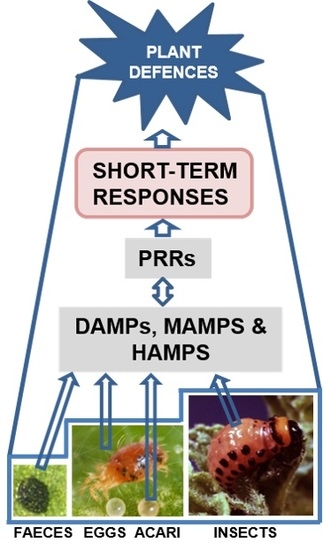
Plant Perception and Short-Term Responses to Phytophagous Insects and Mites
Abstract
:1. Introduction
2. First Signals: Elicitors and Effectors
2.1. Elicitors from the Plant Side
2.2. Elicitors/Effectors from the Pest Side
3. Plant Perception
4. Early Plant Signaling Events
5. Integrated Pest Management Practices
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| cATP | chloroplastic ATP synthase γ-subunit sequence |
| CDPK | Ca2+-binding protein kinase |
| DAMP | damage-associated molecular pattern |
| FAC | fatty acid–amino conjugate |
| GABA | 4-aminobutyrate |
| HAMP | herbivore-associated molecular pattern |
| HypSys | hydoxyproline-rich systemin |
| JA | jasmonic acid |
| MAMP | microbe-associated molecular pattern |
| MAP kinase | mitogen-activated protein kinase |
| OS | oral secretion |
| Pep | plant-elicitor peptide |
| PEPR | Pep receptor |
| PRR | pattern recognition receptor |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| SA | salicylic acid |
| Sys | systemin |
References
- DeLucia, E.H.; Nabity, P.D.; Zavala, J.A.; Berenbaum, M.R. Climate change: Resetting plant-insect interaction. Plant Physiol. 2012, 160, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Ximenez-Embun, M.G.; Castañera, P.; Ortego, F. Drought stress in tomato increases the performance of adapted and non-adapted strains of Tetranychus urticae. J. Insect Physiol. 2017, 96, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Ortego, F. Physiological adaptation of the insect gut to herbivory. In Arthropod-plant Interactions; Springer: Dordrecht, The Netherlands; Hidelberg, Germany; New York, NY, USA; London, UK, 2012; pp. 75–88. ISBN 9-789400-738720. [Google Scholar]
- Duso, C.; Castagnoli, M.; Simoni, S.M.; Angeli, G. The impact of eriphyoids on crops: Recent issue on Aculops schlechtendali, Clepitrimerus vitis and Aculops lycopersici. Exp. Appl. Acarol. 2010, 51, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Bensoussan, N.; Santamaria, M.E.; Zhurov, V.; Diaz, I.; Grbic, M.; Grbic, V. Plant-herbivore interaction: Dissection of the cellular pattern of Tetranychus urticae: Toward understanding cell biology of plant-pest interaction. Front. Plant Sci. 2016, 7, 1105. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Baldwin, I.T. New insights into plant responses to the attack from insect herbivore. Ann. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Martinez, M.; Cambra, I.; Grbic, V.; Diaz, I. Understanding plant defence responses against herbivore attacks: An essential first step towards the development of sustainable resistance against pests. Transgenic Res. 2013, 22, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Muroi, A.; Ramadan, A.; Nishihara, M.; Yamamoto, M.; Ozawa, R.; Takabayashi, J.; Arimura, G. The composite effect of transgenic plant volatiles for acquired immunity to herbivory caused by inter-plant communications. PLoS ONE 2011, 6, e24594. [Google Scholar] [CrossRef] [PubMed]
- Schuman, M.C.; Baldwin, I.T. The layers of plant responses to insect herbivores. Ann. Rev. Entomol. 2016, 61, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, D.A.; Jander, G. The role of protein effectors in plant-aphid interactions. Curr. Opin. Plant Biol. 2013, 17, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Liu, C.; Wang, Y.; Chen, R.; Tang, M.; Du, B.; Zhu, L.; He, G. Herbivore-induced callose deposition on the sieve plates of rice: And important mechanism for host resistance. Plant Physiol. 2008, 146, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Huffaker, A.; Carroll, M.J.; Alborn, H.T.; Ali, J.G.; Teal, P.E.A. An amino acid substitution inhibits specialist herbivore production of an antagonist effector and recovers insect-induced plant defences. Plant Physiol. 2012, 160, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.J.; Appel, H.M.; Carlson, J.E.; DeMoraes, C.M.; Mescher, M.C.; Schultz, J.C. Within-plant signalling via volatiles overcomes vascular constraints on systemic signalling and primes responses against herbivores. Ecol. Lett. 2007, 10, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Reymond, P. Perception, signaling and molecular basis of oviposition-mediated plant responses. Planta 2013, 238, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Fatouros, N.E. Plant responses to insect egg deposition. Ann. Rev. Entomol. 2015, 60, 493. [Google Scholar] [CrossRef] [PubMed]
- Duran-Flores, D.; Heil, M. Sources of specificity in plant damaged-self recognition. Curr. Opin. Plant Biol. 2016, 32, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Varsani, S.; Louis, J. Altering plant defences: Herbivore-associated molecular patterns and effector arsenal of chewing herbivores. Mol. Plant Microbe Interact. 2018, 31, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Zebelo, S.A.; Maffei, M.E. Role of early signalling events in plant-insect interactions. J. Exp. Bot. 2015, 66, 435–448. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Acevedo, F.E.; Rivera-Vega, L.J.; Chung, S.H.; Ray, S.; Felton, G.W. Cues from chewing insects—The intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 2015, 26, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Mithofer, A.; Boland, W. Plant defense against herbivores: Chemical aspects. Ann. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Barah, P.; Bones, A.M. Multidimensional approaches for studying plant defence against insects: From ecology to omics and synthetic biology. J. Exp. Bot. 2015, 66, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.P.; Wunsche, H.; Mitra, S.; Zavala, J.A.; Muck, A.; Svatos, A.; Baldwin, I.T. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant’s proteome. Plant Physiol. 2006, 142, 1621–1641. [Google Scholar] [CrossRef] [PubMed]
- Kempema, L.A.; Cui, X.; Holzer, F.M.; Walling, L.L. Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol. 2007, 143, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Duceppe, M.O.; Cloutier, C.; Michaud, D. Wounding, insect chewing and phloem sap feeding differentially alter the leaf proteome of potato. Solanum tuberosum L. Proteom. Sci. 2012, 10, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhurov, V.; Navarro, M.; Bruinsma, K.A.; Arbona, V.; Santamaria, M.E.; Cazaux, M.; Wybouw, N.; Osborne, E.J.; Ens, C.; Rioja, C.; et al. Reciprocal responses in the interaction between Arabidopsis and the cell-content-feeding chelicerate herbivore spider mite. Plant Physiol. 2014, 164, 384–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Verrall, S.R.; Hancock, R.D. Systematic analysis of phloem-feeding insect-induced transcriptional reprogramming in Arabidopsis highlights common features and reveals distinct responses to specialist and generalist insects. J. Exp. Bot. 2015, 66, 495–512. [Google Scholar] [CrossRef] [PubMed]
- Hettenhausen, C.; Baldwin, I.T.; Wu, J. Nicotiana attenuata MPK4 suppresses a novel jasmonic acid (JA) signaling-independent defense pathway against the specialist insect Manduca sexta, but is not required for the resistance to the generalist Spodoptera littoralis. New Phytol. 2013, 199, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Marti, G.; Erb, M.; Boccard, J.; Glauser, G.; Doyen, G.R.; Villard, N.; Robert, C.A.; Turlings, T.C.; Rudaz, S.; Wolfender, J.L. Metabolomics reveals herbivore-induced metabolites of resistance and susceptibility in maize leaves and roots. Plant Cell Environ. 2013, 36, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Rieu, I.; Mariani, C.; van Dam, N.M. How plants handle multiple stresses: Hormonal interactions undelaying responses to abiotic stress and insect herbivory. Plant Mol. Biol. 2016, 91, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Lassueur, S.; Ponzio, C.; Gols, R.; Dicke, M.; Reymond, P. Combined biotic stresses trigger similar transcriptomic responses but contrasting resistance against chewing herbivore in Brassica nigra. BMC Plant Biol. 2017, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Appel, H.M.; Cocroft, R.B. Plants respond to leaf vibrations caused by insect herbivore. Oecologia 2014, 175, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Bown, A.W.; Hall, D.E.; MacGregor, K.B. Insect footsteps on leaves stimulate the accumulation of 4-aminobutyrate and can be visualized through increased chlorophyll fluorescence and superoxide production. Plant Physiol. 2002, 129, 1430–1434. [Google Scholar] [CrossRef] [PubMed]
- Hogenhout, S.A.; Bos, J.I. Effector proteins that modulate plant-insect interactions. Curr. Opin. Plant Biol. 2011, 14, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Alves, P.C.M.S.; Ahmad, I.; Gaffoor, I.; Acevedo, F.E.; Peiffer, M.; Jin, S.; Han, Y.; Shakeel, S.; Felton, G.W.; et al. Turnabout is Fair play: Herbivory-induced plant chitinases excreted in fall armyworm frass suppress herbivore defenses in maize. Plant Physiol. 2016, 171, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.A.; Pierce, G. Systemins: A functionally defined family of peptide signals that regulate defensive genes in Solanaceae species. Proc. Natl. Acad. Sci. USA 2003, 100, 14577–14580. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Huffaker, A. Endogenous peptide elicitors in higher plants. Curr. Opin. Plant Biol. 2011, 14, 351–157. [Google Scholar] [CrossRef] [PubMed]
- Scheer, J.M.; Ryan, C.A., Jr. The systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR receptor kinase family. Proc. Natl. Acad. Sci. USA 2002, 99, 9585–9590. [Google Scholar] [CrossRef] [PubMed]
- McGurl, B.; Orozco-Cardenas, M.; Pearce, G.; Ryan, C.A. Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase-inhibitor synthesis. Proc. Natl. Acad. Sci. USA 1994, 91, 405–409. [Google Scholar] [CrossRef]
- Degenhardt, D.C.; Refi-Hind, S.; Stratmann, J.W.; Lincoln, D.E. Systemin and jasmonic acid regulate constitutive and herbivore-induced systemic volatile emissions in tomato, Solanum lycopersicum. Phytochemistry 2010, 71, 2014–2037. [Google Scholar] [CrossRef] [PubMed]
- Coppola, M.; Corrado, G.; Coppola, V.; Cascone, P.; Martinelli, R.; Digilio, M.C.; Pennacchio, F.; Rao, R. Prosystemin overexpression in tomato enhances resistance to different biotic stresses by activating genes of multiple signaling pathways. Plant Mol. Biol. Rep. 2015, 33, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- McGurl, B.; Pearce, G.; Orozco-Cardenas, M.; Ryan, C.A. Structure, expression, and antisense inhibition of the systemin precursor gene. Science 1992, 255, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Q.; Jiang, H.L.; Li, C.Y. Systemin/jasmonate-mediated systemic defense signalling in tomato. Mol. Plant 2011, 4, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Narvaez-Vasquez, J.; Pearce, G.; Ryan, C.A. The plant cell wall matrix harbous a precursor of defense signalling peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 12974–12977. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Koramutla, M.K.; Negi, M.; Pearce, G.; Ryan, C.A. Hydroxyproline-rich glycopeptide signals in potato elicit signalling associated with defense against insects and pathogens. Plant Sci. 2013, 207, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, A.; Pearce, G.; Ryan, C.A. An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc. Natl. Acad. Sci. USA 2006, 103, 10098–10103. [Google Scholar] [CrossRef] [PubMed]
- Huffaker, A.; Pearce, G.; Veyrat, N.; Erb, M.; Turlings, T.C.J.; Sartor, R.; Shen, Z.; Briggs, S.P.; Vaughan, M.M.; Alborn, H.T.; et al. Plant elicitor peptides are conserved signals regulating direct and indirect antiherbivore defense. Proc. Natl. Acad. Sci. USA 2013, 110, 5707–5712. [Google Scholar] [CrossRef] [PubMed]
- Klauser, D.; Desurmont, G.A.; Glauser, G.; Vallat, A.; Flury, P.; Boller, T.; Turlings, T.C.J.; Bartels, S. The Arabidopsis pep-PRR system is induced by herbivore feeding and contributes to JA-mediated plant defence against herbivory. J. Exp. Bot. 2015, 66, 5327–5336. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; LeClere, S.; Carroll, M.J.; Alborn, H.T.; Teal, P.E.A. Cowpea chloroplastic ATP synthase is the source of multiple plant defense elicitors during insect herbivory. Plant Physiol. 2007, 144, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G.; VanDoorn, A.; Baldwin, I.T. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011, 16, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, N.; Aboshi, T.; Abe, H.; Nishida, R.; Alborn, H.T.; Tumlinson, J.H.; Mori, N. Active role of fatty acid amino acid conjugates in nitrogen metabolism in Spodoptera litura larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 18058–18063. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Truitt, A.C.; We, H.X.; Pare, P.W. A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell 2004, 16, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Shinya, T.; Hojo, Y.; Desaki, Y.; Christeller, J.T.; Okada, K.; Sibuya, N.; Galis, I. Modulation of plant defense responses to herbivores by simultaneous recognition of different herbivore-associated elicitors in rice. Sci. Rep. 2016, 6, 32537. [Google Scholar] [CrossRef] [PubMed]
- Dinh, S.T.; Baldwin, I.T.; Galis, I. The HERVIBORE ELICITOR-REGULATED1 gene enhances abscisic acid levels and defenses against herbivores in Nicotiana attenuata plants. Plant Physiol. 2013, 162, 2106–2124. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.T.; Hansen, T.V.; Jones, T.H.; Bennett, D.C.; Tumlinson, J.H.; Schmelz, E.A.; Teal, P.E.A. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc. Natl. Acad. Sci. USA 1997, 104, 12976–12981. [Google Scholar] [CrossRef] [PubMed]
- Musser, R.O.; Hum-Musser, S.M.; Eichenseer, H.; Peiffer, M.; Ervin, G.; Murphy, J.B.; Felton, G.W. Herbivory: Caterpillar saliva beats plant defences. Nature 2002, 416, 599–600. [Google Scholar] [CrossRef] [PubMed]
- Bede, J.C.; Musser, R.O.; Felton, G.W.; Korth, K.L. Caterpillar herbivory and salivary enzymes decrease transcript levels of Medicago truncatula genes encoding early enzymes in terpenoid biosynthesis. Plant Mol. Biol. 2006, 60, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Weech, M.H.; Chapleau, M.; Pan, L.; Ide, C.; Bede, J.C. Caterpillar saliva interferes with induced Arabidopsis thaliana defence responses via the systemic acquired resistance pathway. J. Exp. Bot. 2008, 59, 2437–2448. [Google Scholar] [CrossRef] [PubMed]
- Diezel, C.; von Dahl, C.C.; Gaquerel, E.; Baldwin, I.T. Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol. 2009, 150, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Mutti, N.S.; Louis, J.; Pappan, L.K.; Begum, K.; Chen, M.S.; Park, Y.; Dittmer, N.; Marshall, J.; Reese, J.C.; Reeck, G.R. A protein from the salivary glands of the pea aphid, Acyrtosiphon pisum, is essential in feeding on a host plant. Proc. Natl. Acad. Sci. USA 2008, 105, 9965–9969. [Google Scholar] [CrossRef] [PubMed]
- Pitino, M.; Hogenhout, S.A. Aphid protein effectors promote aphid colonization in a plant species-specific manner. Plant Microbe Interact. 2013, 26, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Van Bel, A.J.; Will, T. Functional evaluation of proteins in watery and gel saliva of aphids. Front. Plant Sci. 2016, 7, 1840. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, D.A.; De Vos, M.; Jander, G. Suppression of plant defences by a Myzus persicae (green peach aphid) salivary effector protein. Mol. Plant Microbe Interact. 2014, 27, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Atamian, H.S.; Chaudhary, R.; Cin, V.D.; Bao, E.; Girke, T.; Kaloshian, I. In planta expression of potato aphid Macrosiphum euphorbiae effectors Me10 and Me23 enhances aphid fecundity. Mol. Plant Microbe Interact. 2013, 26, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kettles, G.J.; Kaloshian, I. The potato aphid salivary effector Me47 is a glutathione-S-transferase involved in modifying plant responses to aphid infestation. Front. Plant Sci. 2016, 7, 1142. [Google Scholar] [CrossRef] [PubMed]
- Will, T.; Tjallingii, W.F.; Thönnessen, A.; van Bel, A.J. Molecular sabotage of plant defense by aphid saliva. Proc. Natl. Acad. Sci. USA 2007, 104, 10536–10541. [Google Scholar] [CrossRef] [PubMed]
- Furch, A.C.U.; van Bel, A.J.E.; Will, T. Aphid salivary proteases are capable of degrading sieve-tube proteins. J. Exp. Bot. 2015, 66, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, R.; Subramanyam, S.; Zhao, C.; Chen, M.S.; Harris, M.; Stuart, J.J. Avirulence effector discovery in a plant galling and plant parasitic arthropod, the Hessian fly (Mayetiola destructor). PLoS ONE 2014, 9, e100958. [Google Scholar] [CrossRef] [PubMed]
- Stuart, J. Insect effectors and gene-for-gene interactions with host plants. Curr. Opin. Insect Sci. 2015, 9, 56–61. [Google Scholar] [CrossRef]
- Jonckheere, W.; Dermauw, W.; Zhurov, V.; Wybouw, N.; Van den Bulcke, J.; Villarroel, C.A.; Greenhalgh, R.; Grbic, M.; Schuurink, R.C.; Tirry, L.; et al. The salivary protein repertoire of the polyphagous spider mite Tetranychus urticae: A quest for effectors. Mol. Cell. Proteom. 2016, 15, 3594–3613. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, C.A.; Jonckheere, W.; Alba, J.M.; Glas, J.J.; Dermauw, W.; Haring, M.A.; Van Leeuwen, T.; Schuurink, R.C.; Kant, M.R. Salivary proteins of spider mites suppress defenses in Nicotiana benthamiana and promote mite reproduction. Plant J. 2016, 86, 119–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doss, R.P.; Oliver, J.E.; Proebsting, W.M.; Potter, S.W.; Kuy, S.; Clement, S.L.; Williamson, R.T.; Carney, J.R.; De Vilbiss, E.D. Bruchins: Insect-derived plant regulators that stimulate neoplasm formation. Proc. Natl. Acad. Sci. USA 2000, 97, 6218–6223. [Google Scholar] [CrossRef] [PubMed]
- Fatourus, N.E.; Bukovinszkine´kiss, G.; Kalkerss, L.A.; Gamborena, R.S.; Dick, M.; Hilker, M. Oviposition-induced plant cues: Do they arrest Trichogramma wasps during host location? Entomol. Exp. Appl. 2005, 11, 207–215. [Google Scholar] [CrossRef]
- Fatourus, N.E.; Broekgaarden, C.; Bukovinszkine´kiss, G.; van Loon, J.J.A.; Mumm, R.; Huigens, M.E.; Dicke, M.; Hilker, M. Male-derived butterfly anti-aphrodisiac mediates induced indirect plant defense. Proc. Natl. Acad. Sci. USA 2008, 105, 10033–10038. [Google Scholar] [CrossRef] [PubMed]
- Blenn, B.; Bandoly, M.; Kuffner, A.; Otte, T.; Geisehardt, S.; Fatourus, N.E.; Hiker, M. Insect egg deposition induces indirect defense and epicuticular wax changes in Arabidopsis thaliana. J. Chem. Ecol. 2012, 38, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Bruessow, F.; Gouhier-Darimont, C.; Buchala, A.; Metraux, J.P.; Reymond, P. Insect eggs suppress plant defence against chewing herbivores. Plant J. 2010, 62, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Basu, S.; Rivera-Vega, L.J.; Acevedo, F.E.; Felton, G.W.; Luthe, D.S. Lessons from the far end: Catepillar FRASS-induced defences in maize, cabbage, and tomato. J. Chem. Ecol. 2016, 42, 1130–1141. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Rosa, C.; Scully, E.D.; Peiffer, M.; Tooker, J.F.; Hoover, K.; Luthe, D.S.; Felton, G.W. Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. USA 2013, 110, 15728–15733. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Scully, E.D.; Peiffer, M.; Greib, M.; Rosa, C.; Hoover, K.; Felton, G.W. Host plant species determines symbiotic bacterial community mediating suppression of plant defences. Sci. Rep. 2017, 7, 39690. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.; Schimmel, B.C. J.; Lamers, M.M.; Wybouw, N.; Groot, A.T.; Kant, M.R. Independent effects of a herbivore’s bacterial symbionts on its performance and induced plant defences. Int. J. Mol. Sci. 2017, 18, 182. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Hatt, S.; He, K.; Chen, J.; Francis, F.; Wang, Z. Endosymbionts have also been found to affect aphid-plant interactions. J. Asia Pac. Entomol. 2017, 20, 794–801. [Google Scholar] [CrossRef]
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Gilardoni, P.A.; Hettenhausen, C.; Baldwin, I.T.; Bonaventure, G. Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory. Plant Cell 2011, 23, 3512–3532. [Google Scholar] [CrossRef] [PubMed]
- Mantelin, S.; Peng, H.C.; Li, B.; Atamian, H.S.; Takken, F.L.; Kaloshian, I. The receptor-like kinase SlSERK1 is required for Mi-1-mediated resistance to potato aphids in tomato. Plant J. 2011, 67, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.C.; Mantelin, S.; Hicks, G.R.; Takken, F.L.; Kaloshian, I. The conformation of a plasma membrane-localized somatic embryogenesis receptor kinase complex is altered by a potato aphid-derived effector. Plant Phsyiol. 2016, 171, 2211–2222. [Google Scholar] [CrossRef] [PubMed]
- Gouhier-Darimont, C.; Schmiesing, A.; Bonnet, C.; Lassueur, S.; Reymond, P. Signalling of Arabidopsis thaliana response to Pieris brassicae eggs shares similarities with PAMP-triggered immunity. J. Exp. Bot. 2013, 64, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Prince, D.C.; Drurey, C.; Zipfel, C.; Hoggenhout, S.A. The leucine-rich repeat receptor–like kinase BRASSINOSTEROID INSENSITIVE-1 ASSOCIATED KINASE1 and Cytochrome P450 PHYTOALEXIN DEFICIENT3 contributes to innate immunity to aphids in Arabidopsis. Plant Physiol. 2014, 164, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithofer, A.; Boland, W. Before gene expression: Early events in plant-insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Huffaker, A.; Bryan, A.C.; Tax, F.E.; Ryan, C.A. PEPR2 is a second receptor for the Pep1 and Pep2 peptides and contributes to defense responses in Arabidopsis. Plant Cell 2010, 22, 508–522. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Walker, R.K.; Zhao, Y.; Berkowitz, G.A. Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and down-stream immune signaling in plants. Proc. Natl. Acad. Sci. USA 2012, 27, 19852–19857. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Einig, E.; Almeida-Trapp, M.; Labert, M.; Fliegmann, J.; Mitofer, A.; Kalbacher, H.; Felix, G. The systemin receptor SYR1 enhances resistance of tomato against herbivorous insects. Nat. Plants 2018, 4, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.M.; Chang, C.; Schaller, G.E. Perception of ethylene by plants—Ethylene receptors. Ann. Plant Rev. 2012, 44, 117–145. [Google Scholar]
- Groen, S.C.; Whiteman, N.K. The evolution of ethylene signaling in plant chemical ecology. J. Chem. Ecol. 2014, 40, 700–716. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Ozawa, R.; Nishioka, T.; Boland, W.; Koch, T.; Ehnemann, F.K.; Takabayashi, J. Herbivore-induced volatiles induce the emission of ethylene in neighboring lima bean plants. Plant J. 2002, 29, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xi, J.; Du, L.; Suttle, J.C.; Poovaiah, B.W. Coupling calcium/calmodulin-mediated signaling and herbivore-induced plant response through calmodulin-binding transcription factor AtSR1/CAMTA3. Plant Mol. Biol. 2012, 79, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Hettenhausen, C.; Sun, G.; He, Y.; Zhuang, H.; Sun, T.; Oi, J.; Wu, J. Genome-wide identification of calcium-dependent protein kinases in soybean and analyses of their transcriptional responses to insect herbivory and drought stress. Sci. Rep. 2016, 6, 18973. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Hettenhausen, C.; Baldwin, I.T.; Wu, J. Silencing Nicotiana attenuata calcium-dependent protein kinases, CDPK4 and CDPK5, strongly up-regulates wound- and herbivory-induced jasmonic acid accumulations. Plant Physiol. 2012, 159, 1591–1607. [Google Scholar] [CrossRef] [PubMed]
- Kandoth, P.K.; Ranf, S.; Pancholi, S.S.; Jayanty, S.; Walls, M.D.; Miller, W.; Howe, G.A.; Lincoln, D.E.; Stratmann, J.W. Tomato MAKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc. Natl. Acad. Sci. USA 2007, 104, 12205–12210. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Carroll, M.J.; LeClere, S.; Phipps, S.M.; Meredith, J.; Chourey, P.S.; Alborn, H.T.; Teal, P.E.A. Fragments of ATP synthase mediate plant perception of insect attack. Proc. Natl. Acad. Sci. USA 2006, 103, 8894–8899. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Camejo, D.; Guzman-Cedeño, A.; Moreno, A. Reactive oxygen species, essential molecules, during plant-pathogen interactions. Plant Physiol. Biochem. 2016, 103, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Bittner, N.; Trauere-Kizilelma, U.; Hilker, M. Early plant defence against insect attack: Involvement of reactive oxygen species in plant responses to insect egg deposition. Planta 2017, 245, 993–1007. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Cambra, I.; Martinez, M.; Pozancos, C.; Gonzalez-Melendi, P.; Grbic, V.; Castañera, P.; Ortego, F.; Diaz, I. Gene pyramiding of peptidase inhibitors enhances plant resistance to the spider mite Tetranychus urticae. PLoS ONE 2012, 7, e43011. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, M.E.; Martinez, M.; Arnaiz, A.; Ortego, F.; Grbic, V.; Diaz, I. MATI, a novel protein involved in the regulation of herbivore-associated signalling pathways. Front. Plant Sci. 2017, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Block, A.; Christensen, S.A.; Hunter, C.T.; Alborn, H.T. Herbivore-derived fatty-acid amides elicit reactive oxygen species burst in plants. J. Exp. Bot. 2017. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, L.; Wuensche, H.; Baldwin, I.T. Narboh D, a respiratory burst oxidase homolog in Nicotiana attenuata, is required for late defense responses after herbivore attack. J. Int. Plant Biol. 2013, 55, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Moloi, M.J.; van der Westhuizen, A.J. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J. Plant Physiol. 2006, 163, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Ozawa, R.; Maffei, M.E. Recent advances in plant early signaling in response to herbivory. Int. J. Mol. Sci. 2011, 12, 3623–3739. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, J.; Jiang, L.; Wu, H.; Xiao, Y.; Liu, Y.; Du, Y.; Liu, C.; Wan, J. Nitric oxide production is associated with response to Brown planthopper infestation in rice. J. Plant Physiol. 2011, 168, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Wunsche, H.; Baldwin, I.T.; Wu, J. S-Nitrosoglutathione reductase (GSNOR) mediates the biosynthesis of jasmonic acid and ethylene induced by feeding of the insect herbivore Manduca sexta and is important for jasmonate-elicited responses in Nicotiana attenuata. J. Exp. Bot. 2011, 62, 4605–4616. [Google Scholar] [CrossRef] [PubMed]
- Kawazu, K.; Mochizuki, A.; Sato, Y.; Sugeno, W.; Murata, M.; Seo, S.; Mitsuhara, I. Different expression profiles of jasmonic acid and salicylic acid inducible genes in the tomato plant against herbivores with various feeding modes. Arthropod-Plant Interact. 2012, 6, 221–230. [Google Scholar] [CrossRef]
- Schmiesing, A.; Emonet, A.; Gouhier-Darimont, C.; Reymond, P. Arabidopsis MYC transcription factors are the target of hormonal salicylic acid/jasmonic acid cross talk in response to Pieris brassicae egg extract. Plant Physiol. 2016, 170, 2432–2443. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Meldau, S.; Howe, G.A. Role of phytohormones in insect specific plant reactions. Trends Plant Sci. 2012, 17, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Galis, I.; Gaquerel, E.; pandey, S.P.; Baldwin, IT. Molecular mechanism underlying plant memory in JA-mediated defence responses. Plant Cell Environ. 2009, 32, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Baurle, I.; Geiselhart, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C.; et al. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2016, 91, 1102–1117. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T.; Schultz, J.C. Rapid changes in tree leaf chemistry induced by damage—evidence for communication between plants. Science 1983, 221, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Pearse, I.S.; Hughes, K.; Shiojiri, K.; Ishizaki, S.; Karban, R. Interplant volatile signaling in willows: Revisiting the original talking trees. Oecologia 2013, 172, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Sugimoto, K.; Ramadam, A.; Arimura, G.-I. Memory of plant communications for priming anti-herbivore responses. Sci. Rep. 2013, 3, 1872. [Google Scholar] [CrossRef] [PubMed]
- Rasman, S.; De Vos, M.; Casteel, C.L.; Tian, D.; Halitschke, R.; Sun, J.Y.; Agrawal, A.A.; Felton, G.W.; Jander, G. Herbivory in the previous generation primes plants for enhanced insect resistance. Plant Physiol. 2012, 158, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Hassanali, A.; Herren, H.; Khan, Z.R; Pickett, J.A.; Woodcock, C.M. Integrated pest management: The push-pull approach for controlling insect pests and weeds of cereals, and its potential for other agricultural systems including animal husbandry. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 611–621. [Google Scholar] [CrossRef] [PubMed]
- McKinlay, R.; Plant, J.A.; Bell, J.N.; Voulvoulis, N. Calculating human exposure to endocrine disrupting pesticides via agricultural and non-agricultural exposure routes. Sci. Total Environ. 2008, 398, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Delaunois, B.; Farace, G.; Jeandet, P.; Clément, C.; Baillieul, F.; Dorey, S.; Cordelier, S. Elicitors as alternative strategy to pesticides in grapevine? Current knowledge on their mode of action from controlled conditions to vineyard. Environ. Sci. Pollut. Res. 2014, 21, 4837–4846. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [PubMed]
- Tamiru, A.; Zeyaur, R.K. Volatile Semiochemical Mediated Plant Defense in Cereals: A novel strategy for crop protection. Agronomy 2017, 7, 58. [Google Scholar]
- Xu, Q.; Hatt, S.; Lopes, T.; Zhang, Y.; Bodson, B.; Chen, J.; Francis, F. A push–pull strategy to control aphids combines intercropping with semiochemical releases. J. Pest Sci. 2018, 91, 93–103. [Google Scholar] [CrossRef]
- Alba, J.M.; Bleeker, P.M.; Glas, J.J.; Schimmel, B.C.J.; Wijk, M.; Sabellis, M.W.; Schuurink, R.C.; Kant, M.R. The impact of induced plant volatiles on plant-arthropod interactions. In Arthropod-Plant Interactions; Springer: Dordrecht, The Netherlands; Hidelberg, Germany; New York, NY, USA; London, UK, 2012; pp. 15–69. ISBN 9-789400-738720. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamaria, M.E.; Arnaiz, A.; Gonzalez-Melendi, P.; Martinez, M.; Diaz, I. Plant Perception and Short-Term Responses to Phytophagous Insects and Mites. Int. J. Mol. Sci. 2018, 19, 1356. https://doi.org/10.3390/ijms19051356
Santamaria ME, Arnaiz A, Gonzalez-Melendi P, Martinez M, Diaz I. Plant Perception and Short-Term Responses to Phytophagous Insects and Mites. International Journal of Molecular Sciences. 2018; 19(5):1356. https://doi.org/10.3390/ijms19051356
Chicago/Turabian StyleSantamaria, M. Estrella, Ana Arnaiz, Pablo Gonzalez-Melendi, Manuel Martinez, and Isabel Diaz. 2018. "Plant Perception and Short-Term Responses to Phytophagous Insects and Mites" International Journal of Molecular Sciences 19, no. 5: 1356. https://doi.org/10.3390/ijms19051356
APA StyleSantamaria, M. E., Arnaiz, A., Gonzalez-Melendi, P., Martinez, M., & Diaz, I. (2018). Plant Perception and Short-Term Responses to Phytophagous Insects and Mites. International Journal of Molecular Sciences, 19(5), 1356. https://doi.org/10.3390/ijms19051356