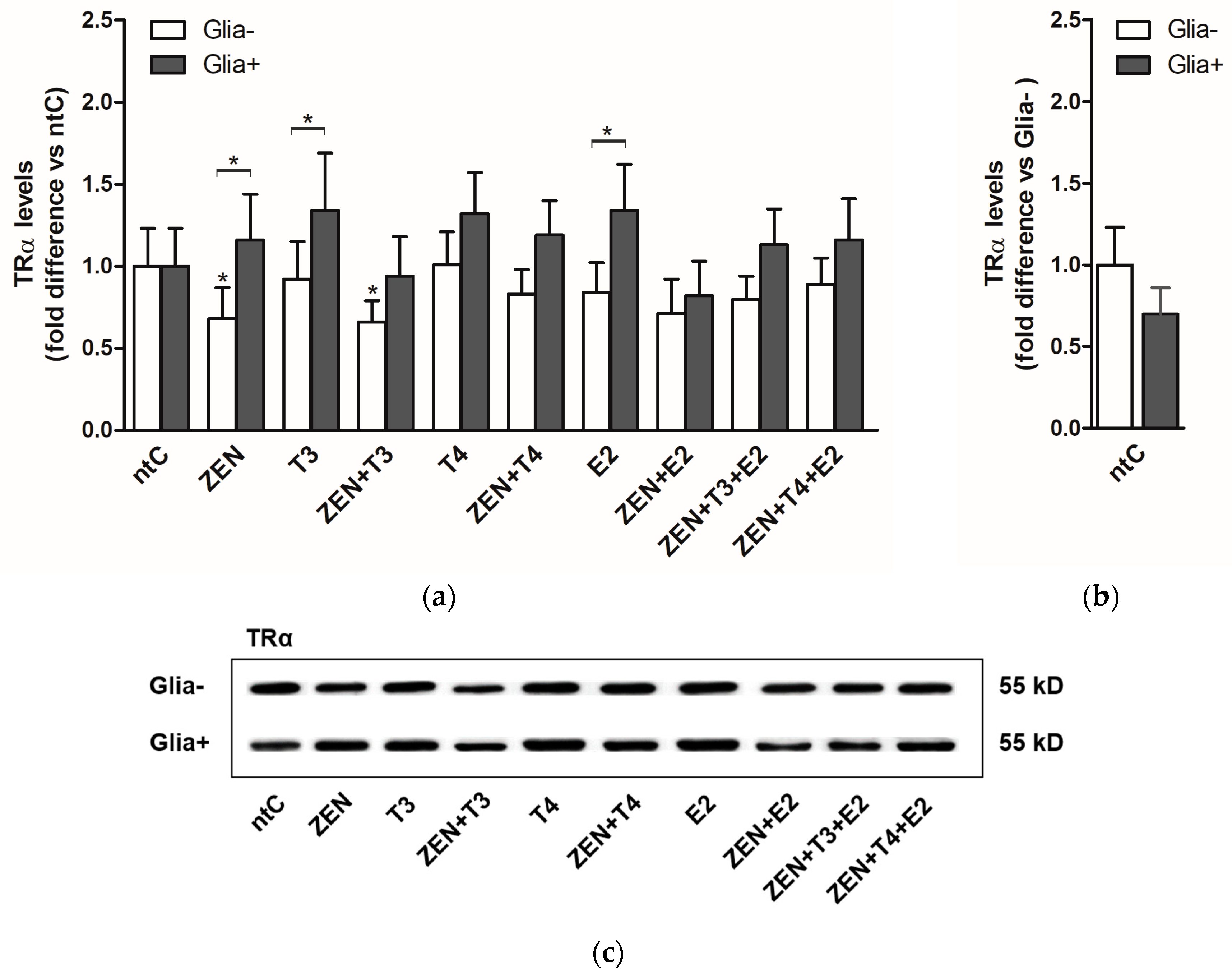
2.1.2. TRα Protein Levels
2.2. Expression of Thyroid Hormone Receptor Beta (TRβ)
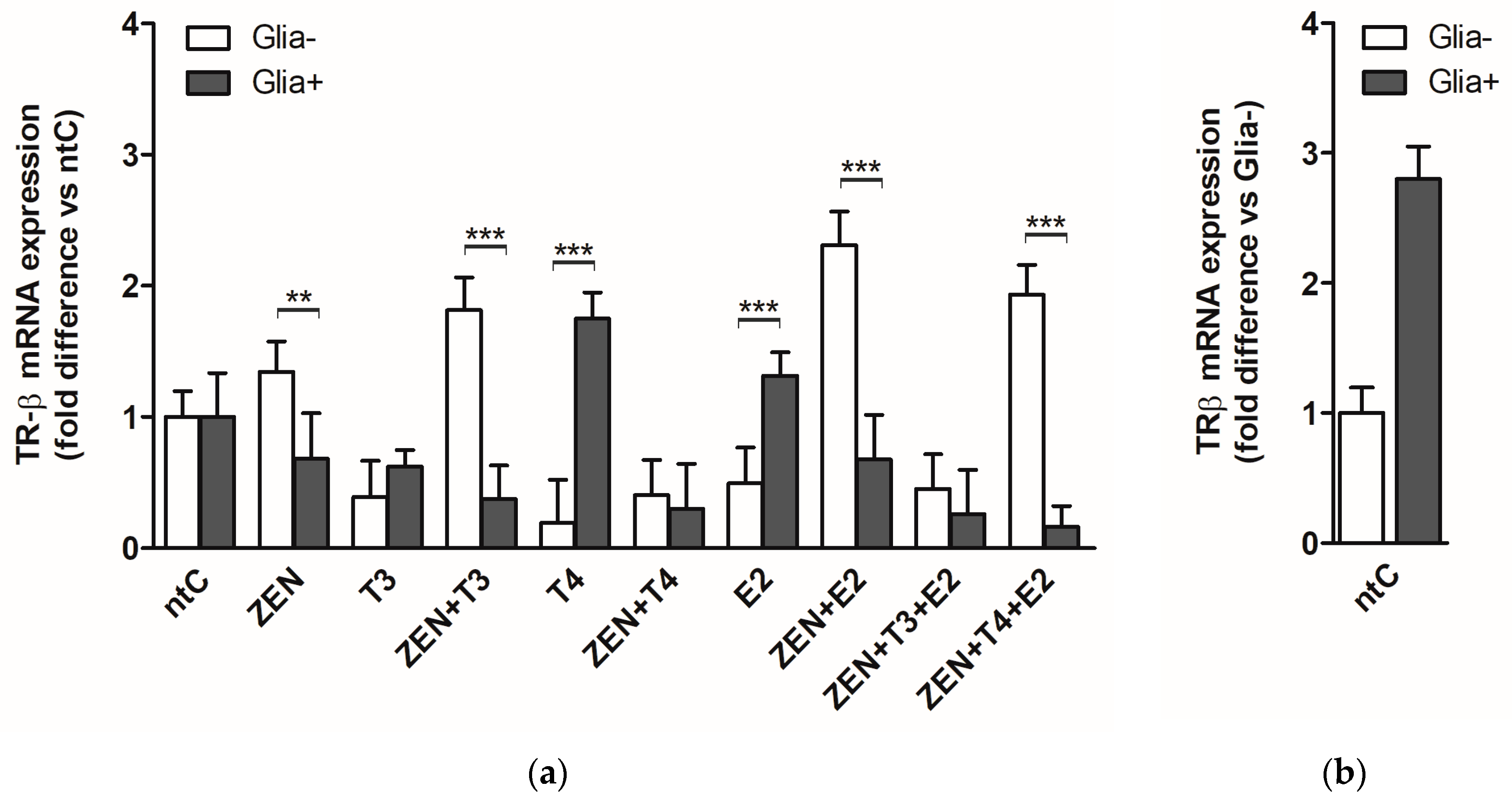
2.2.1. TRβ mRNA Expression
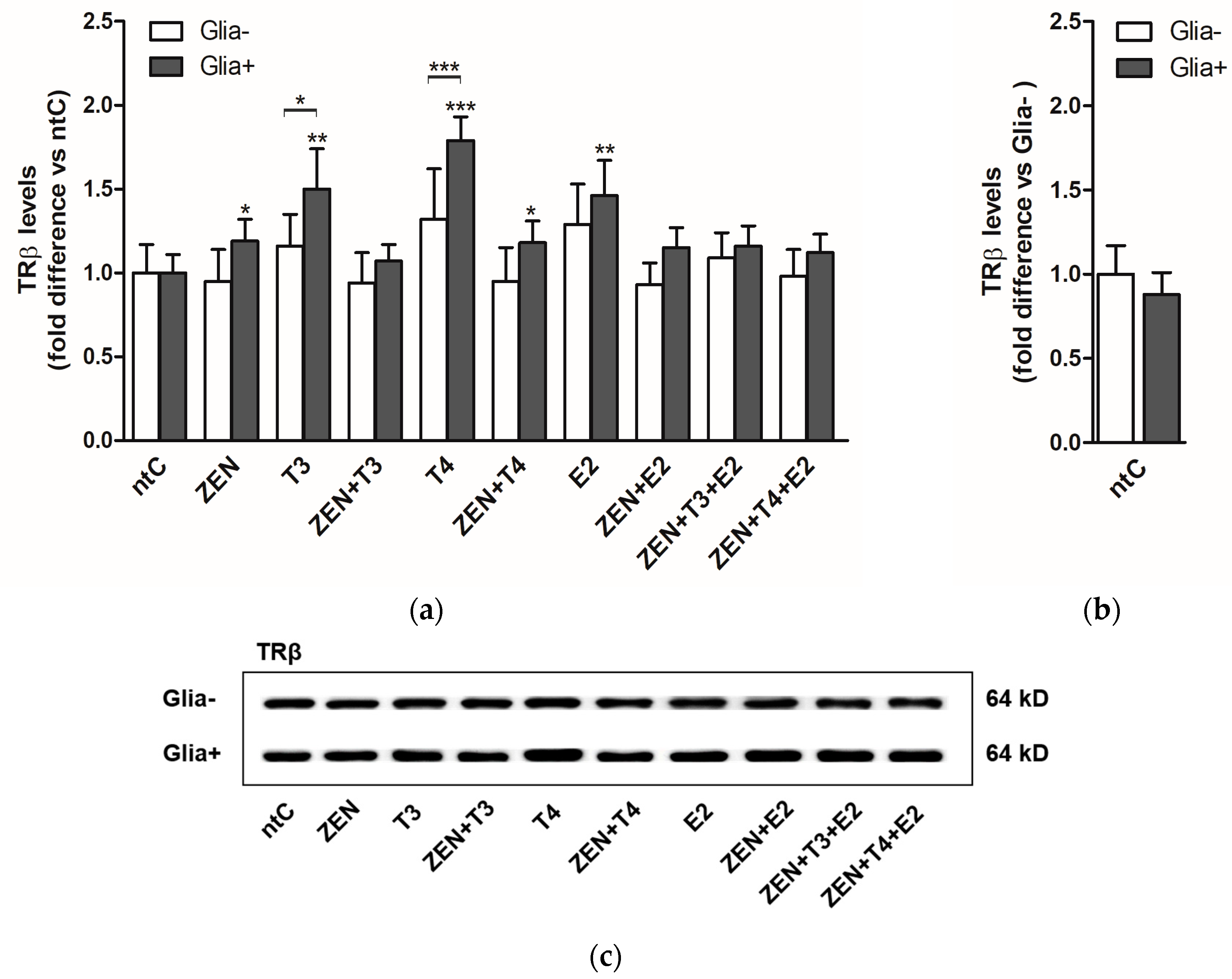
2.2.2. TRβ Protein Levels
3. Discussion
4. Materials and Methods
4.1. Antibodies, Reagents, and Materials
4.2. Animals
4.3. Preparation and Culture of Cerebellar Granule Cells
4.4. Treatments
4.5. Revers Transcription- and Quantitative-RT-PCR
4.6. Western Blot Analysis
4.7. Data Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| ZEN | zearalenone |
| TRα, TRβ | thyroid receptor alpha, thyroid receptor beta |
| T4 | l-thyroxine |
| T3 | 3,3′,5-triiodo-l-thyronine |
| E2 | 17β-estradiol |
References
- Maragos, C.M. Zearalenone occurrence and human exposure. World Mycotoxin J. 2010, 3, 369–383. [Google Scholar] [CrossRef]
- Shin, B.S.; Hong, S.H.; Bulitta, J.B.; Lee, J.B.; Hwang, S.W.; Kim, H.J.; Yang, S.D.; Yoon, H.-S.; Kim, D.J.; Lee, B.M.; et al. Physiologically based pharmacokinetics of zearalenone. J. Toxicol. Environ. Health A 2009, 72, 1395–1405. [Google Scholar] [CrossRef] [PubMed]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Edite Bezerra da Rocha, M.; Freire, F.D.C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Weaver, G.A.; Kurtz, H.J.; Mirocha, C.J.; Bates, F.Y.; Behrens, J.C.; Robinson, T.S.; Gipp, W.F. Mycotoxin-induced abortions in swine. Can. Vet. J. Rev. Vét. Can. 1978, 19, 72–74. [Google Scholar]
- Zinedine, A.; Soriano, J.M.; Man, J.; Molto, J.C. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Weaver, G.A.; Kurtz, H.J.; Behrens, J.C.; Robison, T.S.; Seguin, B.E.; Bates, F.Y.; Mirocha, C.J. Effect of zearalenone on dairy cows. Am. J. Vet. Res. 1986, 47, 1826–1828. [Google Scholar] [PubMed]
- Belhassen, H.; Jiménez-Díaz, I.; Arrebola, J.P.; Ghali, R.; Ghorbel, H.; Olea, N.; Hedili, A. Zearalenone and its metabolites in urine and breast cancer risk: A case-control study in Tunisia. Chemosphere 2015, 128, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Baudrimont, I.; Hassen, W.; Ouanes, Z.; Mobio, T.A.; Anane, R.; Creppy, E.E.; Bacha, H. DNA fragmentation, apoptosis and cell cycle arrest induced by zearalenone in cultured DOK, Vero and Caco-2 cells: Prevention by Vitamin E. Toxicology 2003, 192, 237–248. [Google Scholar] [CrossRef]
- Hassen, W.; Ayed-Boussema, I.; Oscoz, A.A.; De Cerain Lopez, A.; Bacha, H. The role of oxidative stress in zearalenone-mediated toxicity in Hep G2 cells: Oxidative DNA damage, gluthatione depletion and stress proteins induction. Toxicology 2007, 232, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem, I.; Prola, A.; Boussabbeh, M.; Guilbert, A.; Bacha, H.; Lemaire, C.; Abid-Essefi, S. Activation of ER stress and apoptosis by α- and β-zearalenol in HCT116 cells, protective role of Quercetin. Neurotoxicology 2016, 53, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Abid-Essefi, S.; Bouaziz, C.; El Golli-Bennour, E.; Ouanes, Z.; Bacha, H. Comparative study of toxic effects of zearalenone and its two major metabolites α-zearalenol and β-zearalenol on cultured human Caco-2 cells. J. Biochem. Mol. Toxicol. 2009, 23, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Doi, K.; Uetsuka, K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int. J. Mol. Sci. 2011, 12, 5213–5237. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.H.; Deng, H.D.; Deng, Y.T.; Deng, J.L.; Zuo, Z.C.; Yu, S.M.; Shen, L.H.; Cui, H.M.; Xu, Z.W.; Hu, Y.C. Effect of the Fusarium toxins, zearalenone and deoxynivalenol, on the mouse brain. Environ. Toxicol. Pharmacol. 2016, 46, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Venkataramana, M.; Chandra Nayaka, S.; Anand, T.; Rajesh, R.; Aiyaz, M.; Divakara, S.T.; Murali, H.S.; Prakash, H.S.; Lakshmana Rao, P.V. Zearalenone induced toxicity in SHSY-5Y cells: The role of oxidative stress evidenced by N-acetyl cysteine. Food Chem. Toxicol. 2014, 65, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wong, T.Y.; Chan, F.L.; Chen, S.; Leung, L.K. Assessing the effect of food mycotoxins on aromatase by using a cell-based system. Toxicol. In Vitro 2014, 28, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.-M.; Wang, X.-L.; Wang, G.-M.; Zhang, W.-J.; Zhu, L.; Gao, S.; Yang, D.-J.; Qin, Y.; Liang, Q.-J.; Chen, Y.-L.; et al. A stress-induced cellular aging model with postnatal neural stem cells. Cell Death Dis. 2014, 5, e1116. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, Y.; Liu, Y.; Yang, N.; Zuo, P. Phytoestrogen α-zearalanol ameliorates memory impairment and neuronal DNA oxidation in ovariectomized mice. Clinics 2013, 68, 1255–1262. [Google Scholar] [CrossRef]
- Dong, Y.-L.; Yue, Y.; Liu, F.-H.; Lang, S.-Y.; Zhang, X.-C.; Dai, S.-L.; Ge, Q.-S.; Zuo, P.-P. Treatment with phytoestrogen α-zearalanol might protect neurons of hippocampus in ovariectomized rats. Endocrine 2006, 30, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-L.; Zuo, P.-P.; Li, Q.; Liu, F.-H.; Dai, S.-L.; Ge, Q.-S. Protective effects of phytoestrogen α-zearalanol on β amyloid25-35 induced oxidative damage in cultured rat hippocampal neurons. Endocrine 2007, 32, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, P.M.; Bonni, A. Cultures of Cerebellar Granule Neurons. Cold Spring Harb. Protoc. 2008, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dana, K.; Liliana, M. Cell Culture of Primary Cerebellar Granule Cells. In Mouse Cell Culture Methods and Protocols; Ward, A., Tosh, D., Eds.; Springer Protocols: Berlin, Germany, 2010; Volume 633, pp. 233–239. ISBN 978-1-58829-772-3. [Google Scholar]
- Scalise, T.; Gyorffy, A.; Toth, I.; Kiss, D.; Somogyi, V.; Goszleth, G.; Bartha, T.; Frenyó, L.; Zsarnovszky, A. Ligand-induced changes in Oestrogen and thyroid hormone receptor expression in the developing rat cerebellum: A comparative quantitative PCR and Western blot study. Acta Vet. Hung. 2012, 60, 263–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullur, R.; Liu, Y.Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Forrest, D.; Visser, T.J. Thyroid hormone signaling. Biochim. Biophys. Acta 2013, 1830, 3859. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R.; Obregon, M.J.; Ruiz de Ona, C.; Escobar del Rey, F.; Morreale de Escobar, G. Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J. Clin. Investig. 1990, 86, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, T.L.; Correa-Medina, M.; Campos, M.P.O.; Wittmann, G.; Werneck-de-Castro, J.P.; Drigo, R.A.; Mora-Garzon, M.; Ueta, C.B.; Caicedo, A.; Fekete, C.; et al. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J. Clin. Investig. 2013, 123, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, F.; Picou, F.; Fauquier, T.; Flamant, F. Thyroid hormone action in cerebellum and cerebral cortex development. J. Thyroid Res. 2011, 2011, 145762. [Google Scholar] [CrossRef] [PubMed]
- Galton, V.A.; Schneider, M.J.; Clark, A.S.; St Germain, D.L. Life without thyroxine to 3,5,3′-triiodothyronine conversion: Studies in mice devoid of the 5′-deiodinases. Endocrinology 2009, 150, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Dezonne, R.S.; Lima, F.R.S.; Trentin, A.G.; Gomes, F.C. Thyroid Hormone and Astroglia: Endocrine Control of the Neural Environment. J. Neuroendocrinol. 2015, 27, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.J.; Lin, H.Y.; Lansing, L.; Mohamed, S.N.; Davis, F.B.; Mousa, S.; Davis, P.J. Integrin αVβ3 contains a cell surface receptor site for thyroid hormone that is linked to activation of mitogen-activated protein kinase and induction of angiogenesis. Endocrinology 2005, 146, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.J.; Davis, F.B.; Cody, V. Membrane receptors mediating thyroid hormone action. Trends Endocrinol. Metab. 2005, 16, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.R.; Gervais, A.; Colin, C.; Izembart, M.; Neto, V.M.; Mallat, M. Regulation of microglial development: A novel role for thyroid hormone. J. Neurosci. 2001, 21, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Trentin, A.G. Thyroid hormone and astrocyte morphogenesis. J. Endocrinol. 2006, 189, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Manzano, J.; Bernal, J.; Morte, B. Influence of thyroid hormones on maturation of rat cerebellar astrocytes. Int. J. Dev. Neurosci. 2007, 25, 171–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzano, J.; Cuadrado, M.; Morte, B.; Bernal, J. Influence of thyroid hormone and thyroid hormone receptors in the generation of cerebellar γ-aminobutyric acid-ergic interneurons from precursor cells. Endocrinology 2007, 148, 5746–5751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jocsak, G.; Kiss, D.S.; Toth, I.; Goszleth, G.; Bartha, T.; Frenyo, L.V.; Horvath, T.L.; Zsarnovszky, A. Comparison of Individual and Combined Effects of Four Endocrine Disruptors on Estrogen Receptor β Transcription in Cerebellar Cell Culture: The Modulatory Role of Estradiol and Triiodo-Thyronine. Int. J. Environ. Res. Public Health 2016, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Zoeller, R.T. Environmental chemicals as thyroid hormone analogues: New studies indicate that thyroid hormone receptors are targets of industrial chemicals? Mol. Cell. Endocrinol. 2005, 242, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.-Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 2011, 139, 10–16. [Google Scholar] [CrossRef]
- Le Guevel, R.; Pakdel, F. Assessment of oestrogenic potency of chemicals used as growth promoter by in-vitro methods. Hum. Reprod. 2001, 16, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Luongo, D.; De Luna, R.; Russo, R.; Severino, L. Effects of four Fusarium toxins (fumonisin B(1), α-zearalenol, nivalenol and deoxynivalenol) on porcine whole-blood cellular proliferation. Toxicon 2008, 52, 156–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, L.J.; Smith, T.K. Effect of dietary alfalfa on zearalenone toxicity and metabolism in rats and swine. J. Anim. Sci. 1982, 55, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Vlata, Z.; Porichis, F.; Tzanakakis, G.; Tsatsakis, A.; Krambovitis, E. A study of zearalenone cytotoxicity on human peripheral blood mononuclear cells. Toxicol. Lett. 2006, 165, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.Z.; Yang, Z.B.; Yang, W.R.; Gao, J.; Liu, F.X.; Broomhead, J.; Chi, F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J. Anim. Sci. 2011, 89, 3008–3015. [Google Scholar] [CrossRef] [PubMed]
- Adori, M.; Kiss, E.; Barad, Z.; Barabás, K.; Kiszely, E.; Schneider, A.; Kövesdi, D.; Sziksz, E.; Abrahám, I.M.; Matkó, J.; et al. Estrogen augments the T cell-dependent but not the T-independent immune response. Cell. Mol. Life Sci. 2010, 67, 1661–1674. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Gilkeson, G. Estrogen receptors in immunity and autoimmunity. Clin. Rev. Allergy Immunol. 2011, 40, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, H.; Kouro, T.; Yokota, T.; Comp, P.C.; Kincade, P.W. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc. Natl. Acad. Sci. USA 2001, 98, 15131–15136. [Google Scholar] [CrossRef] [PubMed]
- Hueza, I.M.; Raspantini, P.C.F.; Raspantini, L.E.R.; Latorre, A.O.; Górniak, S.L. Zearalenone, an estrogenic mycotoxin, is an immunotoxic compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Salah-Abbès, J.; Abbès, S.; Houas, Z.; Abdel-Wahhab, M.A.; Oueslati, R. Zearalenone induces immunotoxicity in mice: Possible protective effects of radish extract (Raphanus sativus). J. Pharm. Pharmacol. 2008, 60, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Borutova, R.; Faix, S.; Placha, I.; Gresakova, L.; Cobanova, K.; Leng, L. Effects of deoxynivalenol and zearalenone on oxidative stress and blood phagocytic activity in broilers. Arch. Anim. Nutr. 2008, 62, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Yegani, M.; Smith, T.K.; Leeson, S.; Boermans, H.J. Effects of feeding grains naturally contaminated with Fusarium mycotoxins on performance and metabolism of broiler breeders. Poult. Sci. 2006, 85, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Lioi, M.B.; Santoro, A.; Barbieri, R.; Salzano, S.; Ursini, M.V. Ochratoxin A and zearalenone: A comparative study on genotoxic effects and cell death induced in bovine lymphocytes. Mutat. Res. 2004, 557, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Hashiba, Y.; Ohtsuka, H.; Kohiruimaki, M.; Masui, M.; Kawamura, S.; Endo, H.; Ogata, Y. Effects of mycotoxins on mitogen-stimulated proliferation of bovine peripheral blood mononuclear cells. J. Vet. Med. Sci. 2008, 70, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.A.; Miller, K. Inhibitory effect of deoxynivalenol, 3-acetyldeoxynivalenol and zearalenone on induction of rat and human lymphocyte proliferation. Toxicol. Lett. 1984, 23, 215–221. [Google Scholar] [CrossRef]
- Forsell, J.H.; Pestka, J.J. Relation of 8-ketotrichothecene and zearalenone analog structure to inhibition of mitogen-induced human lymphocyte blastogenesis. Appl. Environ. Microbiol. 1985, 50, 1304–1307. [Google Scholar] [PubMed]
- Tola, S.; Bureau, D.P.; Hooft, J.M.; Beamish, F.W.H.; Sulyok, M.; Krska, R.; Encarnação, P.; Petkam, R. Effects of Wheat Naturally Contaminated with Fusarium Mycotoxins on Growth Performance and Selected Health Indices of Red Tilapia (Oreochromis niloticus × O. mossambicus). Toxins 2015, 7, 1929–1944. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.; Mirocha, C.J.; Abbas, H.K.; Johansson, B. Metabolism of high concentrations of dietary zearalenone by young male turkey poults. Poult. Sci. 1986, 65, 1905–1910. [Google Scholar] [CrossRef] [PubMed]
- Conková, E.; Laciaková, A.; Pástorová, B.; Seidel, H.; Kovác, G. The effect of zearalenone on some enzymatic parameters in rabbits. Toxicol. Lett. 2001, 121, 145–149. [Google Scholar] [CrossRef]
- Maaroufi, K.; Chekir, L.; Creppy, E.E.; Ellouz, F.; Bacha, H. Zearalenone induces modifications of haematological and biochemical parameters in rats. Toxicon 1996, 34, 535–540. [Google Scholar] [CrossRef]
- Sun, L.-H.; Lei, M.-Y.; Zhang, N.-Y.; Gao, X.; Li, C.; Krumm, C.S.; Qi, D.-S. Individual and combined cytotoxic effects of aflatoxin B1, zearalenone, deoxynivalenol and fumonisin B1 on BRL 3A rat liver cells. Toxicon 2015, 95, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Denli, M.; Blandon, J.C.; Guynot, M.E.; Salado, S.; Pérez, J.F. Efficacy of activated diatomaceous clay in reducing the toxicity of zearalenone in rats and piglets. J. Anim. Sci. 2015, 93, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Yin, S.; Liu, M.; Zhang, Y.; Gao, R.; Shi, B.; Shan, A.; Chen, Z. Modified halloysite nanotubes and the alleviation of kidney damage induced by dietary zearalenone in swine. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Ouanes, Z.; Abid, S.; Ayed, I.; Anane, R.; Mobio, T.; Creppy, E.E.; Bacha, H. Induction of micronuclei by Zearalenone in Vero monkey kidney cells and in bone marrow cells of mice: Protective effect of Vitamin E. Mutat. Res. 2003, 538, 63–70. [Google Scholar] [CrossRef]
- Zatecka, E.; Ded, L.; Elzeinova, F.; Kubatova, A.; Dorosh, A.; Margaryan, H.; Dostalova, P.; Korenkova, V.; Hoskova, K.; Peknicova, J. Effect of zearalenone on reproductive parameters and expression of selected testicular genes in mice. Reprod. Toxicol. 2014, 45, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, J. Related factors of sperm DNA damage: Advances in studies. Zhonghua Nan Ke Xue 2015, 21, 675–680. [Google Scholar] [PubMed]
- Weidner, M.; Hüwel, S.; Ebert, F.; Schwerdtle, T.; Galla, H.J.; Humpf, H.U. Influence of T-2 and HT-2 Toxin on the Blood-Brain Barrier In Vitro: New Experimental Hints for Neurotoxic Effects. PLoS ONE 2013, 8, e60484. [Google Scholar] [CrossRef] [PubMed]
- Fénichel, P.; Chevalier, N. Perturbateurs endocriniens environnementaux: De nouveaux diabétogènes? C. R. Biol. 2017, 340, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.N.; Salleh, B.; Saad, B.; Abbas, H.K.; Abel, C.A.; Shier, W.T. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010, 29, 3–26. [Google Scholar] [CrossRef]
- Gauger, K.J.; Kato, Y.; Haraguchi, K.; Lehmler, H.J.; Robertson, R.W.; Bansal, R.; Zoeller, R.T. Polychlorinated biphenyls (PCBs) exert thyroid hormone-like effects in the fetal rat brain but do not bind to thyroid hormone receptors. Environ. Health Perspect. 2004, 112, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Crofton, K.M. PCBs, Thyroid Hormones, and Ototoxicity in Rats: Cross-Fostering Experiments Demonstrate the Impact of Postnatal Lactation Exposure. Toxicol. Sci. 2000, 57, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Segura, L.M.; Chowen, J.A.; Naftolin, F. Endocrine glia: Roles of glial cells in the brain actions of steroid and thyroid hormones and in the regulation of hormone secretion. Front. Neuroendocrinol. 1996, 17, 180–211. [Google Scholar] [CrossRef] [PubMed]
- Dudazy-Gralla, S.; Nordström, K.; Hofmann, P.J.; Meseh, D.A.; Schomburg, L.; Vennström, B.; Mittag, J. Identification of thyroid hormone response elements in vivo using mice expressing a tagged thyroid hormone receptor α1. Biosci. Rep. 2013, 33, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Freindorf, M.; Furlani, T.R.; Kong, J.; Cody, V.; Davis, F.B.; Davis, P.J. Combined QM/MM study of thyroid and steroid hormone analogue interactions with αv3β integrin. J. Biomed. Biotechnol. 2012, 2012, 959057. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.P.; De Velasco, E.M.F.; Mizuno, F.; Scappini, E.L.; Gloss, B.; Erxleben, C.; Williams, J.G.; Stapleton, H.M.; Gentile, S.; Armstrong, D.L. A rapid cytoplasmic mechanism for pi3 kinase regulation by the nuclear thyroid hormone receptor, TRb, and genetic evidence for its role in the maturation of mouse hippocampal synapses in vivo. Endocrinology 2014, 155, 3713–3724. [Google Scholar] [CrossRef] [PubMed]
- Peeters, R.P.; Visser, T.J. Metabolism of Thyroid Hormone; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- McDonald, J.W.; Goldberg, M.P.; Gwag, B.J.; Chi, S.-I.; Choi, D.W. Cyclosporine induces neuronal apoptosis and selective oligodendrocyte death in cortical cultures. Ann. Neurol. 1996, 40, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Rosene, M.L.; Wittmann, G.; Arrojo E Drigo, R.; Singru, P.S.; Lechan, R.M.; Bianco, A.C. Inhibition of the type 2 iodothyronine deiodinase underlies the elevated plasma TSH associated with amiodarone treatment. Endocrinology 2010, 151, 5961–5970. [Google Scholar] [CrossRef] [PubMed]
- Cenik, C.; Cenik, E.S.; Byeon, G.W.; Grubert, F.; Candille, S.I.; Spacek, D.; Alsallakh, B.; Tilgner, H.; Araya, C.L.; Tang, H.; et al. Integrative analysis of RNA, translation, and protein levels reveals distinct regulatory variation across humans. Genome Res. 2015, 25, 1610–1621. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into regulation of protein abundance from proteomics and transcriptomis analyses. Nat. Rev. Genet. 2013, 13, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Strait, K.A.; Schwartz, H.L.; Seybold, V.S.; Ling, N.C.; Oppenheimer, J.H. Immunofluorescence localization of thyroid hormone receptor protein β 1 and variant α 2 in selected tissues: Cerebellar Purkinje cells as a model for β 1 receptor-mediated developmental effects of thyroid hormone in brain. Proc. Natl. Acad. Sci. USA 1991, 88, 3887–3891. [Google Scholar] [CrossRef] [PubMed]
- Maher, S.K.; Wojnarowicz, P.; Ichu, T.-A.; Veldhoen, N.; Lu, L.; Lesperance, M.; Propper, C.R.; Helbing, C.C. Rethinking the biological relationships of the thyroid hormones, l-thyroxine and 3,5,3′-triiodothyronine. Comp. Biochem. Physiol. Part D Genom. Proteom. 2016, 18, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Carlson, D.J.; Strait, K.A.; Schwartz, H.L.; Oppenheimer, J.H. Thyroid hormone receptor isoform content in cultured type 1 and type 2 astrocytes. Endocrinology 1996, 137, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Oetting, A.; Yen, P.M. New insights into thyroid hormone action. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Freitas, B.C.G.; Gereben, B.; Castillo, M.; Kalló, I.; Zeöld, A.; Egri, P.; Liposits, Z.; Zavacki, A.M.; Maciel, R.M.B.; Jo, S.; et al. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J. Clin. Investig. 2010, 120, 2206–2217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, J.; Friesema, E.C.H.; Milici, C.; Visser, T.J. Thyroid Hormone Transporters in Health and Disease. Thyroid 2005, 15, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Wirth, E.K.; Schweizer, U.; Köhrle, J. Transport of thyroid hormone in brain. Front. Endocrinol. 2014, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, D.; Kusuhara, H.; Taniguchi, H.; Ishikawa, S.; Nozaki, Y.; Aburatani, H.; Sugiyama, Y. Functional Characterization of Rat Brain-specific Organic Anion Transporter (OATP14) at the Blood-Brain Barrier: High affinity transporter for thyroxine. J. Biol. Chem. 2003, 278, 43489–43495. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.M.; Ritchie, J.W.A. Tissue uptake of thyroid hormone by amino acid transporters. Best Pract. Res. Clin. Endocrinol. Metab. 2007, 21, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Roberts, L.M.; Woodford, K.; Zhou, M.; Black, D.S.; Haggerty, J.E.; Tate, E.H.; Grindstaff, K.K.; Mengesha, W.; Raman, C.; Zerangue, N. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 2008, 149, 6251–6261. [Google Scholar] [CrossRef] [PubMed]
- Domènech-Estévez, E.; Baloui, H.; Repond, C.; Rosafio, K.; Médard, J.-J.; Tricaud, N.; Pellerin, L.; Chrast, R. Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. J. Neurosci. 2015, 35, 4151–4156. [Google Scholar] [CrossRef] [PubMed]
- Jayarama-Naidu, R.; Johannes, J.; Meyer, F.; Wirth, E.K.; Schomburg, L.; Köhrle, J.; Renko, K. A Nonradioactive Uptake Assay for Rapid Analysis of Thyroid Hormone Transporter Function. Endocrinology 2015, 156, 2739–2745. [Google Scholar] [CrossRef] [PubMed]
- Tachampa, K.; Takeda, M.; Khamdang, S.; Noshiro-Kofuji, R.; Tsuda, M.; Jariyawat, S.; Endou, H. Interactions of organic anion transporters and organic cation transporters with mycotoxins. J. Pharm. Sci. 2008, 106, 435–443. [Google Scholar] [CrossRef]
- Cozzini, P.; Dellafiora, L. In silico approach to evaluate molecular interaction between mycotoxins and the estrogen receptors ligand binding domain: A case study on zearalenone and its metabolites. Toxicol. Lett. 2012, 214, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Molina, R.; Frischknecht, R.; Maldonado, H.; Seidenbecher, C.I.; Gundelfinger, E.D.; Hetz, C.; de la Luz Aylwin, M.; Schneider, P.; Quest, A.F.G.; Leyton, L. Astrocytic αvβ3 integrin inhibits neurite outgrowth and promotes retraction of neuronal processes by clustering thy-1. PLoS ONE 2012, 7, e34295. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Reddy, D.S. Integrins as receptor targets for neurological disorders. Pharmacol. Ther. 2012, 134, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.K.; Kennedy, P.R.; Belcher, S.M. Simplified serum- and steroid-free culture conditions for high-throughput viability analysis of primary cultures of cerebellar granule neurons. J. Neurosci. Methods 2001, 110, 45–55. [Google Scholar] [CrossRef]
- Gallo, V.; Kingsbury, A.; Balázs, R.; Jørgensen, O.S. The Role of Depolarization in the Survival and Differentiation of Cerebellar Granule Cells in Culture. J. Neurosci. 1987, 7, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Thangnipon, W.; Kingsbury, A.; Webb, M.; Balazs, R. Observations on rat cerebellar cells in vitro: Influence of substratum, potassium concentration and relationship between neurones and astrocytes. Dev. Brain Res. 1983, 11, 177–189. [Google Scholar] [CrossRef]
- Sayed-Ahmed, A.; Kulcsar, M.; Rudas, P.; Bartha, T. Expression and localisation of leptin and leptin receptor in the mammary gland of the dry and lactating non-pregnant cow. Acta Vet. Hung. 2004, 52, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Billon, N.; Jolicoeur, C.; Tokumoto, Y.; Vennström, B.; Raff, M. Normal timing of oligodendrocyte development depends on thyroid hormone receptor α 1 (TRα1). EMBO J. 2002, 21, 6452–6460. [Google Scholar] [CrossRef] [PubMed]
- Kariv, R.; Enden, A.; Zvibel, I.; Rosner, G.; Brill, S.; Shafritz, D.A.; Halpern, Z.; Oren, R. Triiodothyronine and interleukin-6 (IL-6) induce expression of HGF in an immortalized rat hepatic stellate cell line. Liver Int. 2003, 23, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiss, D.S.; Ioja, E.; Toth, I.; Barany, Z.; Jocsak, G.; Bartha, T.; Horvath, T.L.; Zsarnovszky, A. Comparative Analysis of Zearalenone Effects on Thyroid Receptor Alpha (TRα) and Beta (TRβ) Expression in Rat Primary Cerebellar Cell Cultures. Int. J. Mol. Sci. 2018, 19, 1440. https://doi.org/10.3390/ijms19051440
Kiss DS, Ioja E, Toth I, Barany Z, Jocsak G, Bartha T, Horvath TL, Zsarnovszky A. Comparative Analysis of Zearalenone Effects on Thyroid Receptor Alpha (TRα) and Beta (TRβ) Expression in Rat Primary Cerebellar Cell Cultures. International Journal of Molecular Sciences. 2018; 19(5):1440. https://doi.org/10.3390/ijms19051440
Chicago/Turabian StyleKiss, David Sandor, Eniko Ioja, Istvan Toth, Zoltan Barany, Gergely Jocsak, Tibor Bartha, Tamas L. Horvath, and Attila Zsarnovszky. 2018. "Comparative Analysis of Zearalenone Effects on Thyroid Receptor Alpha (TRα) and Beta (TRβ) Expression in Rat Primary Cerebellar Cell Cultures" International Journal of Molecular Sciences 19, no. 5: 1440. https://doi.org/10.3390/ijms19051440
APA StyleKiss, D. S., Ioja, E., Toth, I., Barany, Z., Jocsak, G., Bartha, T., Horvath, T. L., & Zsarnovszky, A. (2018). Comparative Analysis of Zearalenone Effects on Thyroid Receptor Alpha (TRα) and Beta (TRβ) Expression in Rat Primary Cerebellar Cell Cultures. International Journal of Molecular Sciences, 19(5), 1440. https://doi.org/10.3390/ijms19051440