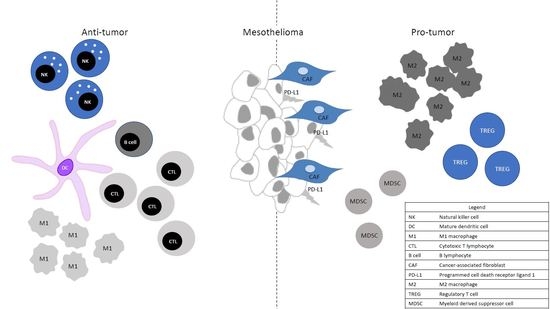
Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma
Abstract
:1. Introduction
2. Tumor Microenvironment (TME) in Mesothelioma
3. Macrophages
4. Myeloid-Derived Suppressor Cells
5. T Cells and Natural Killer Cells in Mesothelioma
6. Regulatory T Cells (Tregs)
7. B Lymphocytes
8. Cancer-Associated Fibroblasts (CAFs)
9. PD-L1 Expression and Other Immune Checkpoints
10. Discussion
Author Contributions
Conflicts of Interest
References
- Carbone, M.; Ly, B.H.; Dodson, R.F.; Pagano, I.; Morris, P.T.; Dogan, U.A.; Gazdar, A.F.; Pass, H.I.; Yang, H. Malignant mesothelioma: Facts, myths, and hypotheses. J. Cell. Physiol. 2012, 227, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Attanoos, R.L.; Gibbs, A.R. Pathology of malignant mesothelioma. Histopathology 1997, 30, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Brambilla, E.; Burke, A.P.; Marx, A.; Nicholson, A.G. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J. Thorac. Oncol. 2015, 10, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, D.J.; Flores, R.M.; Jaklitsch, M.T.; Richards, W.G.; Strauss, G.M.; Corson, J.M.; DeCamp, M.M.; Swanson, S.J.; Bueno, R.; Lukanich, J.M.; et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: Results in 183 patients. J. Thorac. Cardiovasc. Surg. 1999, 117, 54–63. [Google Scholar] [CrossRef]
- Mossman, B.T.; Shukla, A.; Heintz, N.H.; Verschraegen, C.F.; Thomas, A.; Hassan, R. New insights into understanding the mechanisms, pathogenesis, and management of malignant mesotheliomas. Am. J. Pathol. 2013, 182, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Bograd, A.J.; Suzuki, K.; Vertes, E.; Colovos, C.; Morales, E.A.; Sadelain, M.; Adusumilli, P.S. Immune responses and immunotherapeutic interventions in malignant pleural mesothelioma. Cancer Immunol. Immunother. 2011, 60, 1509–1527. [Google Scholar] [CrossRef] [PubMed]
- Yap, T.A.; Aerts, J.G.; Popat, S.; Fennell, D.A. Novel insights into mesothelioma biology and implications for therapy. Nat. Rev. Cancer 2017, 17, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Hegmans, J.P.; Aerts, J.G. Immunomodulation in cancer. Curr. Opin. Pharmacol. 2014, 17, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Veltman, J.D.; Lambers, M.E.; van Nimwegen, M.; Hendriks, R.W.; Hoogsteden, H.C.; Aerts, J.G.; Hegmans, J.P. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer 2010, 10, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veltman, J.D.; Lambers, M.E.H.; van Nimwegen, M.; Hendriks, R.W.; Hoogsteden, H.C.; Hegmans, J.P.; Aerts, J.G. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br. J. Cancer 2010, 103, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Veltman, J.D.; Lambers, M.E.H.; van Nimwegen, M.; de Jong, S.; Hendriks, R.W.; Hoogsteden, H.C.; Aerts, J.G.; Hegmans, J.P. Low-dose cyclophosphamide synergizes with dendritic cell-based immunotherapy in antitumor activity. J. Biomed. Biotechnol. 2010, 2010, 798467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Most, R.G.; Currie, A.J.; Mahendran, S.; Prosser, A.; Darabi, A.; Robinson, B.W.S.; Nowak, A.K.; Lake, R.A. Tumor eradication after cyclophosphamide depends on concurrent depletion of regulatory T cells: A role for cycling TNFR2-expressing effector-suppressor T cells in limiting effective chemotherapy. Cancer Immunol. Immunother. 2009, 58, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Pradel, L.P.; Ooi, C.-H.; Romagnoli, S.; Cannarile, M.A.; Sade, H.; Rüttinger, D.; Ries, C.H. Macrophage Susceptibility to Emactuzumab (RG7155) Treatment. Mol. Cancer Ther. 2016, 15, 3077–3086. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, F.; Ayaub, E.; Parthasarathy, P.; Ackermann, M.; Inman, M.D.; Kolb, M.R.J.; Wollin, L.; Ask, K.; Tandon, K. Nintedanib attenuates the polarization of profibrotic macrophages through the inhibition of tyrosine phosphorylation on CSF1 receptor. Am. J. Respir. Crit. Care Med. 2017, 195, A2397. [Google Scholar]
- Burt, B.M.; Rodig, S.J.; Tilleman, T.R.; Elbardissi, A.W.; Bueno, R.; Sugarbaker, D.J. Circulating and tumor-infiltrating myeloid cells predict survival in human pleural mesothelioma. Cancer 2011, 117, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, R.; Lievense, L.A.; Maat, A.P.; Hendriks, R.W.; Hoogsteden, H.C.; Bogers, A.J.; Hegmans, J.P.; Aerts, J.G. Ratio of intratumoral macrophage phenotypes is a prognostic factor in epithelioid malignant pleural mesothelioma. PLoS ONE 2014, 9, e106742. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, R.; Lievense, L.A.; Robertus, J.-L.; Hendriks, R.W.; Hoogsteden, H.C.; Hegmans, J.P.; Aerts, J.G. Intratumoral macrophage phenotype and CD8+ T lymphocytes as potential tools to predict local tumor outgrowth at the intervention site in malignant pleural mesothelioma. Lung Cancer 2015, 88, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Marcq, E.; Siozopoulou, V.; De Waele, J.; van Audenaerde, J.; Zwaenepoel, K.; Santermans, E.; Hens, N.; Pauwels, P.; van Meerbeeck, J.P.; Smits, E.L.J. Prognostic and predictive aspects of the tumor immune microenvironment and immune checkpoints in malignant pleural mesothelioma. Oncoimmunology 2017, 6, e1261241. [Google Scholar] [CrossRef] [PubMed]
- Schürch, C.M.; Forster, S.; Brühl, F.; Yang, S.H.; Felley-Bosco, E.; Hewer, E. The “don’t eat me” signal CD47 is a novel diagnostic biomarker and potential therapeutic target for diffuse malignant mesothelioma. Oncoimmunology 2018, 7, e1373235. [Google Scholar] [CrossRef] [PubMed]
- Mandruzzato, S.; Solito, S.; Falisi, E.; Francescato, S.; Chiarion-Sileni, V.; Mocellin, S.; Zanon, A.; Rossi, C.R.; Nitti, D.; Bronte, V.; et al. IL4Rα+ Myeloid-Derived Suppressor Cell Expansion in Cancer Patients. J. Immunol. 2009, 182, 6562–6568. [Google Scholar] [CrossRef] [PubMed]
- Burt, B.M.; Bader, A.; Winter, D.; Rodig, S.J.; Bueno, R.; Sugarbaker, D.J. Expression of Interleukin-4 Receptor Alpha in Human Pleural Mesothelioma Is Associated with Poor Survival and Promotion of Tumor Inflammation. Clin. Cancer Res. 2012, 18, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Saito, K.; Shiiba, K.; Ohuchi, A.; Saigenji, K.; Nagura, H.; Ohtani, H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998, 58, 3491–3494. [Google Scholar] [PubMed]
- Zhang, L.; Conejo-Garcia, J.R.; Katsaros, D.; Gimotty, P.A.; Massobrio, M.; Regnani, G.; Makrigiannakis, A.; Gray, H.; Schlienger, K.; Liebman, M.N.; et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003, 348, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, K.; Haensch, W.; Röefzaad, C.; Schlag, P.M. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001, 61, 3932–3936. [Google Scholar] [PubMed]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Friedman, K.M.; Prieto, P.A.; Devillier, L.E.; Gross, C.A.; Yang, J.C.; Wunderlich, J.R.; Rosenberg, S.A.; Dudley, M.E. Tumor-specific CD4+ melanoma tumor-infiltrating lymphocytes. J. Immunother. 2012, 35, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F.; Finotto, S. The emerging role of T cell cytokines in non-small cell lung cancer. Cytokine Growth Factor Rev. 2012, 23, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef] [PubMed]
- Leigh, R.A.; Webster, I. Lymphocytic infiltration of pleural mesothelioma and its significance for survival. S. Afr. Med. J. 1982, 61, 1007–1009. [Google Scholar] [PubMed]
- Mudhar, H.S.; Wallace, W.A.H. No relationship between tumour infiltrating lymphocytes and overall survival is seen in malignant mesothelioma of the pleura. Eur. J. Surg. Oncol. 2002, 28, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Hegmans, J.P.J.J. Mesothelioma environment comprises cytokines and T-regulatory cells that suppress immune responses. Eur. Respir. J. 2006, 27, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Cunningham, K.S.; Yun, Z.; Tsao, M.-S.; Zhang, L.; Keshavjee, S.; Johnston, M.R.; de Perrot, M. Impact of tumor-infiltrating T cells on survival in patients with malignant pleural mesothelioma. J. Thorac. Cardiovasc. Surg. 2008, 135, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Oizumi, S.; Kikuchi, E.; Shinagawa, N.; Konishi-Sakakibara, J.; Ishimine, A.; Aoe, K.; Gemba, K.; Kishimoto, T.; Torigoe, T.; et al. CD8+ tumor-infiltrating lymphocytes predict favorable prognosis in malignant pleural mesothelioma after resection. Cancer Immunol. Immunother. 2010, 59, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Jones, R.E.; Liu, H.; Lizotte, P.H.; Ivanova, E.V.; Kulkarni, M.; Herter-Sprie, G.S.; Liao, X.; Santos, A.A.; Bittinger, M.A.; et al. Cytotoxic T Cells in PD-L1-Positive Malignant Pleural Mesotheliomas Are Counterbalanced by Distinct Immunosuppressive Factors. Cancer Immunol. Res. 2016, 4, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kadota, K.; Sima, C.S.; Sadelain, M.; Rusch, V.W.; Travis, W.D.; Adusumilli, P.S. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol. Immunother. 2011, 60, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 2014, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- DeLong, P.; Carroll, R.G.; Henry, A.C.; Tanaka, T.; Ahmad, S.; Leibowitz, M.S.; Sterman, D.H.; June, C.H.; Albelda, S.M.; Vonderheide, R.H. Regulatory T cells and cytokines in malignant pleural effusions secondary to mesothelioma and carcinoma. Cancer Biol. Ther. 2005, 4, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, H.; Kadota, K.; Nitadori, J.; Aerts, J.G.; Woo, K.M.; Sima, C.S.; Travis, W.D.; Jones, D.R.; Krug, L.M.; Adusumilli, P.S. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology 2015, 4, e1009285. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.S.; Righi, L.; Koeppen, H.; Zou, W.; Izzo, S.; Grosso, F.; Libener, R.; Loiacono, M.; Monica, V.; Buttigliero, C.; et al. Molecular and Histopathological Characterization of the Tumor Immune Microenvironment in Advanced Stage of Malignant Pleural Mesothelioma. J. Thorac. Oncol. 2018, 13, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Baglole, C.J.; Ray, D.M.; Bernstein, S.H.; Feldon, S.E.; Smith, T.J.; Sime, P.J.; Phipps, R.P. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunol. Investig. 2006, 35, 297–325. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, W.; Yamada, T.; Matsumoto, K.; Sakai, K.; Bando, Y.; Uehara, H.; Nishioka, Y.; Sone, S.; Iwakiri, S.; et al. Pleural Mesothelioma Instigates Tumor-Associated Fibroblasts To Promote Progression via a Malignant Cytokine Network. Am. J. Pathol. 2011, 179, 1483–1493. [Google Scholar] [CrossRef]
- Lo, A.; Wang, L.-C.; Scholler, J.; Monslow, J.; Avery, D.; Newick, K.; O’Brien, S.; Evans, R.A.; Bajor, D.J.; Clendenin, C.; et al. Tumor-Promoting Desmoplasia Is Disrupted by Depleting FAP-Expressing Stromal Cells. Cancer Res. 2015, 75, 2800–2810. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.; Warn, A.; Newman, P.; Perry, L.J.; Ball, R.Y.; Warn, R.M. Immunoreactivity for hepatocyte growth factor/scatter factor and its receptor, met, in human lung carcinomas and malignant mesotheliomas. J. Pathol. 1996, 180, 389–394. [Google Scholar] [CrossRef]
- Gandini, S.; Massi, D.; Mandalà, M. PD-L1 expression in cancer patients receiving anti PD-1/PD-L1 antibodies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2016, 100, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef]
- Combaz-Lair, C.; Galateau-Sallé, F.; McLeer-Florin, A.; Le Stang, N.; David-Boudet, L.; Duruisseaux, M.; Ferretti, G.R.; Brambilla, E.; Lebecque, S.; Lantuejoul, S. Immune biomarkers PD-1/PD-L1 and TLR3 in malignant pleural mesotheliomas. Hum. Pathol. 2016, 52, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Cedrés, S.; Ponce-Aix, S.; Zugazagoitia, J.; Sansano, I.; Enguita, A.; Navarro-Mendivil, A.; Martinez-Marti, A.; Martinez, P.; Felip, E. Analysis of Expression of Programmed Cell Death 1 Ligand 1 (PD-L1) in Malignant Pleural Mesothelioma (MPM). PLoS ONE 2015, 10, e0121071. [Google Scholar] [CrossRef] [PubMed]
- Inaguma, S.; Lasota, J.; Wang, Z.; Czapiewski, P.; Langfort, R.; Rys, J.; Szpor, J.; Waloszczyk, P.; Okoń, K.; Biernat, W.; et al. Expression of ALCAM (CD166) and PD-L1 (CD274) independently predicts shorter survival in malignant pleural mesothelioma. Hum. Pathol. 2018, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, A.S.; Roden, A.C.; Peikert, T.; Sheinin, Y.M.; Harrington, S.M.; Krco, C.J.; Dong, H.; Kwon, E.D. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J. Thorac. Oncol. 2014, 9, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Khanna, S.; Thomas, A.; Abate-Daga, D.; Zhang, J.; Morrow, B.; Steinberg, S.M.; Orlandi, A.; Ferroni, P.; Schlom, J.; Guadagni, F.; et al. Malignant Mesothelioma Effusions Are Infiltrated by CD3+ T Cells Highly Expressing PD-L1 and the PD-L1+ Tumor Cells within These Effusions Are Susceptible to ADCC by the Anti-PD-L1 Antibody Avelumab. J. Thorac. Oncol. 2016, 11, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Cedrés, S.; Ponce-Aix, S.; Pardo-Aranda, N.; Navarro-Mendivil, A.; Martinez-Marti, A.; Zugazagoitia, J.; Sansano, I.; Montoro, M.A.; Enguita, A.; Felip, E. Analysis of expression of PTEN/PI3K pathway and programmed cell death ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). Lung Cancer 2016, 96, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D.; Kovacsovics-Bankowski, M.; Morris, N.; Walker, E.; Chisholm, L.; Floyd, K.; Walker, J.; Gonzalez, I.; Meeuwsen, T.; Fox, B.A.; et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013, 73, 7189–7198. [Google Scholar] [CrossRef] [PubMed]
| Surface Marker | Present on |
|---|---|
| CD3 | T lymphocytes |
| CD4 | T helper cells |
| CD8 | Cytotoxic T cells |
| CD11b | Monocytes, macrophages, MDSCs, NK cells, eosinophils, neutrophils, basophils, dendritic cells, mast cells, CD8+ T cells, B cells |
| CD16 | Natural killer cells, myeloid cells, monocytes, neutrophils |
| CD19 | B cells |
| CD20 | B cells |
| CD33 | Myeloid cells |
| CD45 | Leucocytes |
| CD45RO | T effector and memory cells |
| CD56 | Natural killer cells |
| CD68 | Macrophages |
| CD163 | M2 macrophages |
| CD206 | M2 macrophages |
| Foxp3+CD4+CD25+ | Regulatory T cells |
| Study | n | CD68+ | Coefficient of Variation (CV) * | CD163+ | Coefficient of Variation (CV) * |
|---|---|---|---|---|---|
| [18] | 52 | 25.2 ± 9.3%, (epithelial) 29.7 ± 10.2%, (non-epithelial) | 0.37, (epithelial) 0.34, (non-epithelial) | n.a. *** | n.a. *** |
| [19] | 16 | 211.3 ** ± 80.2, (surgery) 213.9 ** ± 100.4, (non-surgery) | 0.37, (surgery) 0.47, (non-surgery) | 168.3 ** ± 80.2, (surgery) 164.1 ** ± 82.5, (non-surgery) | 0.48, (surgery) 0.50, (non-surgery) |
| [20] | 20 | 202 ** | Range 45–408 | 153 ** | Range 42–422 |
| [21] | 54 | Present in all specimens | n.a. *** | n.a. *** | n.a. *** |
| [22] | 40 | Heavy infiltration | n.a. *** | Heavy infiltration | n.a. *** |
| Surface Marker | Mean (Cell Count per Field) | Standard Deviation | Coefficient of Variation (CV) * |
|---|---|---|---|
| CD3+ | 232.16 | 114.1 | 0.49 |
| CD4+ | 119.9 | 94.2 | 0.79 |
| CD8+ | 73.1 | 40.2 | 0.55 |
| CD25+ | 17.5 | 12.6 | 0.72 |
| FOXP3+ | 21.8 | 19.0 | 0.87 |
| CD45RO+ | 115.7 | 56.2 | 0.49 |
| Surface Marker | Mean (Cell Count per Field) | Standard Deviation | Coefficient of Variation (CV) * | Range | Median |
|---|---|---|---|---|---|
| CD4+ | 51.1 | 41.8 | 0.82 | 0.2–159.7 | 37.3 |
| CD8+ | 103.3 | 106.9 | 1.03 | 8.8–547.5 | 64.5 |
| CD56+ | 5.4 | 8.3 | 1.54 | 0.0–41.8 | 1.8 |
| Study | PD-L1 Antibody | n | Positivity (%) | PD-L1 Positive (n (%)) | PD-L1 Positive in Epithelioid (n (%)) | PD-L1 Positive in Non-Epithelioid (n (%)) | Survival in PD-L1+ (Months) | Survival in PD-L1- (Months) | p Value |
|---|---|---|---|---|---|---|---|---|---|
| [53] | 5H1-A3 | 106 | ≥5 | 42 (40) | 14/68 (21) | 37/38 (97) | 5 | 14.5 | <0.0001 |
| [51] | E1L3N | 77 | >1% | 16 (21) | 7/53 (13) | 9/24 (38) | 4.8 | 16.3 | 0.012 |
| [26] | E1L3N | 39 | ≥1% | 18 (46) | 8/26 (31) | 10/13 (77) | shorter | longer | 0.15 |
| [50] | E1L3N | 58 | ≥1% | 17 (29) | 8/34 (24) | 9/24 (38) | n.a.* | n.a.* | n.a.* |
| [50] | SP142 | 58 | ≥1% | 10 (17) | 4/34 (12) | 6/24 (25) | 4 | 13 | 0.016 |
| [54] | rabbit | 65 | ≥5% | 41 (63) | n.a.* | n.a.* | 23.0 | 33.3 | 0.35 |
| [21] | SP142 | 54 | ≥1% | 35 (65) | n.a.* | More in sarcomatoid | n.a.* | n.a.* | n.a.* |
| [52] | E1L3N | 175 | ≥5% | 57 (33) | 46/148 (31) | 11/27 (41) | 6 | 18 | <0.01 |
| [42] | SP142 | 99 | >1% | 16 (16) | 5/75 (6.7) | 9/24 (38) | shorter | longer |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minnema-Luiting, J.; Vroman, H.; Aerts, J.; Cornelissen, R. Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2018, 19, 1041. https://doi.org/10.3390/ijms19041041
Minnema-Luiting J, Vroman H, Aerts J, Cornelissen R. Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma. International Journal of Molecular Sciences. 2018; 19(4):1041. https://doi.org/10.3390/ijms19041041
Chicago/Turabian StyleMinnema-Luiting, Jorien, Heleen Vroman, Joachim Aerts, and Robin Cornelissen. 2018. "Heterogeneity in Immune Cell Content in Malignant Pleural Mesothelioma" International Journal of Molecular Sciences 19, no. 4: 1041. https://doi.org/10.3390/ijms19041041