Molecular Binding Contributes to Concentration Dependent Acrolein Deposition in Rat Upper Airways: CFD and Molecular Dynamics Analyses
Abstract
:1. Introduction
2. Results
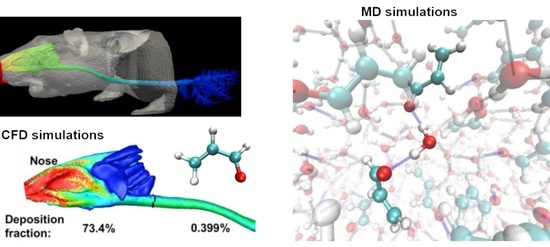
2.1. Airflow
2.2. Deposition Fraction
2.3. Surface Deposition Distribution
2.4. Computation vs. In Vitro Experiment
2.5. Binding Potential Analysis
2.6. Molecular Dynamics Simulations
3. Discussion
4. Materials and Methods
4.1. Rat Airway Model
4.2. Computational Flow-Vapor Transport Models
4.3. Molecular Dynamic (MD) Simulations
5. Conclusions
- Acrolein–water compounds were predicted to have either one (i.e., acrolein–water) or two (i.e., acrolein-water-acrolein) hydrogen bonds.
- The fraction of hydrogen-bonded acrolein compounds over free acrolein molecules increased with larger acrolein concentrations and higher media polarity.
- The decreased acrolein-compound diffusivity due to molecular binding lowered the total deposition rate and altered the deposition distribution in the rat airway.
- Molecule binding cannot explain the entire decrease in acrolein uptake that had been observed experimentally in rats, and acts as a secondary mechanism contributing to the deposition decrease of chemicals with high polarity.
- Consideration of concentration-dependent diffusivities is recommended in inhalation dosimetry predictions for improved accuracy.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Facchinetti, F.; Amadei, F.; Geppetti, P.; Tarantini, F.; Di Serio, C.; Dragotto, A.; Gigli, P.M.; Catinella, S.; Civelli, M.; Patacchini, R. Alpha,beta-unsaturated aldehydes in cigarette smoke release inflammatory mediators from human macrophages. Am. J. Respir. Cell Mol. Biol. 2007, 37, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Stein, Y.S.; Antal, M.J.; Jones, M. A study of the gas-phase pyrolysis of glycerol. J. Anal. Appl. Pyrolysis 1983, 4, 283–296. [Google Scholar] [CrossRef]
- Eiserich, J.P.; van der Vliet, A.; Handelman, G.J.; Halliwell, B.; Cross, C.E. Dietary antioxidants and cigarette smoke-induced biomolecular damage: A complex interaction. Am. J. Clin. Nutr. 1995, 62, 1490s–1500s. [Google Scholar] [CrossRef] [PubMed]
- Bein, K.; Leikauf, G.D. Acrolein—A pulmonary hazard. Mol. Nutr. Food Res. 2011, 55, 1342–1360. [Google Scholar] [CrossRef] [PubMed]
- Yeager, R.P.; Kushman, M.; Chemerynski, S.; Weil, R.; Fu, X.; White, M.; Callahan-Lyon, P.; Rosenfeldt, H. Proposed Mode of Action for Acrolein Respiratory Toxicity Associated with Inhaled Tobacco Smoke. Toxicol. Sci. 2016, 151, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Burcham, P.C.; Thompson, C.A.; Henry, P.J. Chapter 1—Invited Review: Acrolein and the Lung: Chemical, Molecular, and Pathological Aspects. In Advances in Molecular Toxicology; Fishbein, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 4, pp. 1–36. [Google Scholar]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of mcrolein moxicity: Relevance to human disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Moretto, N.; Volpi, G.; Pastore, F.; Facchinetti, F. Acrolein effects in pulmonary cells: Relevance to chronic obstructive pulmonary disease. Ann. N. Y. Acad. Sci. 2012, 1259, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.B. Uptake of acrolein in the upper respiratory tract of the F344 rat. Inhal. Toxicol. 1996, 8, 387–403. [Google Scholar] [CrossRef]
- Struve, M.F.; Wong, V.A.; Marshall, M.W.; Kimbell, J.S.; Schroeter, J.D.; Dorman, D.C. Nasal uptake of inhaled acrolein in rats. Inhal. Toxicol. 2008, 20, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Achanta, S.; Jordt, S.E. TRPA1: Acrolein meets its target. Toxicol. Appl. Pharmacol. 2017, 324, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, M.K.; Avila, D.; Zhang, J.; Barve, S.; Arteel, G.; McClain, C.; Joshi-Barve, S. Acrolein cytotoxicity in hepatocytes involves endoplasmic reticulum stress, mitochondrial dysfunction and oxidative stress. Toxicol. Appl. Pharmacol. 2012, 265, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Lee, J.D.; Lee, B.D.; Chae, S.W.; Park, M.K. Effect of acrolein, a hazardous air pollutant in smoke, on human middle ear epithelial cells. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.R. Temperature distribution inside a burning cigarette. Nature 1974, 247, 405–406. [Google Scholar] [CrossRef]
- Skog, K.; Steineck, G.; Augustsson, K.; Jagerstad, M. Effect of cooking temperature on the formation of heterocyclic amines in fried meat products and pan residues. Carcinogenesis 1995, 16, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Benson, J.M.; Irvin, C.; Irshad, H.; Cheng, Y.-S. Particle Size Distribution and Inhalation Dose of Shower Water under Selected Operating Conditions. Inhal. Toxicol. 2007, 19, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Si, X.; Kim, J.W.; Berlinski, A. Simulation of airflow and aerosol deposition in the nasal cavity of a 5-year-old child. J. Aerosol Sci. 2011, 42, 156–173. [Google Scholar] [CrossRef]
- Zhou, Y.; Xi, J.; Simpson, J.; Irshad, H.; Cheng, Y.-S. Aerosol Deposition in a Nasopharyngolaryngeal Replica of a 5-Year-Old Child. Aerosol Sci. Technol. 2013, 47, 275–282. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, M.; Xi, J.; Irshad, H.; Cheng, Y.-S. Nasal Deposition in Infants and Children. J. Aerosol Med. 2014, 26, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Kim, J.; Si, X.A.; Corley, R.A.; Zhou, Y. Modeling of inertial deposition in scaled models of rat and human nasal airways: Towards in vitro regional dosimetry in small animals. J. Aerosol Sci. 2016, 99, 78–93. [Google Scholar] [CrossRef]
- Corley, R.A.; Kabilan, S.; Kuprat, A.P.; Carson, J.P.; Minard, K.R.; Jacob, R.E.; Timchalk, C.; Glenny, R.; Pipavath, S.; Cox, T.; et al. Comparative Computational Modeling of Airflows and Vapor Dosimetry in the Respiratory Tracts of Rat, Monkey, and Human. Toxicol. Sci. 2012, 128, 500–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Villalta, P.W.; Wang, M.; Hecht, S.S. Detection and Quantitation of Acrolein-Derived 1,N(2)-Propanodeoxyguanosine Adducts in Human Lung by Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry. Chem. Res. Toxicol. 2007, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Deshlahra, P.; Conway, J.; Wolf, E.E.; Schneider, W.F. Influence of Dipole–Dipole Interactions on Coverage-Dependent Adsorption: CO and NO on Pt(111). Langmuir 2012, 28, 8408–8417. [Google Scholar] [CrossRef] [PubMed]
- Lemkul, J.A.; Huang, J.; MacKerell, A.D. Induced Dipole–Dipole Interactions Influence the Unfolding Pathways of Wild-Type and Mutant Amyloid β-Peptides. J. Phys. Chem. B 2015, 119, 15574–15582. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.; Grabowski, S.J.; Kar, T. Influence of hybridization and substitution on the properties of the CH···O hydrogen bond. J. Phys. Chem. A 2001, 105, 10607–10612. [Google Scholar] [CrossRef]
- Georg, H.C.; Coutinho, K.; Canuto, S. A sequential Monte Carlo quantum mechanics study of the hydrogen-bond interaction and the solvatochromic shift of the n-pi* transition of acrolein in water. J. Chem. Phys. 2005, 123, 124307. [Google Scholar] [CrossRef] [PubMed]
- Malde, A.K.; Zuo, L.; Breeze, M.; Stroet, M.; Poger, D.; Nair, P.C.; Oostenbrink, C.; Mark, A.E. An Automated Force Field Topology Builder (ATB) and Repository: Version 1.0. J. Chem. Theory Comput. 2011, 7, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Marian, C.; O’Connor, R.J.; Djordjevic, M.; Rees, V.W.; Hatsukami, D.K.; Shields, P.G. Reconciling Human Smoking Behavior and Machine Smoking Patterns: Implications for Understanding Smoking Behavior and the Impact on Laboratory Studies. Cancer Epidemiol. Biomarkers Prev. 2009, 18, 3305–3320. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.E.; Sarangapani, R. Clearance concepts applied to the metabolism of inhaled vapors in tissues lining the nasal cavity. Inhal. Toxicol. 1999, 11, 873–897. [Google Scholar] [PubMed]
- McRobbie, D.W.; Pritchard, S.E. Studies of the human oropharyngeal airspaces using magnetic resonance imaging. III. The effects of device resistance with forced maneuver and tidal breathing on upper airway geometry. J. Aerosol Med. 2005, 18, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Talaat, K.; Xi, J. Computational modeling of aerosol transport, dispersion, and deposition in rhythmically expanding and contracting terminal alveoli. J. Aerosol Sci. 2017, 112, 19–33. [Google Scholar] [CrossRef]
- Naseri, A.; Shaghaghian, S.; Abouali, O.; Ahmadi, G. Numerical investigation of transient transport and deposition of microparticles under unsteady inspiratory flow in human upper airways. Respir. Physiol. Neurobiol. 2017, 244, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Scheinherr, A.; Bailly, L.; Boiron, O.; Legou, T.; Giovanni, A.; Caillibotte, G.; Pichelin, M. Glottal motion and its impact on the respiratory flow. Comput. Methods Biomech. Biomed. Eng. 2012, 15 (Suppl. 1), 69–71. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Wang, Z.; Talaat, K.; Glide-Hurst, C.; Dong, H. Numerical study of dynamic glottis and tidal breathing on respiratory sounds in a human upper airway model. Sleep Breath. 2017, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.J.; Schuh, A.; Amine-Eddine, G.; McConnell, K.; Loew, W.; Fleck, R.J.; Woods, J.C.; Dumoulin, C.L.; Amin, R.S. Assessing the relationship between movement and airflow in the upper airway using computational fluid dynamics with motion determined from magnetic resonance imaging. Clin. Biomech. 2017. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mitchell, J.; Chen, Y.; Yim, W.; Chu, W.; Wang, R.C. Study of the upper airway of obstructive sleep apnea patient using fluid structure interaction. Respir. Physiol. Neurobiol. 2018, 249, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Frederix, E.M.A.; Kuczaj, A.K.; Nordlund, M.; Bělka, M.; Lizal, F.; Jedelský, J.; Elcner, J.; Jícha, M.; Geurts, B.J. Simulation of size-dependent aerosol deposition in a realistic model of the upper human airways. J. Aerosol Sci. 2018, 115, 29–45. [Google Scholar] [CrossRef]
- Xi, J.; Yuan, J.E.; Si, X.A. Simulation study of electric-guided delivery of 0.4 µm monodisperse and polydisperse aerosols to the ostiomeatal complex. Comput. Biol. Med. 2016, 72, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Kim, J.; Si, X.A.; Zhou, Y. Hygroscopic aerosol deposition in the human upper respiratory tract under various thermo-humidity conditions. J. Environ. Sci. Health A 2013, 48, 1790–1805. [Google Scholar] [CrossRef] [PubMed]
- Longest, P.W.; Xi, J. Condensational growth may contribute to the enhanced deposition of cigarette smoke particles in the upper respiratory tract. Aerosol Sci. Technol. 2008, 42, 579–602. [Google Scholar] [CrossRef]
- Kim, J.W.; Xi, J.; Si, X.A. Dynamic growth and deposition of hygroscopic aerosols in the nasal airway of a 5-year-old child. Int. J. Numer. Methods Biomed. Eng. 2013, 29, 17–39. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Si, X.; Longest, P.W. Electrostatic Charge Effects on Pharmaceutical Aerosol Deposition in Human Nasal-Laryngeal Airways. Pharmaceutics 2013, 6, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Si, X.A.; Gaide, R. Electrophoretic Particle Guidance Significantly Enhances Olfactory Drug Delivery: A Feasibility Study. PLoS ONE 2014, 9, e86593. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Berlinski, A.; Zhou, Y.; Greenberg, B.; Ou, X. Breathing resistance and ultrafine particle deposition in nasal–laryngeal airways of a newborn, an infant, a child, and an adult. Ann. Biomed. Eng. 2012, 40, 2579–2595. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Tawhai, M.H.; Hoffman, E.A.; Lin, C.-L. On intra- and intersubject variabilities of airflow in the human lungs. Phys. Fluids 2009, 21, 101901. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xi, J.; Langenderfer, J.E. Sensitivity Analysis and Uncertainty Quantification in Pulmonary Drug Delivery of Orally Inhaled Pharmaceuticals. J. Pharm. Sci. 2017, 106, 3303–3315. [Google Scholar] [CrossRef] [PubMed]
- Minard, K.R.; Kuprat, A.P.; Kabilan, S.; Jacob, R.E.; Einstein, D.R.; Carson, J.P.; Corley, R.A. Phase-contrast MRI and CFD modeling of apparent (3)He gas flow in rat pulmonary airways. J. Magn. Reson. 2012, 221, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lizal, F.; Jedelsky, J.; Morgan, K.; Bauer, K.; Llop, J.; Cossio, U.; Kassinos, S.; Verbanck, S.; Ruiz-Cabello, J.; Santos, A.; et al. Experimental methods for flow and aerosol measurements in human airways and their replicas. Eur. J. Pharm. Sci. 2018, 113, 95–131. [Google Scholar] [CrossRef] [PubMed]
- Menache, M.G.; Hanna, L.M.; Gross, E.A.; Lou, S.R.; Zinreich, S.J.; Leopold, D.A.; Jarabek, A.M.; Miller, F.J. Upper respiratory tract surface areas and volumes of laboratory animals and humans: Considerations for dosimetry models. J. Toxicol. Environ. Health 1997, 50, 475–506. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, D.C. Turbulence Modeling for CFD, 2nd ed.; DCW Industries, Inc.: La Canada, CA, USA, 1998. [Google Scholar]
- Kim, J.; Xi, J.; Si, X.; Berlinski, A.; Su, W.C. Hood Nebulization: Effects of Head Direction and Breathing Mode on Particle Inhalability and Deposition in a 7-Month-Old Infant Model. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, J.; Dong, H.; Zhao, K. Computational modeling and validation of human nasal airflow under various breathing conditions. J. Biomech. 2017, 64, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Chen, J.; Chen, Q. On the turbulence models and turbulent Schmidt number in simulating stratified flows. J. Build. Perform. Simu. 2016, 9, 134–148. [Google Scholar] [CrossRef]
- Longest, P.W.; Xi, J. Computational investigation of particle inertia effects on submicron aerosol deposition in the respiratory tract. J. Aerosol Sci. 2007, 38, 111–130. [Google Scholar] [CrossRef]
- Xi, J.; Longest, P.W. Numerical predictions of submicrometer aerosol deposition in the nasal cavity using a novel drift flux approach. Int. J. Heat Mass. Tran. 2008, 51, 5562–5577. [Google Scholar] [CrossRef]
- Tian, G.; Longest, P.W. Development of a CFD boundary condition to model transient vapor absorption in the respiratory airways. J. Biomech. Eng. 2010, 132, 051003. [Google Scholar] [PubMed]
- Tian, G.; Longest, P.W. Application of a new dosimetry program TAOCS to assess transient vapour absorption in the upper airways. Inhal. Toxicol. 2010, 22, 1047–1063. [Google Scholar] [CrossRef] [PubMed]
- Schroeter, J.D.; Kimbell, J.S.; Gross, E.A.; Willson, G.A.; Dorman, D.C.; Tan, Y.-M.; Clewell, H.J., III. Application of physiological computational fluid dynamics models to predict interspecies nasal dosimetry of inhaled acrolein. Inhal. Toxicol. 2008, 20, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38, 27–38. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, Z.; Qiu, H.; Kong, M.G.; Joshi, R.P. Physics of nanoporation and water entry driven by a high-intensity, ultrashort electrical pulse in the presence of membrane hydrophobic interactions. Phys. Rev. E 2013, 87, 032704. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Clarendon Press: Oxford, UK, 1989; p. 385. [Google Scholar]
- Hu, Q.; Joshi, R.P. Analysis of intense, subnanosecond electrical pulse-induced transmembrane voltage in spheroidal cells with arbitrary orientation. IEEE Trans. Biomed. Eng. 2009, 56, 1617–1626. [Google Scholar] [CrossRef] [PubMed]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, J.; Hu, Q.; Zhao, L.; Si, X.A. Molecular Binding Contributes to Concentration Dependent Acrolein Deposition in Rat Upper Airways: CFD and Molecular Dynamics Analyses. Int. J. Mol. Sci. 2018, 19, 997. https://doi.org/10.3390/ijms19040997
Xi J, Hu Q, Zhao L, Si XA. Molecular Binding Contributes to Concentration Dependent Acrolein Deposition in Rat Upper Airways: CFD and Molecular Dynamics Analyses. International Journal of Molecular Sciences. 2018; 19(4):997. https://doi.org/10.3390/ijms19040997
Chicago/Turabian StyleXi, Jinxiang, Qin Hu, Linlin Zhao, and Xiuhua April Si. 2018. "Molecular Binding Contributes to Concentration Dependent Acrolein Deposition in Rat Upper Airways: CFD and Molecular Dynamics Analyses" International Journal of Molecular Sciences 19, no. 4: 997. https://doi.org/10.3390/ijms19040997