Remote Ischemic Preconditioning Does Not Affect the Release of Humoral Factors in Propofol-Anesthetized Cardiac Surgery Patients: A Secondary Analysis of the RIPHeart Study
Abstract
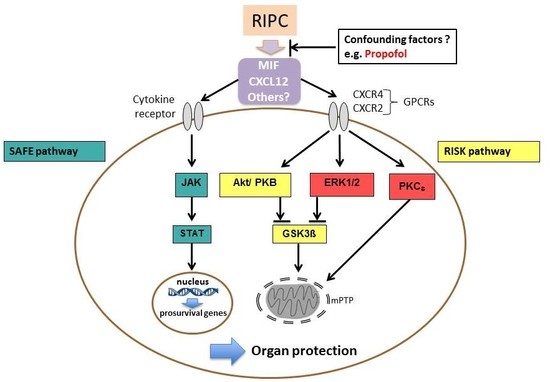
:1. Introduction
2. Results
2.1. Patients
2.2. Cardioprotective Effects of RIPC—Perioperative Troponin T Release
2.3. Serum and Tissue Levels of MIF and Serum Levels of CXCL12 as Potential Mediators of RIPC
2.4. Serum Levels of IL-6, CXCL8, and IL-10
2.5. Western Blot Analysis of Protein Kinases ERK1/2, AKT, GSK-3β, and PKCε for Content and Phosphorylation
3. Discussion
4. Methods and Materials
4.1. Study Design, Patients, Randomization
4.2. Surgical Procedure, Anesthesia, Intervention, Data Collection, and Blinding
4.3. Laboratory Assessment
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Murry, C.E.; Jennings, R.B.; Reimer, K.A. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation 1986, 74, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Barrabes, J.A.; Bøtker, H.E.; Davidson, S.M.; Di Lisa, F.; Downey, J.; Engstrom, T.; Ferdinandy, P.; Carbrera-Fuentes, H.A.; Heusch, G.; Ibanez, B. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016, 111, 70. [Google Scholar] [CrossRef] [PubMed]
- Przyklenk, K.; Bauer, B.; Ovize, M.; Kloner, R.A.; Whittaker, P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993, 87, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Bøtker, H.E.; Przyklenk, K.; Redington, A.; Yellon, D. Remote Ischemic Conditioning. J. Am. Coll. Cardiol. 2015, 65, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Mwamure, P.K.; Venugopal, V.; Harris, J.; Barnard, M.; Grundy, E.; Ashley, E.; Vichare, S.; Di Salvo, C.; Kolvekar, S.; et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: A randomized controlled trial. Lancet 2007, 18, 575–579. [Google Scholar] [CrossRef]
- Thielmann, M.; Kottenberg, E.; Kleinbongard, P.; Wendt, D.; Gedik, N.; Pasa, S.; Price, V.; Tsagakis, K.; Neuhäuser, M.; Peters, J.; Jakob, H. Cradioprotective and prognostic effects of remote iscaemic preconditioning in patients undergoing coronary artery bypass graft: A single centre randomized, double-blind, controlled trial. Lancet 2013, 382, 597–604. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Candilio, L.; Evans, R.; Ariti, C.; Jenkins, D.P.; Kolvekar, S.; Knight, R.; Kunst, G.; Laing, C.; Nicholas, J.; et al. Remote Ischemic Preconditioning and Outcomes of Cardiac Surgery. N. Engl. J. Med. 2015, 373, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Meybohm, P.; Bein, B.; Brosteanu, O.; Cremer, J.; Gruenewald, M.; Stoppe, C.; Coburn, M.; Schaelte, G.; Böning, A.; Niemann, B.; Roesner, J. A multicentered trial of remote ischemic preconditioning for heart surgery. N. Engl. J. Med. 2015, 373, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Kottenberg, E.; Thielmann, M.; Bergmann, L.; Heine, T.; Jakob, H.; Heusch, G.; Peters, J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—A clinical trial. Acta Anaesthesiol. Scand. 2012, 56, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Bosnjak, Z.J.; Ge, Z. The application of remote ischemic conditioning in cardiac surgery. F1000Reserch 2017, 6, 928. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 13, 674–699. [Google Scholar] [CrossRef] [PubMed]
- Bucala, R.; Bernhagen, J. MIF Family Cytokines in Innate Immunity and Homeostasis; Springer: New York, NY, USA, 2017; ISBN 978-3-319-52354-5. [Google Scholar]
- Chatterjee, M.; Borst, O.; Walker, B.; Fotinos, A.; Vogel, S.; Seizer, P.; Mack, A.F.; Alampour-Rajabi, S.; Rath, D.; Geisler, T.; Lang, F. Macrophage migration inhibitory factor limits activation-induced apoptosis of platelets via CXCR7-dependent Akt signaling. Circ. Res. 2014, 115, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Lue, H.; Thiele, M.; Franz, J.; Dahl, E.; Speckgens, S.; Leng, L.; Fingerle-Rowson, G.; Bucala, R.; Lüscher, B.; Bernhagen, J. Macrophage migration inhibitory factor (MIF) promotes cell survival by activation of the Akt pathway and role for CSN5/JAB1 in the control of autocrine MIF activity. Oncogene 2007, 26, 5046–5059. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Jacobs, D.; Emontzpohl, C.; Goetzenich, A.; Soppert, J.; Jarchow, M.; Schindler, L.; Averdunk, L.; Kraemer, S.; Marx, G.; et al. Myocardial Ischemia Induces SDF-1α Release in Cardiac Surgery Patients. J. Cardiovasc. Transl. Res. 2016, 9, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Bautin, A.; Datsenko, S.; Tashkhanov, D.; Gordeev, M.; Rubinchik, V.; Kurapeev, D.; Galagudza, M. Influence of the Anaesthesia Technique on the Cardioprotective Effects of the Remote Ischemic Preconditioning in the Patients Undergoing the Aortic Valve Replacement. Heart 2013, 99, A40–A41. [Google Scholar] [CrossRef]
- Zarbock, A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Martens, S.; Zahn, P.K.; Wolf, B.; Goebel, U.; Schwer, C.I.; Rosenberger, P.; et al. Effect of Remote Ischemic Preconditioning on Kidney Injury Among High-Risk Patients Undergoing Cardiac Surgery: A Randomized Clinical Trial. JAMA 2015, 313, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Y.; Yao, Y.; Zhou, S.; Fang, N.; Wang, W.; Li, L. ß-blockers and volatile anesthetics may attentuate cardioprotection by remote preconditioning in adult cardiac surgery: A metaanalysis of 15 randomized trial. J. Cardiothorac. Vasc. Anesth. 2013, 27, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Zangrillo, A.; Musu, M.; Greco, T.; Di Prima, A.L.; Matteazzi, A.; Testa, V.; Nardelli, P.; Febres, D.; Monaco, F.; Calabrò, M.G.; Ma, J. Additive Effect on Survival of Anaesthetic Cardiac Protection and Remote Ischemic Preconditioning in Cardiac Surgery: A Bayesian Network Meta-Analysis of Randomized Trials. PLoS ONE 2015, 31, e0134264. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Iliodromitis, E.K.; Andreadou, I.; Papalois, A.; Gritsopoulos, G.; Anastasiou-Nana, M.; Kremastinos, D.T.; Yellon, D.M. Investigating the signal transduction pathways underlying remote ischemic conditioning in the porcine heart. Cardiovasc. Drugs Ther. 2012, 26, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. New directions for protecting the heart against ischemia-reperfusion injury: Targeting the reperfusion injury salvage kinase (RISK) pathway. Cardiovasc. Res. 2011, 61, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Lecour, S.; Yellon, D.M. Reperfusion injury salvage kinase and survivor activating factor enhancement pro- survival signaling pathways in ischemic postconditioning: Two sides of the same coin. Antioxid. Redox Signal. 2011, 14, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Skyschally, A.; Gent, S.; Amanakis, G.; Schulte, C.; Kleinbongard, P.; Heusch, G. Across-Species Transfer of Protection by Remote Ischemic Preconditioning with Species-Specific Myocardial Signal Transduction by Reperfusion Injury Salvage Kinase and Survival Activating Factor Enhancement Pathways. Circ. Res. 2015, 117, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Lecour, S. Activation of the protective survivor activating enhancement (SAFE) pathway against reperfusion injury: Does it go beyond the RISK pathway? J. Mol. Cell. Cardiol. 2009, 47, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Musiolik, J.; Kottenberg, E.; Peters, J.; Jakob, H.; Thielmann, M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans. Circ. Res. 2012, 110, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kottenberg, E.; Musiolik, J.; Thielmann, M.; Jakob, H.; Peters, J.; Heusch, G. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2014, 147, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.M.; Selvaraj, P.; He, D.; Boi-Doku, C.; Yellon, R.L.; Vicencio, J.M.; Yellon, D.M. Remote ischaemic preconditioning involves signalling through the SDF-1α/CXCR4 signalling axis. Basic Res. Cardiol. 2013, 108, 377. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, V.; Lue, H.; Kraemer, S.; Korbiel, J.; Krohn, R.; Ohl, K.; Bucala, R.; Weber, C.; Bernhagen, J. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett. 2009, 583, 2749–2757. [Google Scholar] [CrossRef] [PubMed]
- Kucia, M.; Jankowski, K.; Reca, R.; Wysoczynski, M.; Bandura, L.; Allendorf, D.J.; Zhang, J.; Ratajczak, J.; Ratajczak, M.Z. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J. Mol. Histol. 2004, 35, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Bromage, D.I.; Davidson, S.M.; Yellon, D.M. Stromal derived factor 1α: A chemokine that delivers a two-pronged defence of the myocardium. Pharmacol. Ther. 2014, 143, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Hitchon, C.; Wong, K.; Ma, G.; Reed, J.; Lyttle, D.; El-Gabalawy, H. Hypoxia-induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthr. Rheum. 2002, 46, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Stoppe, C.; Grieb, G.; Simons, D.; Rossaint, R.; Bernhagen, J.; Rex, S. High postoperative blood levels of macrophage migration inhibitory factor are associated with less organ dysfunction in patients after cardiac surgery. Mol. Med. 2012, 18, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Goetzenich, A.; Kraemer, S.; Rossaint, R.; Bleilevens, C.; Dollo, F.; Siry, L.; Rajabi-Alampour, S.; Beckers, C.; Soppert, J.; Lue, H.; et al. The role of macrophage migration inhibitory factor in anesthetic induced myocardial preconditioning. PLoS ONE 2014, 9, e92827. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Newman, M.A.; Shehatha, J.S.; Redington, A.N.; Konstantinov, I.E. Remote ischemic conditioning: Evolution of the concept, mechanisms, and clinical application. J. Card. Surg. 2010, 25, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, I.E.; Arab, S.; Li, J.; Coles, J.G.; Boscarino, C.; Mori, A.; Cukerman, E.; Dawood, F.; Cheung, M.M.; Shimizu, M.; et al. The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J. Thorac. Cardiovasc. Surg. 2005, 130, 1326–1332. [Google Scholar] [CrossRef] [PubMed]
- Nederlof, R.; Weber, N.C.; Juffermans, N.P.; de Mol, B.A.; Hollmann, M.W.; Preckel, B.; Zuurbier, C.J. Randomized trial of remote ischemic preconditioning and control treatment for cardioprotection in sevoflurane-anesthetized CABG patients. BMC Anestheiol. 2017, 17, 51. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, J.; Pryds, K.; Salman, R.; Løfgren, B.; Kristiansen, S.B.; Bøtker, H.E. The remote ischemic preconditioning algorithm: Effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res. Cardiol. 2016, 111, 10. [Google Scholar] [CrossRef] [PubMed]
- Candilio, L.; Malik, A.; Hausenloy, D.J. Protection of organs other than the heart by remote ischemic conditioning. J. Cardiovasc. Med. 2013, 14, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Cell Biology of Ischemia/Reperfusion Injury. Int. Rev. Cell Mol. Biol. 2012, 298, 229–317. [Google Scholar] [PubMed]
- Hess, D.C.; Blauenfeldt, R.A.; Andersen, G.; Hougaard, K.D.; Hoda, M.N.; Ding, Y.; Ji, X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015, 12, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Anttila, V.; Haapanen, H.; Yannopoulos, F.; Herajärvi, J.; Anttila, T.; Juvonen, T. Review of remote ischemic preconditioning: From laboratory studies to clinical trials. Scand. Cardiovasc. J. 2016, 50, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Benstoem, C.; Stoppe, C.; Liakopoulos, O.J.; Ney, J.; Hasenclever, D.; Meybohm, P.; Goetzenich, A. Remote ischaemic preconditioning for coronary artery bypass grafting (with or without valve surgery). Cochrane Database Syst. Rev. 2017, 5, CD011719. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Control (n = 21) | RIPC (n = 19) | p-Value |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 67 (45–84) * | 67 (51–80) | 0.860 |
| Male (N, %) | 12 (57) | 14 (74) | 0.287 |
| Height (cm) | 167 (145–185) * | 173 (146–190) | 0.047 |
| Weight (kg) | 77 (57–103) * | 85 (58–117) | 0.158 |
| Logistic EuroSCORE * | 2.2 (0.6–5.5) | 1.7 (0.5–6.2) | 0.892 |
| Comorbidities, No. (%) | |||
| Coronary heart disease | 17 (76) | 12 (63) | 0.221 |
| Hypertension | 17 (76) | 16 (84) | 0.545 |
| COPD | 1 (5) | 2 (11) | 0.514 |
| Diabetes | 8 (38) | 5 (26) | 0.443 |
| Stroke in the past | 6 (29) | 3 (16) | 0.394 |
| Medication, No. (%) | |||
| Beta-blockers | 17 (76) | 14 (74) | 0.600 |
| ACE/AT1 inhibitors | 14 (67) | 14 (74) | 0.645 |
| Statins | 18 (86) | 16 (84) | 0.912 |
| Ca2+-channel blockers | 6 (29) | 2 (11) | 0.165 |
| Aspirin | 17 (81) | 16 (84) | 0.805 |
| Insulin/Metformin | 5 (24) | 2 (11) | 0.545 |
| Diuretics | 11 (52) | 9 (47) | 0.230 |
| Characteristics? | Control Group (n = 21) | RIPC Group (n = 19) | p-Value |
|---|---|---|---|
| Procedure, No. (%) | |||
| CABG only | 11 (52) | 9 (47) | 0.759 |
| Valve only | 1 (5) | 2 (11) | 0.502 |
| Combined | 9 (43) | 8 (42) | 0.962 |
| Intraoperative times, Median (IQR), min | |||
| Time until CPB circulation | 105.48 (65–145) | 106.84 (80–150) | 0.871 |
| CPB in total | 120 (30–232) | 143 (67–247) | 0.063 |
| Aortic cross-clamping | 79 (15–146) | 99 (50–166) | 0.083 |
| Reperfusion on CPB | 32 (6–77) | 38 (9–77) | 0.163 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ney, J.; Hoffmann, K.; Meybohm, P.; Goetzenich, A.; Kraemer, S.; Benstöm, C.; Weber, N.C.; Bickenbach, J.; Rossaint, R.; Marx, G.; et al. Remote Ischemic Preconditioning Does Not Affect the Release of Humoral Factors in Propofol-Anesthetized Cardiac Surgery Patients: A Secondary Analysis of the RIPHeart Study. Int. J. Mol. Sci. 2018, 19, 1094. https://doi.org/10.3390/ijms19041094
Ney J, Hoffmann K, Meybohm P, Goetzenich A, Kraemer S, Benstöm C, Weber NC, Bickenbach J, Rossaint R, Marx G, et al. Remote Ischemic Preconditioning Does Not Affect the Release of Humoral Factors in Propofol-Anesthetized Cardiac Surgery Patients: A Secondary Analysis of the RIPHeart Study. International Journal of Molecular Sciences. 2018; 19(4):1094. https://doi.org/10.3390/ijms19041094
Chicago/Turabian StyleNey, Julia, Katleen Hoffmann, Patrick Meybohm, Andreas Goetzenich, Sandra Kraemer, Carina Benstöm, Nina C. Weber, Johannes Bickenbach, Rolf Rossaint, Gernot Marx, and et al. 2018. "Remote Ischemic Preconditioning Does Not Affect the Release of Humoral Factors in Propofol-Anesthetized Cardiac Surgery Patients: A Secondary Analysis of the RIPHeart Study" International Journal of Molecular Sciences 19, no. 4: 1094. https://doi.org/10.3390/ijms19041094