Towards Understanding Plant Calcium Signaling through Calmodulin-Like Proteins: A Biochemical and Structural Perspective
Abstract
:1. Introduction
2. The EF-Hand: Variations on a Theme
2.1. Architecture of EF-Hands
2.2. The Affinity of EF-Hands for Ca2+
2.3. The Role of Mg2+
3. Structural Consequences of Ca2+ Binding and Conformational Changes
4. Interaction of CMLs with Targets
5. Conclusions
Conflicts of Interest
Abbreviations
| CaM | Calmodulin |
| CML | CaM-like protein |
| CD | Circular dichroism |
| ITC | Isothermal titration calorimetry |
| DSC | Differential scanning calorimetry |
| HIC | Hydrophobic interaction chromatography |
| NMR | Nuclear magnetic resonance |
| Cryo-EM | Cryo-electron microscopy |
| HSQC | Heteronuclear single-quantum coherence |
| ANS | Anilino-8-naphthalene sulfonate |
| SEC | Size-exclusion chromatography |
| LP | Limited proteolysis |
| MM | Molecular modeling |
| CaMBD | CaM-binding domain |
| Kd | Dissociation constant |
References
- Sanders, D.; Brownlee, C.; Harper, J.F. Communicating with calcium. Plant Cell 1999, 11, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.W.; Snedden, W.A. Calmodulin-related proteins step out from the shadow of their namesake. Plant Physiol. 2013, 163, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mazumder, M.; Gupta, N.; Chattopadhyay, S.; Gourinath, S. Crystal structure of arabidopsis thaliana calmodulin7 and insight into its mode of DNA binding. FEBS Lett. 2016, 590, 3029–3039. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Dunand, C.; Snedden, W.; Galaud, J.P. Cam and cml emergence in the green lineage. Trends Plant Sci. 2015, 20, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Edel, K.H.; Kudla, J. Increasing complexity and versatility: How the calcium signaling toolkit was shaped during plant land colonization. Cell Calcium 2015, 57, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Batistic, O.; Kudla, J. Analysis of calcium signaling pathways in plants. Biochim. Biophys. Acta 2012, 1820, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Snedden, W.A.; Fromm, H. Calmodulin, calmodulin-related proteins and plant responses to the environment. Trends Plant Sci. 1998, 3, 299–304. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003, 8, 505–512. [Google Scholar] [CrossRef] [PubMed]
- McCormack, E.; Tsai, Y.C.; Braam, J. Handling calcium signaling: Arabidopsis cams and cmls. Trends Plant Sci. 2005, 10, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Astegno, A.; La Verde, V.; Marino, V.; Dell’Orco, D.; Dominici, P. Biochemical and biophysical characterization of a plant calmodulin: Role of the n- and c-lobes in calcium binding, conformational change, and target interaction. Biochimica et Biophysica Acta (BBA) Proteins Proteomics 2016, 1864, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Astegno, A.; Maresi, E.; Marino, V.; Dominici, P.; Pedroni, M.; Piccinelli, F.; Dell’Orco, D. Structural plasticity of calmodulin on the surface of caf2 nanoparticles preserves its biological function. Nanoscale 2014, 6, 15037–15047. [Google Scholar] [CrossRef] [PubMed]
- Halling, D.B.; Liebeskind, B.J.; Hall, A.W.; Aldrich, R.W. Conserved properties of individual ca2+-binding sites in calmodulin. Proc. Natl. Acad. Sci. USA 2016, 113, E1216–E1225. [Google Scholar] [CrossRef] [PubMed]
- McCormack, E.; Braam, J. Calmodulins and related potential calcium sensors of arabidopsis. New Phytol. 2003, 159, 585–598. [Google Scholar] [CrossRef]
- Boonburapong, B.; Buaboocha, T. Genome-wide identification and analyses of the rice calmodulin and related potential calcium sensor proteins. BMC Plant Biol. 2007, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Khan, M.R.G.; Song, J.; Munir, S.; Zhang, Y.; Ye, Z.; Wang, T. Genome-wide identification, characterization and expression analysis of calmodulin-like (cml) proteins in tomato (solanum lycopersicum). Plant Physiol. Biochem. 2016, 102, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yue, D.; Wei, W.; Hu, Y.; Feng, J.; Zou, Z. Characterization and functional analysis of calmodulin and calmodulin-like genes in fragaria vesca. Front. Plant Sci. 2016, 7, 1820. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Deng, J.; Qin, Z.; Tang, J.; Shu, M.; Ding, C.; Liu, J.; Hu, C.; Yuan, M.; Huang, Y.; et al. Genome-wide identification and analyses of calmodulins and calmodulin-like proteins in lotus japonicas. Front. Plant Sci. 2017, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Zhang, M.; Zhang, L. Genome-wide identification and expression analysis of calmodulin-like (cml) genes in chinese cabbage (brassica rapa l. Ssp. Pekinensis). BMC Genom. 2017, 18, 842. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xu, L.; Singh, A.; Wang, H.; Du, L.; Poovaiah, B.W. Involvement of calmodulin and calmodulin-like proteins in plant responses to abiotic stresses. Front. Plant Sci. 2015, 6, 600. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Aldon, D.; Galaud, J.P.; Ranty, B. Calmodulin and calmodulin-like proteins in plant calcium signaling. Biochimie 2011, 93, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Aldon, D.; Mbengue, M.; Mazars, C.; Galaud, J.-P. Calcium signalling in plant biotic interactions. Int. J. Mol. Sci. 2018, 19, 665. [Google Scholar] [CrossRef] [PubMed]
- Cheval, C.; Aldon, D.; Galaud, J.P.; Ranty, B. Calcium/calmodulin-mediated regulation of plant immunity. Biochim. Biophys. Acta 2013, 1833, 1766–1771. [Google Scholar] [CrossRef] [PubMed]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.-P.; Thuleau, P.; Mazars, C. Calcium sensors as key hubs in plant responses to biotic and abiotic stresses. Front. Plant Sci. 2016, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhao, Q.; Thao, S.; Frederick, R.O.; Markley, J.L. Solution structure of a calmodulin-like calcium-binding domain from arabidopsis thaliana. J. Biomol. NMR 2004, 30, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Falke, J.J.; Drake, S.K.; Hazard, A.L.; Peersen, O.B. Molecular tuning of ion binding to calcium signaling proteins. Q. Rev. Biophys. 1994, 27, 219–290. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Walsh, M.P.; Vogel, H.J. Structures and metal-ion-binding properties of the ca2+-binding helix-loop-helix ef-hand motifs. Biochem. J. 2007, 405, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, H.; Kretsinger, R.H. Structural and functional diversity of ef-hand proteins: Evolutionary perspectives. Protein Sci. 2017, 26, 1898–1920. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. Meme suite: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Strynadka, N.C.; James, M.N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu. Rev. Biochem. 1989, 58, 951–998. [Google Scholar] [CrossRef] [PubMed]
- Dobney, S.; Chiasson, D.; Lam, P.; Smith, S.P.; Snedden, W.A. The calmodulin-related calcium sensor cml42 plays a role in trichome branching. J. Biol. Chem. 2009, 284, 31647–31657. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.W.; Dobney, S.; Ogunrinde, A.; Chiasson, D.; Mullen, R.T.; Teresinski, H.J.; Singh, P.; Munro, K.; Smith, S.P.; Snedden, W.A. The calmodulin-like protein cml43 functions as a salicylic-acid-inducible root-specific ca(2+) sensor in arabidopsis. Biochem. J. 2014, 457, 127–136. [Google Scholar] [CrossRef] [PubMed]
- La Verde, V.; Trande, M.; D’Onofrio, M.; Dominici, P.; Astegno, A. Binding of calcium and target peptide to calmodulin-like protein cml19, the centrin 2 of arabidopsis thaliana. Int. J. Biol. Macromol. 2018, 108, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Astegno, A.; Bonza, M.C.; Vallone, R.; La Verde, V.; D’Onofrio, M.; Luoni, L.; Molesini, B.; Dominici, P. Arabidopsis calmodulin-like protein cml36 is a calcium (ca2+) sensor that interacts with the plasma membrane ca2+-atpase isoform aca8 and stimulates its activity. J. Biol. Chem. 2017, 292, 15049–15061. [Google Scholar] [CrossRef] [PubMed]
- Vallone, R.; La Verde, V.; D’Onofrio, M.; Giorgetti, A.; Dominici, P.; Astegno, A. Metal binding affinity and structural properties of calmodulin-like protein 14 from arabidopsis thaliana. Protein Sci. 2016, 25, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Ogunrinde, A.; Munro, K.; Davidson, A.; Ubaid, M.; Snedden, W.A. Arabidopsis calmodulin-like proteins, cml15 and cml16 possess biochemical properties distinct from calmodulin and show non-overlapping tissue expression patterns. Front. Plant Sci. 2017, 8, 2175. [Google Scholar] [CrossRef] [PubMed]
- Anandalakshmi, R.; Marathe, R.; Ge, X.; Herr, J.M.; Mau, C.; Mallory, A.; Pruss, G.; Bowman, L.; Vance, V.B. A calmodulin-related protein that suppresses posttranscriptional gene silencing in plants. Science 2000, 290, 142–144. [Google Scholar] [CrossRef] [PubMed]
- Makiyama, R.K.; Fernandes, C.A.H.; Dreyer, T.R.; Moda, B.S.; Matioli, F.F.; Fontes, M.R.M.; Maia, I.G. Structural and thermodynamic studies of the tobacco calmodulin-like rgs-cam protein. Int. J. Biol. Macromol. 2016, 92, 1288–1297. [Google Scholar] [CrossRef] [PubMed]
- Piazza, M.; Taiakina, V.; Guillemette, S.R.; Guillemette, J.G.; Dieckmann, T. Solution structure of calmodulin bound to the target peptide of endothelial nitric oxide synthase phosphorylated at thr495. Biochemistry 2014, 53, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Waters, B.M. Moving magnesium in plant cells. New Phytol. 2011, 190, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Grabarek, Z. Insights into modulation of calcium signaling by magnesium in calmodulin, troponin c and related ef-hand proteins. Biochimica et biophysica acta 2011, 1813, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M.; Dace, A.; Tong, K.I.; Valiveti, A.; Ikura, M.; Ames, J.B. Mg2+ and ca2+ differentially regulate DNA binding and dimerization of dream. J. Biol. Chem. 2005, 280, 18008–18014. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.L.; Jamshidiha, M.; Mo, J.; Ishida, H.; Vogel, H.J. Comparing the calcium binding abilities of two soybean calmodulins: Towards understanding the divergent nature of plant calmodulins. Plant Cell 2013, 25, 4512–4524. [Google Scholar] [CrossRef] [PubMed]
- Malmendal, A.; Evenas, J.; Forsen, S.; Akke, M. Structural dynamics in the c-terminal domain of calmodulin at low calcium levels. J. Mol. Biol. 1999, 293, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Ohki, S.; Ikura, M.; Zhang, M. Identification of mg2+-binding sites and the role of mg2+ on target recognition by calmodulin. Biochemistry 1997, 36, 4309–4316. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Chigri, F.; Flosdorff, S.; Pilz, S.; Kolle, E.; Dolze, E.; Gietl, C.; Vothknecht, U.C. The arabidopsis calmodulin-like proteins atcml30 and atcml3 are targeted to mitochondria and peroxisomes, respectively. Plant Mol. Biol. 2012, 78, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Dolze, E.; Chigri, F.; Howing, T.; Hierl, G.; Isono, E.; Vothknecht, U.C.; Gietl, C. Calmodulin-like protein atcml3 mediates dimerization of peroxisomal processing protease atdeg15 and contributes to normal peroxisome metabolism. Plant Mol. Biol. 2013, 83, 607–624. [Google Scholar] [CrossRef] [PubMed]
- Ruge, H.; Flosdorff, S.; Ebersberger, I.; Chigri, F.; Vothknecht, U.C. The calmodulin-like proteins atcml4 and atcml5 are single-pass membrane proteins targeted to the endomembrane system by an n-terminal signal anchor sequence. J. Exp. Bot. 2016, 67, 3985–3996. [Google Scholar] [CrossRef] [PubMed]
- Won, S.-K.; Lee, Y.-J.; Lee, H.-Y.; Heo, Y.-K.; Cho, M.; Cho, H.-T. Cis-element- and transcriptome-based screening of root hair-specific genes and their functional characterization in arabidopsis. Plant Physiol. 2009, 150, 1459–1473. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.D.; Liao, Y.Y.; Yang, T.J.; Pan, C.Y.; Buckhout, T.J.; Schmidt, W. Coexpression-based clustering of arabidopsis root genes predicts functional modules in early phosphate deficiency signaling. Plant Physiol. 2011, 155, 1383–1402. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Kim, H.S.; Wu, X.; Clouse, S.D.; Zielinski, R.E.; Huber, S.C. Calcium/calmodulin inhibition of the arabidopsis brassinosteroid-insensitive 1 receptor kinase provides a possible link between calcium and brassinosteroid signalling. Biochem. J. 2012, 443, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Perez, M.; Aldon, D.; Galaud, J.P. Respective contribution of cml8 and cml9, two arabidopsis calmodulin-like proteins, to plant stress responses. Plant Signal. Behav. 2017, 12, e1322246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Robe, E.; Jomat, L.; Aldon, D.; Mazars, C.; Galaud, J.P. Cml8, an arabidopsis calmodulin-like protein, plays a role in pseudomonas syringae plant immunity. Plant Cell Physiol. 2017, 58, 307–319. [Google Scholar] [PubMed]
- Park, H.C.; Park, C.Y.; Koo, S.C.; Cheong, M.S.; Kim, K.E.; Kim, M.C.; Lim, C.O.; Lee, S.Y.; Yun, D.J.; Chung, W.S. Atcml8, a calmodulin-like protein, differentially activating cam-dependent enzymes in arabidopsis thaliana. Plant Cell Rep. 2010, 29, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Burstenbinder, K.; Savchenko, T.; Muller, J.; Adamson, A.W.; Stamm, G.; Kwong, R.; Zipp, B.J.; Dinesh, D.C.; Abel, S. Arabidopsis calmodulin-binding protein iq67-domain 1 localizes to microtubules and interacts with kinesin light chain-related protein-1. J. Biol. Chem. 2013, 288, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Campe, R.; Langenbach, C.; Leissing, F.; Popescu, G.V.; Popescu, S.C.; Goellner, K.; Beckers, G.J.; Conrath, U. Abc transporter pen3/pdr8/abcg36 interacts with calmodulin that, like pen3, is required for arabidopsis nonhost resistance. New Phytol. 2016, 209, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-Y.; Shi, D.-Q.; Jia, P.-F.; Tang, J.; Li, H.-J.; Liu, J.; Yang, W.-C. The arabidopsis receptor kinase zar1 is required for zygote asymmetric division and its daughter cell fate. PLoS Genet. 2016, 12, e1005933. [Google Scholar] [CrossRef] [PubMed]
- Leba, L.J.; Cheval, C.; Ortiz-Martin, I.; Ranty, B.; Beuzon, C.R.; Galaud, J.P.; Aldon, D. Cml9, an arabidopsis calmodulin-like protein, contributes to plant innate immunity through a flagellin-dependent signalling pathway. Plant J. 2012, 71, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Magnan, F.; Ranty, B.; Charpenteau, M.; Sotta, B.; Galaud, J.P.; Aldon, D. Mutations in atcml9, a calmodulin-like protein from arabidopsis thaliana, alter plant responses to abiotic stress and abscisic acid. Plant J. 2008, 56, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Perochon, A.; Dieterle, S.; Pouzet, C.; Aldon, D.; Galaud, J.P.; Ranty, B. Interaction of a plant pseudo-response regulator with a calmodulin-like protein. Biochem. Biophys. Res. Commun. 2010, 398, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Scholz, S.S.; Mithofer, A. Multiple calmodulin-like proteins in arabidopsis are induced by insect-derived (spodoptera littoralis) oral secretion. Plant Signal. Behav. 2012, 7, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Leba, L.J.; Perochon, A.; Cheval, C.; Ranty, B.; Galaud, J.P.; Aldon, D. Cml9, a multifunctional arabidopsis thaliana calmodulin-like protein involved in stress responses and plant growth? Plant Signal. Behav. 2012, 7, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Brauer, E.K.; Ahsan, N.; Dale, R.; Kato, N.; Coluccio, A.E.; Piñeros, M.A.; Kochian, L.V.; Thelen, J.J.; Popescu, S.C. The raf-like kinase ilk1 and the high affinity k(+) transporter hak5 are required for innate immunity and abiotic stress response. Plant Physiol. 2016, 171, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Nguyen, H.T.; Kim, S.Y.; Shin, J.S.; Cho, D.H.; Hong, S.B.; Ok, S.H. Cml10, a variant of calmodulin, modulates ascorbic acid synthesis. New Phytol. 2016, 209, 664–678. [Google Scholar] [CrossRef] [PubMed]
- Benjamins, R.; Ampudia, C.S.; Hooykaas, P.J.; Offringa, R. Pinoid-mediated signaling involves calcium-binding proteins. Plant Physiol. 2003, 132, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Aharon, G.S.; Sottosanto, J.B.; Blumwald, E. Vacuolar na+/h+ antiporter cation selectivity is regulated by calmodulin from within the vacuole in a ca2+- and ph-dependent manner. Proc. Natl. Acad. Sci. USA 2005, 102, 16107–16112. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Flury, S.; Kalck, V.; Hohn, B.; Molinier, J. Centrin2 interacts with the arabidopsis homolog of the human xpc protein (atrad4) and contributes to efficient synthesis-dependent repair of bulky DNA lesions. Plant Mol. Biol. 2006, 61, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Molinier, J.; Ramos, C.; Fritsch, O.; Hohn, B. Centrin2 modulates homologous recombination and nucleotide excision repair in arabidopsis. Plant Cell 2004, 16, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Tang, X.; Tian, G.; Wang, F.; Liu, K.; Nguyen, V.; Kohalmi, S.E.; Keller, W.A.; Tsang, E.W.T.; Harada, J.J.; et al. Arabidopsis homolog of the yeast trex-2 mrna export complex: Components and anchoring nucleoporin. Plant J. 2010, 61, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Qiao, Z.; Liu, H.; Acharya, B.R.; Li, C.; Zhang, W. Cml20, an arabidopsis calmodulin-like protein, negatively regulates guard cell aba signaling and drought stress tolerance. Front. Plant Sci. 2017, 8, 824. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Lu, Q.; Kohalmi, S.E.; Rothstein, S.J.; Cui, Y. Evidence that the arabidopsis ubiquitin c-terminal hydrolases 1 and 2 associate with the 26s proteasome and the trex-2 complex. Plant Signal. Behav. 2012, 7, 1415–1419. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Delk, N.A.; Chowdhury, N.I.; Braam, J. Arabidopsis potential calcium sensors regulate nitric oxide levels and the transition to flowering. Plant Signal. Behav 2007, 2, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Delk, N.A.; Johnson, K.A.; Chowdhury, N.I.; Braam, J. Cml24, regulated in expression by diverse stimuli, encodes a potential ca2+ sensor that functions in responses to abscisic acid, daylength, and ion stress. Plant Physiol. 2005, 139, 240–253. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Koo, Y.; Delk, N.A.; Gehl, B.; Braam, J. Calmodulin-related cml24 interacts with atg4b and affects autophagy progression in arabidopsis. Plant J. 2013, 73, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Smigel, A.; Tsai, Y.-C.; Braam, J.; Berkowitz, G.A. Innate immunity signaling: Cytosolic ca(2+) elevation is linked to downstream nitric oxide generation through the action of calmodulin or a calmodulin-like protein. Plant Physiol. 2008, 148, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, S.S.; Wang, M.; Qiao, Z.; Bao, C.C.; Zhang, W. Arabidopsis thaliana calmodulin-like protein cml24 regulates pollen tube growth by modulating the actin cytoskeleton and controlling the cytosolic ca(2+) concentration. Plant Mol. Biol. 2014, 86, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Diao, W.Z.; Yang, X.; Qiao, Z.; Wang, M.; Acharya, B.R.; Zhang, W. Arabidopsis thaliana cml25 mediates the ca regulation of k transmembrane trafficking during pollen germination and tube elongation. Plant Cell Environ. 2015, 38, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Ceserani, T.; Trofka, A.; Gandotra, N.; Nelson, T. Vh1/brl2 receptor-like kinase interacts with vascular-specific adaptor proteins vit and vik to influence leaf venation. Plant J. 2009, 57, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Cao, Y.; Zhang, X.C.; Stacey, G. Lik1, a cerk1-interacting kinase, regulates plant immune responses in arabidopsis. PLoS ONE 2014, 9, e102245. [Google Scholar] [CrossRef] [PubMed]
- Vanderbeld, B.; Snedden, W.A. Developmental and stimulus-induced expression patterns of arabidopsis calmodulin-like genes cml37, cml38 and cml39. Plant Mol. Biol. 2007, 64, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Reichelt, M.; Vadassery, J.; Mithofer, A. Calmodulin-like protein cml37 is a positive regulator of aba during drought stress in arabidopsis. Plant Signal. Behav. 2015, 10, e1011951. [Google Scholar] [CrossRef] [PubMed]
- Scholz, S.S.; Vadassery, J.; Heyer, M.; Reichelt, M.; Bender, K.W.; Snedden, W.A.; Boland, W.; Mithofer, A. Mutation of the arabidopsis calmodulin-like protein cml37 deregulates the jasmonate pathway and enhances susceptibility to herbivory. Mol. Plant 2014, 7, 1712–1726. [Google Scholar] [CrossRef] [PubMed]
- Campos, W.F.; Dressano, K.; Ceciliato, P.H.O.; Guerrero-Abad, J.C.; Silva, A.L.; Fiori, C.S.; Morato do Canto, A.; Bergonci, T.; Claus, L.A.N.; Silva-Filho, M.C.; et al. Arabidopsis thaliana rapid alkalinization factor 1-mediated root growth inhibition is dependent on calmodulin-like protein 38. J. Biol. Chem. 2018, 293, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Lokdarshi, A.; Conner, W.C.; McClintock, C.; Li, T.; Roberts, D. Arabidopsis cml38, a calcium sensor that localizes to ribonucleoprotein complexes under hypoxia stress. Plant Physiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Bender, K.W.; Rosenbaum, D.M.; Vanderbeld, B.; Ubaid, M.; Snedden, W.A. The arabidopsis calmodulin-like protein, cml39, functions during early seedling establishment. Plant J. 2013, 76, 634–647. [Google Scholar] [CrossRef] [PubMed]
- Midhat, U.; Ting, M.K.Y.; Teresinski, H.J.; Snedden, W.A. The calmodulin-like protein, cml39, is involved in regulating seed development, germination, and fruit development in arabidopsis. Plant Mol. Biol. 2018, 96, 375–392. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Cheval, C.; Laohavisit, A.; Hocking, B.; Chiasson, D.; Olsson, T.S.G.; Shirasu, K.; Faulkner, C.; Gilliham, M. A calmodulin-like protein regulates plasmodesmal closure during bacterial immune responses. New Phytol. 2017, 215, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chiasson, D.; Ekengren, S.; Martin, G.; Dobney, S.; Snedden, W. Calmodulin-like proteins from arabidopsis and tomato are involved in host defense against pseudomonas syringae pv. Tomato. Plant Mol. Biol. 2005, 58, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Vadassery, J.; Reichelt, M.; Hause, B.; Gershenzon, J.; Boland, W.; Mithofer, A. Cml42-mediated calcium signaling coordinates responses to spodoptera herbivory and abiotic stresses in arabidopsis. Plant Physiol. 2012, 159, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Truman, W.; Liu, X.; Bethke, G.; Zhou, M.; Myers, C.; Katagiri, F.; Glazebrook, J. Different modes of negative regulation of plant immunity by calmodulin-related genes. Plant Physiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- De Castro, E.; Sigrist, C.J.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. Scanprosite: Detection of prosite signature matches and prorule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Kumar, P.; Bae, H. Genomics and evolutionary aspect of calcium signaling event in calmodulin and calmodulin-like proteins in plants. BMC Plant Biol. 2017, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Rogozin, I.B.; Carmel, L.; Csuros, M.; Koonin, E.V. Origin and evolution of spliceosomal introns. Biol. Direct 2012, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Chard, P.S.; Bleakman, D.; Christakos, S.; Fullmer, C.S.; Miller, R.J. Calcium buffering properties of calbindin d28k and parvalbumin in rat sensory neurones. J. Physiol. 1993, 472, 341–357. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.L.; Kim, J.; Truong, K.; Sherman, M.; Yuan, T.; Ikura, M. Calmodulin target database. J. Struct. Funct. Genom. 2000, 1, 8–14. [Google Scholar] [CrossRef]
- Yamniuk, A.P.; Vogel, H.J. Calmodulin’s flexibility allows for promiscuity in its interactions with target proteins and peptides. Mol. Biotechnol. 2004, 27, 33–57. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Bunick, C.G.; Chazin, W.J. Target selectivity in ef-hand calcium binding proteins. Biochimica et Biophysica Acta (BBA) Molecular Cell Research 2004, 1742, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Astegno, A.; Capitani, G.; Dominici, P. Functional roles of the hexamer organization of plant glutamate decarboxylase. Biochim. Biophys. Acta 2015, 9, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Gut, H.; Dominici, P.; Pilati, S.; Astegno, A.; Petoukhov, M.V.; Svergun, D.I.; Grutter, M.G.; Capitani, G. A common structural basis for ph- and calmodulin-mediated regulation in plant glutamate decarboxylase. J. Mol. Biol. 2009, 392, 334–351. [Google Scholar] [CrossRef] [PubMed]
- Tidow, H.; Nissen, P. Structural diversity of calmodulin binding to its target sites. FEBS J. 2013, 280, 5551–5565. [Google Scholar] [CrossRef] [PubMed]
- Garrigos, M.; Deschamps, S.; Viel, A.; Lund, S.; Champeil, P.; Moller, J.V.; le Maire, M. Detection of ca(2+)-binding proteins by electrophoretic migration in the presence of ca2+ combined with 45ca2+ overlay of protein blots. Anal. Biochem. 1991, 194, 82–88. [Google Scholar] [CrossRef]
- Chinpongpanich, A.; Wutipraditkul, N.; Thairat, S.; Buaboocha, T. Biophysical characterization of calmodulin and calmodulin-like proteins from rice, oryza sativa L. Acta Biochim Biophys. Sin. 2011, 43, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, X.; Duanmu, H.; Zhu, D.; Yu, Y.; Cao, L.; Liu, A.; Jia, B.; Xiao, J.; Zhu, Y. Gscml27, a gene encoding a calcium-binding ef-hand protein from glycine soja, plays differential roles in plant responses to bicarbonate, salt and osmotic stresses. PLoS ONE 2015, 10, e0141888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Tanaka, T.; Ikura, M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat. Struct. Biol. 1995, 2, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Ikura, M.; Ames, J.B. Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: Two ways to promote multifunctionality. Proc. Natl. Acad. Sci. USA 2006, 103, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Babu, Y.S.; Bugg, C.E.; Cook, W.J. Structure of calmodulin refined at 2.2 a resolution. J. Mol. Biol. 1988, 204, 191–204. [Google Scholar] [CrossRef]
- Yuan, T.; Ouyang, H.; Vogel, H.J. Surface exposure of the methionine side chains of calmodulin in solution. A nitroxide spin label and two-dimensional nmr study. J. Biol. Chem. 1999, 274, 8411–8420. [Google Scholar] [CrossRef] [PubMed]
- DeFalco, T.A.; Bender, K.W.; Snedden, W.A. Breaking the code: Ca2+ sensors in plant signalling. Biochem. J. 2010, 425, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Vogel, H.J. Calmodulin: A versatile calcium mediator protein. Biochem. Cell Biol. 1994, 72, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Popescu, S.C.; Popescu, G.V.; Bachan, S.; Zhang, Z.; Seay, M.; Gerstein, M.; Snyder, M.; Dinesh-Kumar, S.P. Differential binding of calmodulin-related proteins to their targets revealed through high-density arabidopsis protein microarrays. Proc. Natl. Acad. Sci. USA 2007, 104, 4730–4735. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, J.; Nacry, P.; Christodoulidou, A.; Drevensek, S.; Camilleri, C.; Amiour, N.; Parcy, F.; Pastuglia, M.; Bouchez, D. Arabidopsis tonneau1 proteins are essential for preprophase band formation and interact with centrin. Plant Cell 2008, 20, 2146–2159. [Google Scholar] [CrossRef] [PubMed]
- Yamniuk, A.P.; Vogel, H.J. Structural investigation into the differential target enzyme regulation displayed by plant calmodulin isoforms. Biochemistry 2005, 44, 3101–3111. [Google Scholar] [CrossRef] [PubMed]
- Rainaldi, M.; Yamniuk, A.P.; Murase, T.; Vogel, H.J. Calcium-dependent and -independent binding of soybean calmodulin isoforms to the calmodulin binding domain of tobacco mapk phosphatase-1. J. Biol. Chem. 2007, 282, 6031–6042. [Google Scholar] [CrossRef] [PubMed]
- Villarroel, A.; Taglialatela, M.; Bernardo-Seisdedos, G.; Alaimo, A.; Agirre, J.; Alberdi, A.; Gomis-Perez, C.; Soldovieri, M.V.; Ambrosino, P.; Malo, C.; et al. The ever changing moods of calmodulin: How structural plasticity entails transductional adaptability. J. Mol. Biol. 2014, 426, 2717–2735. [Google Scholar] [CrossRef] [PubMed]
- Barbato, G.; Ikura, M.; Kay, L.E.; Pastor, R.W.; Bax, A. Backbone dynamics of calmodulin studied by 15n relaxation using inverse detected two-dimensional nmr spectroscopy: The central helix is flexible. Biochemistry 1992, 31, 5269–5278. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.S.; Masuta, C.; Yamada, S.; Shimura, H.; Kashihara, Y.; Wada, T.S.; Meguro, A.; Goto, K.; Tadamura, K.; Sueda, K.; et al. Tobacco calmodulin-like protein provides secondary defense by binding to and directing degradation of virus rna silencing suppressors. Proc. Natl. Acad. Sci. USA 2012, 109, 10113–10118. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.A.; Tirone, F.; Durussel, I.; Firanescu, C.; Blouquit, Y.; Duchambon, P.; Craescu, C.T. Calcium and magnesium binding to human centrin 3 and interaction with target peptides. Biochemistry 2005, 44, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Piirainen, H.; Hellman, M.; Tossavainen, H.; Permi, P.; Kursula, P.; Jaakola, V.-P. Human adenosine a2a receptor binds calmodulin with high affinity in a calcium-dependent manner. Biophys. J. 2015, 108, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, X.; Mueller, G.A.; Sobhany, M.; DeRose, E.F.; Zhang, Y.; London, R.E.; Birnbaumer, L. Crystal structure of calmodulin binding domain of orai1 in complex with ca2+ calmodulin displays a unique binding mode. J. Biol. Chem. 2012, 287, 43030–43041. [Google Scholar] [CrossRef] [PubMed]
- Majava, V.; Petoukhov, M.V.; Hayashi, N.; Pirila, P.; Svergun, D.I.; Kursula, P. Interaction between the c-terminal region of human myelin basic protein and calmodulin: Analysis of complex formation and solution structure. BMC Struct. Biol. 2008, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Vogel, H. Structural basis for the regulation of l-type voltage-gated calcium channels: Interactions between the n-terminal cytoplasmic domain and ca2+-calmodulin. Front. Mol. Neurosci. 2012, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Ikura, M.; Clore, G.M.; Gronenborn, A.M.; Zhu, G.; Klee, C.B.; Bax, A. Solution structure of a calmodulin-target peptide complex by multidimensional nmr. Science 1992, 256, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Meador, W.E.; Means, A.R.; Quiocho, F.A. Target enzyme recognition by calmodulin: 2.4 a structure of a calmodulin-peptide complex. Science 1992, 257, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.M.; Schneider, D.M.; Strobel, L.A.; Van Berkum, M.F.; Means, A.R.; Wand, A.J. Characterization of the secondary structure of calmodulin in complex with a calmodulin-binding domain peptide. Biochemistry 1992, 31, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Hoeflich, K.P.; Ikura, M. Calmodulin in action: Diversity in target recognition and activation mechanisms. Cell 2002, 108, 739–742. [Google Scholar] [CrossRef]

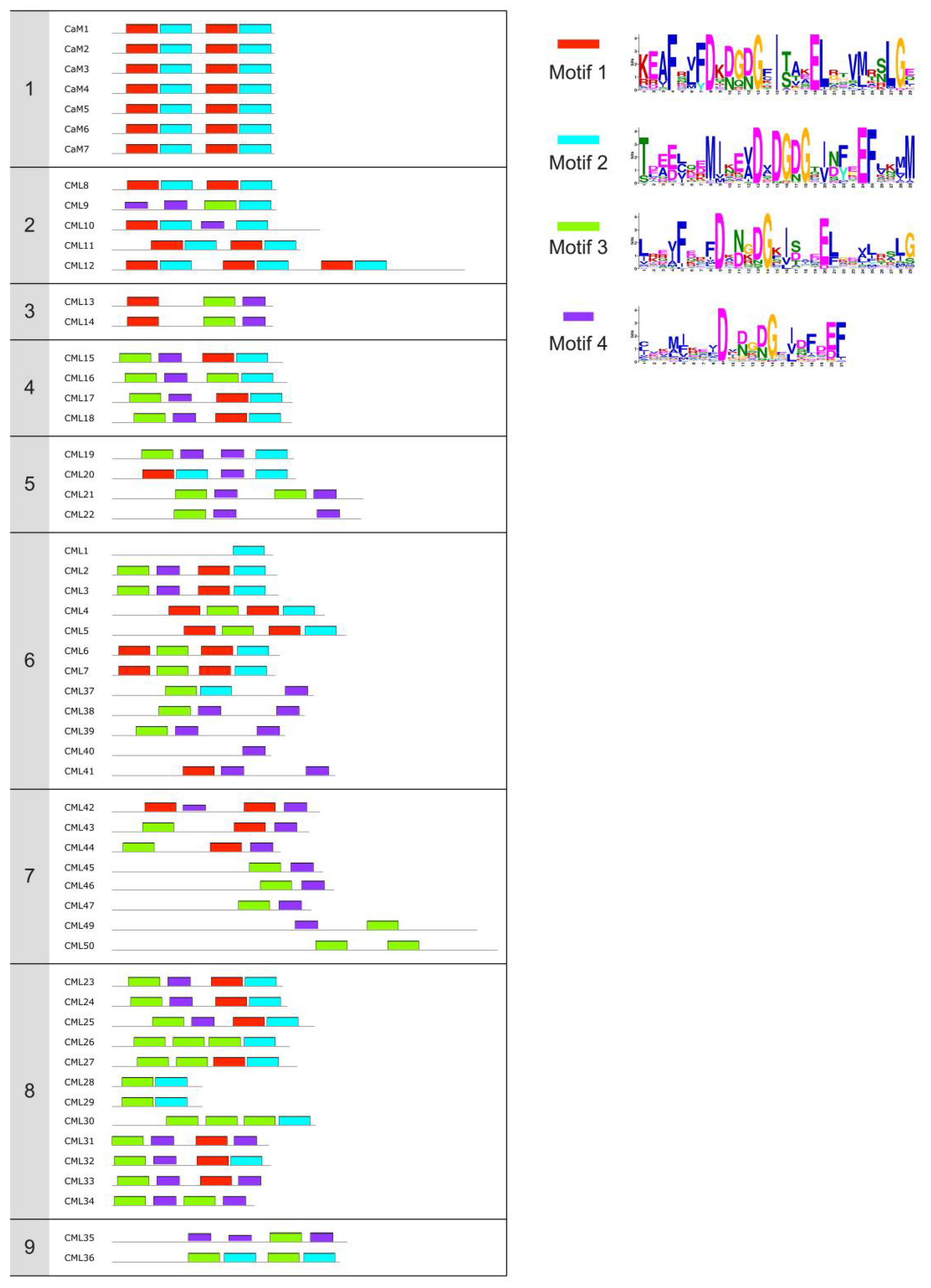
| Name 1 | Accession number | EF-hands 2 | Experimental Ca2+-binding sites 3 | Biochemical and structural characterization 4 | Identified target | Putative role | Refs |
|---|---|---|---|---|---|---|---|
| CML1 | At3g59450 | 1 | ? | ? | ? | ? | |
| CML2 | At4g12860 | 4 | ? | ? | ? | ? | |
| CML3 | At3g07490 | 4 | ? | Gel shift, HIC | AtDEG15 | ? | [46,47] |
| CML4 | At3g59440 | 4 | ? | Gel shift, HIC | ? | ? | [48] |
| CML5 | At2g43290 | 4 | ? | Gel shift, HIC | ? | ? | [48] |
| CML6 | At4g03290 | 4 | ? | ? | ? | ? | |
| CML7 | At1g05990 | 4 | ? | ? | ? | Development (Root hair elongation) | [49,50] |
| CML8 | At4g14640 | 4 | ? | HIC, radioactive Ca2+-binding assay | BRI1, ZAR1, IQD1, PEN3 | Plant immunity (Positive regulation) | [51,52,53,54,55,56,57] |
| CML9 | At3g51920 | 4 | ? | ? | PPR2, IQD1, PEN3, ILK1 | Signaling hub 5 | [52,55,56,58,59,60,61,62,63] |
| CML10 | At2g41090 | 4 | ? | Gel shift | PM-MUTASE | Abiotic stress (Oxidative stress) | [64] |
| CML11 | At3g22930 | 4 | ? | ? | ? | ? | |
| CML12 | At2g41100 | 6 | ? | ? | PINOID, PEN3 | Development; Plant immunity | [56,65] |
| CML13 | At1g12310 | 3 | ? | ? | ? | ? | |
| CML14 | At1g62820 | 3 | 1 | NMR, ITC, DSC, Gel shift, ANS, SEC, LP, MM | ? | ? | [34] |
| CML15 | At1g18530 | 4 | 2 | Gel Shift, CD, ANS, ITC, HIC, MM | ? | ? | [35] |
| CML16 | At3g25600 | 4 | 3 | Gel Shift, CD, ANS, ITC, HIC, MM | ? | ? | [35] |
| CML17 | At1g32250 | 4 | ? | ? | ? | ? | |
| CML18 | At3g03000 | 4 | ? | ? | NHX1, CBP60C | Abiotic stress (Salt) | [66] |
| CML19 | At4g37010 | 4 | 4 | NMR, ITC, Gel shift, ANS, SEC, CD, LP | RAD4, SAC3b, DSS1 | Abiotic stress (UV-damage) | [32,67,68,69] |
| CML20 | At3g50360 | 4 | ? | Gel shift | TON1, SAC3, UCH | Abiotic stress (Drought Stress) | [69,70,71] |
| CML21 | At4g26470 | 4 | ? | ? | ? | ? | |
| CML22 | At3g24110 | 4 | ? | ? | ? | ? | |
| CML23 | At1g66400 | 4 | ? | ? | ? | Development (Flowering) | [72] |
| CML24 | At5g37770 | 4 | ? | Gel shift, HIC | ATG4b | Signaling hub5 | [72,73,74,75,76] |
| CML25 | At1g24620 | 4 | ? | Gel shift, HIC | ? | Development (Root, Pollen tube) | [77] |
| CML26 | At1g73630 | 4 | ? | ? | ? | ? | |
| CML27 | At1g18210 | 4 | ? | ? | ? | ? | |
| CML28 | At3g03430 | 2 | ? | ? | ? | ? | |
| CML29 | At5g17480 | 2 | ? | ? | ? | ? | |
| CML30 | At2g15680 | 4 | ? | Gel shift, HIC | ? | ? | [46] |
| CML31 | At2g36180 | 4 | ? | ? | ? | ? | |
| CML32 | At5g17470 | 4 | ? | ? | ? | ? | |
| CML33 | At3g03400 | 4 | ? | ? | ? | ? | |
| CML34 | At3g03410 | 4 | ? | NMR | ? | ? | [24] |
| CML35 | At2g41410 | 4 | ? | ? | TTL3 | ? | [78] |
| CML36 | At3g10190 | 4 | 4 | NMR, ITC, DSC, Gel shift, ANS, SEC, LP, CD | ACA8, CERK1 | ? | [33,79] |
| CML37 | At5g42380 | 4 | ? | Gel shift, CD, ANS | PEN3 | Signaling hub5 | [56,80,81,82] |
| CML38 | At1g76650 | 4 | ? | Gel shift | RALF1, PEN3 | Signaling hub5 | [56,80,83,84] |
| CML39 | At1g76640 | 4 | ? | Gel shift | ? | Development (Seed, Fruit) | [80,85,86] |
| CML40 | At3g01830 | 2 | ? | ? | ? | ? | |
| CML41 | At3g50770 | 4 | ? | Gel Shift | ? | Plant immunity | [87] |
| CML42 | At4g20780 | 3 | 3 | CD, ITC, ANS, NMR, HIC, Gel shift | KIC | Signaling hub 5 | [30,88,89] |
| CML43 | At5g44460 | 3 | 3 | CD, ITC, ANS, NMR, DSC, HIC, Gel shift | ? | Plant Immunity (positive regulation) | [31,88] |
| CML44 | At1g21550 | 3 | ? | ? | ? | ? | |
| CML45 | At3g29000 | 3 | ? | ? | ? | ? | |
| CML46 | At5g39670 | 3 | ? | ? | ? | Plant Immunity (negative regulation) | [90] |
| CML47 | At3g47480 | 2 | ? | ? | ? | Plant Immunity (negative regulation) | [90] |
| CML48 | At2g27480 | 2 | ? | ? | ? | ? | |
| CML49 | At3g10300 | 2 | ? | ? | ? | ? | |
| CML50 | At5g04170 | 2 | ? | ? | ? | ? |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
La Verde, V.; Dominici, P.; Astegno, A. Towards Understanding Plant Calcium Signaling through Calmodulin-Like Proteins: A Biochemical and Structural Perspective. Int. J. Mol. Sci. 2018, 19, 1331. https://doi.org/10.3390/ijms19051331
La Verde V, Dominici P, Astegno A. Towards Understanding Plant Calcium Signaling through Calmodulin-Like Proteins: A Biochemical and Structural Perspective. International Journal of Molecular Sciences. 2018; 19(5):1331. https://doi.org/10.3390/ijms19051331
Chicago/Turabian StyleLa Verde, Valentina, Paola Dominici, and Alessandra Astegno. 2018. "Towards Understanding Plant Calcium Signaling through Calmodulin-Like Proteins: A Biochemical and Structural Perspective" International Journal of Molecular Sciences 19, no. 5: 1331. https://doi.org/10.3390/ijms19051331




