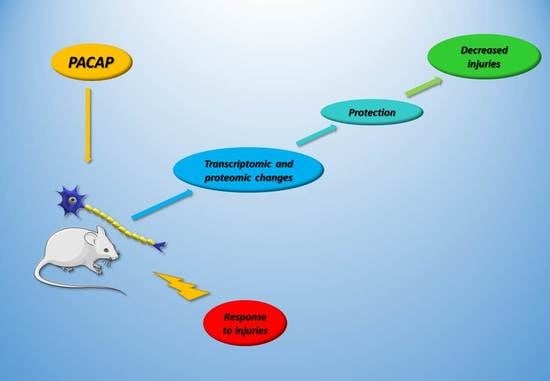
Review on PACAP-Induced Transcriptomic and Proteomic Changes in Neuronal Development and Repair
Abstract
:1. General Overview
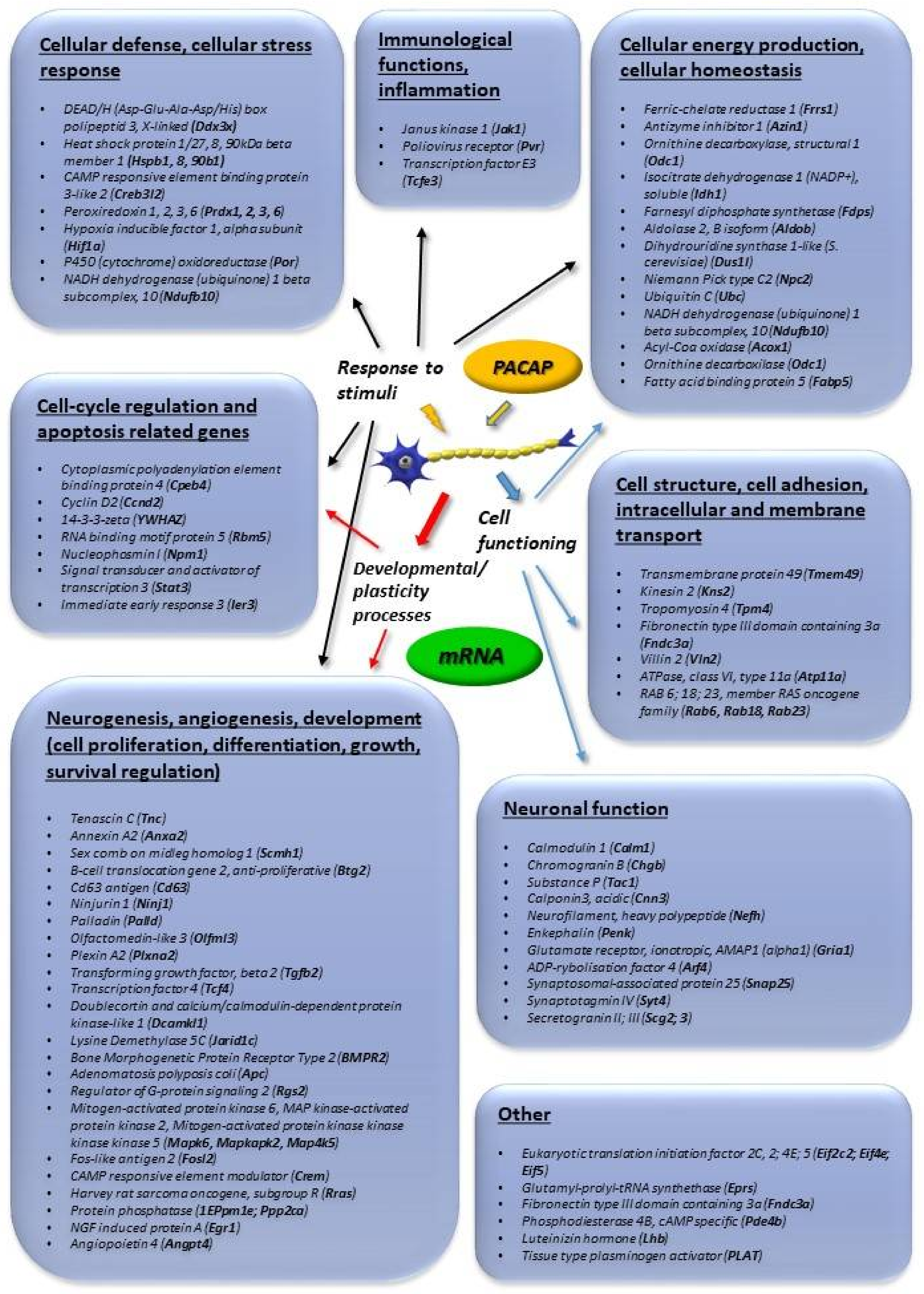
2. Involvement of PACAP in Neuronal Developmental Processes
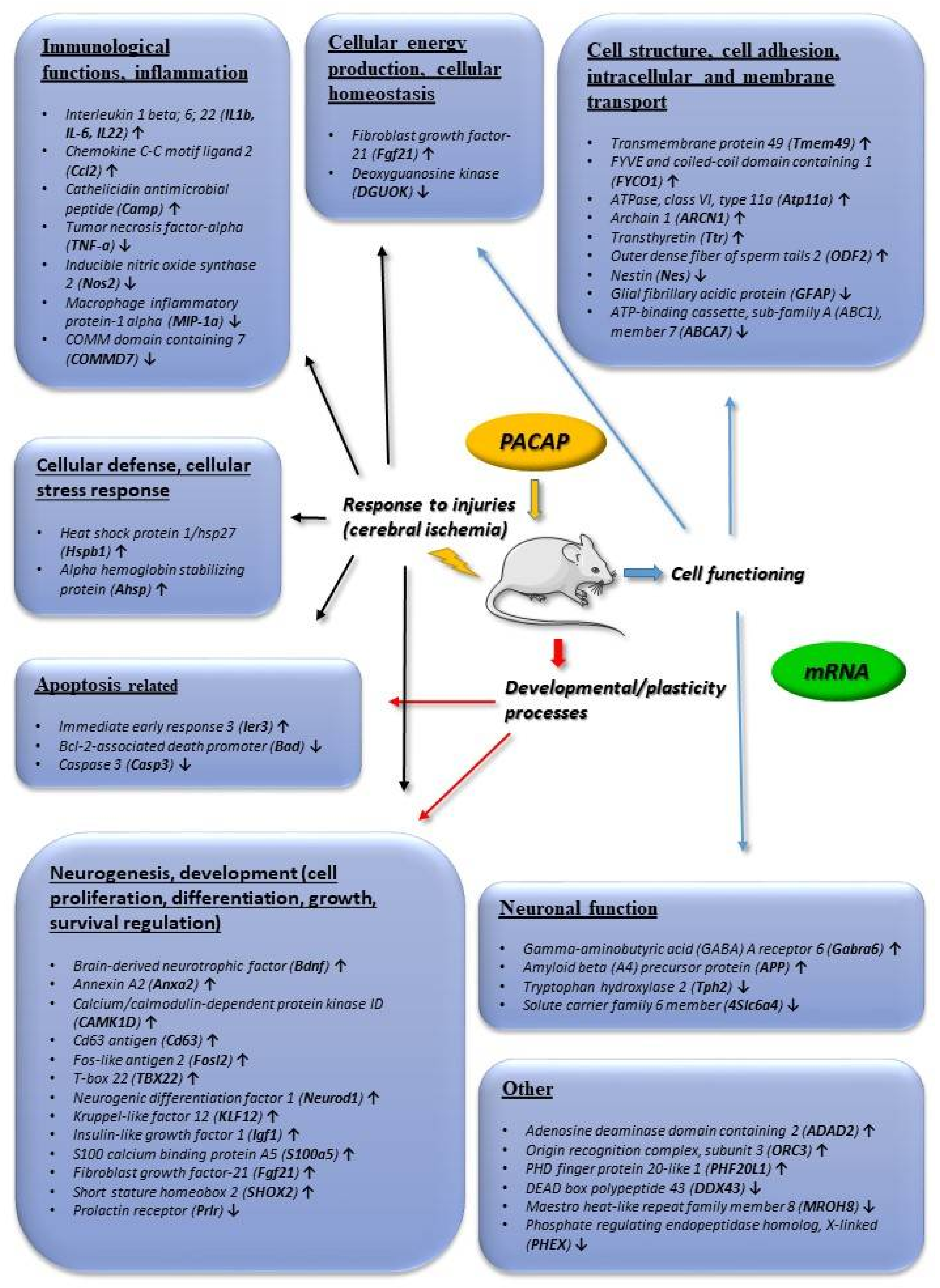
3. Neuroprotective Effects of PACAP
Acknowledgments
Conflicts of Interest
References
- Jolivel, V.; Basille, M.; Aubert, N.; de Jouffrey, S.; Ancian, P.; Le Bigot, J.F.; Noack, P.; Massonneau, M.; Fournier, A.; Vaudry, H.; et al. Distribution and functional characterization of pituitary adenylate cyclase-activating polypeptide receptors in the brain of non-human primates. Neuroscience 2009, 160, 434–451. [Google Scholar] [CrossRef] [PubMed]
- Pirger, Z.; Krajcs, N.; Kiss, T. Occurrence, distribution, and physiological function of pituitary adenylyl cyclase-activating polypeptide in invertebrate species. In Pituitary Adenylate Cyclase Activating Polypeptide —PACAP; Reglodi, D., Tamas, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 19–31. [Google Scholar]
- Egri, P.; Fekete, C.; Denes, A.; Reglodi, D.; Hashimoto, H.; Fulop, B.D.; Gereben, B. Pituitary adenylate cyclase-activating polypeptide (PACAP) regulates the hypothalamo-pituitary-thyroid (HPT) axis via type 2 deiodinase in male mice. Endocrinology 2016, 157, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, H.; Oride, A.; Tselmeg, M.; Sukhbaatar, U.; Kyo, S. Role of PACAP and its PACAP type I receptor in the central control of reproductive hormones. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 375–387. [Google Scholar]
- Garami, A.; Pakai, E.; Rumbus, Z.; Solymar, M. The role of pacap in the regulation of body temperature. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer: Cham, Switzerland, 2016; pp. 239–257. [Google Scholar]
- Banki, E.; Kovacs, K.; Nagy, D.; Juhasz, T.; Degrell, P.; Csanaky, K.; Kiss, P.; Jancso, G.; Toth, G.; Tamas, A. Molecular mechanisms underlying the nephroprotective effects of PACAP in diabetes. J. Mol. Neurosci. 2014, 54, 300–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekar, R.; Wang, L.; Chow, B.K. Central control of feeding behavior by the secretin, PACAP, and glucagon family of peptides. Front. Endocrinol. 2017, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.M.; Maunze, B.; Block, M.E.; Frenkel, M.M.; Reilly, M.J.; Kim, E.; Chen, Y.; Li, Y.; Baker, D.A.; Liu, Q.-S. Pituitary adenylate-cyclase activating polypeptide regulates hunger-and palatability-induced binge eating. Front. Neurosci. 2016, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Nakamachi, T.; Watanabe, J.; Sugiyama, K.; Ohtaki, H.; Murai, N.; Sasaki, S.; Xu, Z.; Hashimoto, H.; Seki, T. Pituitary adenylate cyclase-activating polypeptide (PACAP) is involved in adult mouse hippocampal neurogenesis after stroke. J. Mol. Neurosci. 2016, 59, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, V.; Sardone, L.M.; Chisari, M.; Licata, F.; Volsi, G.L.; Perciavalle, V.; Ciranna, L.; Costa, L. A subnanomolar concentration of pituitary adenylate cyclase-activating polypeptide (PACAP) pre-synaptically modulates glutamatergic transmission in the rat hippocampus acting through acetylcholine. Neuroscience 2017, 340, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Caselli, R.J.; Baxter, L.; Serrano, G.; Yin, J.; Beach, T.G.; Reiman, E.M.; Shi, J. Association of pituitary adenylate cyclase–activating polypeptide with cognitive decline in mild cognitive impairment due to Alzheimer disease. JAMA Neurol. 2015, 72, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Kirry, A.J.; Herbst, M.R.; Poirier, S.E.; Maskeri, M.M.; Rothwell, A.C.; Twining, R.C.; Gilmartin, M.R. Pituitary adenylate-cyclase activating-polypeptide (PACAP) signaling in the prefrontal cortex modulates cued fear learning, but not spatial working memory, in female rats. Neuropharmacology 2018, 133, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Seki, T.; Shioda, S. Pacap and neural development. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer: Cham, Switzerland, 2016; pp. 65–82. [Google Scholar]
- Nakamachi, T.; Farkas, J.; Watanabe, J.; Ohtaki, H.; Dohi, K.; Arata, S.; Shioda, S. Role of PACAP in neural stem/progenitor cell and astrocyte: From neural development to neural repair. Curr. Pharm. Des. 2011, 17, 973–984. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A. Multiple actions of pituitary adenylyl cyclase activating peptide in nervous system development and regeneration. Dev. Neurosci. 2002, 24, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Somogyvari-Vigh, A.; Reglodi, D. Pituitary adenylate cyclase activating polypeptide: A potential neuroprotective peptide. Curr. Pharm. Des. 2004, 10, 2861–2889. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the protective effects of PACAP in models of neurodegenerative diseases in vitro and in vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef] [PubMed]
- Shioda, S.; Nakamachi, T. PACAP as a neuroprotective factor in ischemic neuronal injuries. Peptides 2015, 72, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Kiss, P.; Szabadfi, K.; Atlasz, T.; Gabriel, R.; Horvath, G.; Szakaly, P.; Sandor, B.; Lubics, A.; Laszlo, E. PACAP is an endogenous protective factor—Insights from PACAP-deficient mice. J. Mol. Neurosci. 2012, 48, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, H.; Nakamachi, T.; Dohi, K.; Aizawa, Y.; Takaki, A.; Hodoyama, K.; Yofu, S.; Hashimoto, H.; Shintani, N.; Baba, A. Pituitary adenylate cyclase-activating polypeptide (PACAP) decreases ischemic neuronal cell death in association with IL-6. Proc. Natl. Acad. Sci. USA 2006, 103, 7488–7493. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikawa, D.; Nakamachi, T.; Tsuchida, M.; Wada, Y.; Hori, M.; Farkas, J.; Yoshikawa, A.; Kagami, N.; Imai, N.; Shioda, S. The neuroprotective effect of endogenous PACAP on spinal cord injury. J. Mol. Neurosci. 2012, 48, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Szabadfi, K.; Atlasz, T.; Kiss, P.; Danyadi, B.; Tamas, A.; Helyes, Z.; Hashimoto, H.; Shintani, N.; Baba, A.; Toth, G. Mice deficient in pituitary adenylate cyclase activating polypeptide (PACAP) are more susceptible to retinal ischemic injury in vivo. Neurotox. Res. 2012, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Manavalan, S.; Getachew, B.; Manaye, K.F.; Khundmiri, S.J.; Csoka, A.B.; McKinley, R.; Tamas, A.; Reglodi, D.; Tizabi, Y. PACAP protects against ethanol and nicotine toxicity in SH-SY5Y cells: Implications for drinking-smoking co-morbidity. Neurotox. Res. 2017, 32, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Renaud, J.; Tamas, A.; Tizabi, Y.; Socias, S.B.; del-Bel, E.; Raisman-Vozari, R. Novel tactics for neuroprotection in Parkinson’s disease: Role of antibiotics, polyphenols and neuropeptides. Prog. Neurobiol. 2017, 155, 120–148. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Gonzalez, B.J.; Basille, M.; Yon, L.; Fournier, A.; Vaudry, H. Pituitary adenylate cyclase-activating polypeptide and its receptors: From structure to functions. Pharmacol. Rev. 2000, 52, 269–324. [Google Scholar] [PubMed]
- Manecka, D.-L.; Boukhzar, L.; Falluel-Morel, A.; Lihrmann, I.; Anouar, Y. PACAP signaling in neuroprotection. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer: Cham, Switzerland, 2016; pp. 549–561. [Google Scholar]
- Vaczy, A.; Reglodi, D.; Somoskeoy, T.; Kovács, K.; Lokos, E.; Szabo, E.; Tamas, A.; Atlasz, T. The protective role of PAC1-receptor agonist maxadilan in BCCAO-induced retinal degeneration. J. Mol. Neurosci. 2016, 60, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, H.; Shioda, S. PACAP regulation of inflammatory and free radical networks in neuronal and nonneuronal diseases. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 671–690. [Google Scholar]
- Moody, T.W.; Nuche-Berenguer, B.; Jensen, R.T. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Doan, N.-D.; Chatenet, D.; Létourneau, M.; Vaudry, H.; Vaudry, D.; Fournier, A. Receptor-independent cellular uptake of pituitary adenylate cyclase-activating polypeptide. Biochim. Biophys. Acta 2012, 1823, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Samal, B.; Gerdin, M.J.; Huddleston, D.; Hsu, C.-M.; Elkahloun, A.G.; Stroth, N.; Hamelink, C.; Eiden, L.E. Meta-analysis of microarray-derived data from PACAP-deficient adrenal gland in vivo and PACAP-treated chromaffin cells identifies distinct classes of PACAP-regulated genes. Peptides 2007, 28, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Eiden, L.E.; Samal, B.; Gerdin, M.J.; Mustafa, T.; Vaudry, D.; Stroth, N. Discovery of pituitary adenylate cyclase-activating polypeptide-regulated genes through microarray analyses in cell culture and in vivo. Ann. N. Y. Acad. Sci. 2008, 1144, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ali, D.; Samal, B.; Mustafa, T.; Eiden, L.E. Neuropeptides, growth factors, and cytokines: A cohort of informational molecules whose expression is up-regulated by the stress-associated slow transmitter PACAP in chromaffin cells. Cell. Mol. Neurobiol. 2010, 30, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Samal, B.; Ait-Ali, D.; Bunn, S.; Mustafa, T.; Eiden, L.E. Discrete signal transduction pathway utilization by a neuropeptide (PACAP) and a cytokine (TNF-α) first messenger in chromaffin cells, inferred from coupled transcriptome-promoter analysis of regulated gene cohorts. Peptides 2013, 45, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Dejda, A.; Seaborn, T.; Bourgault, S.; Touzani, O.; Fournier, A.; Vaudry, H.; Vaudry, D. PACAP and a novel stable analog protect rat brain from ischemia: Insight into the mechanisms of action. Peptides 2011, 32, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Nakamachi, T.; Shibato, J.; Rakwal, R.; Shioda, S.; Numazawa, S. Unraveling the specific ischemic core and penumbra transcriptome in the permanent middle cerebral artery occlusion mouse model brain treated with the neuropeptide PACAP38. Microarrays 2015, 4, 2–24. [Google Scholar] [CrossRef] [PubMed]
- Lebon, A.; Seyer, D.; Cosette, P.; Coquet, L.; Jouenne, T.; Chan, P.; Leprince, J.; Fournier, A.; Vaudry, H.; Gonzalez, B.J.; et al. Identification of proteins regulated by PACAP in PC12 cells by 2D gel electrophoresis coupled to mass spectrometry. Ann. N. Y. Acad. Sci. 2006, 1070, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Gasperini, L.; Piubelli, C.; Carboni, L. Proteomics of rat hypothalamus, hippocampus and pre-frontal/frontal cortex after central administration of the neuropeptide PACAP. Mol. Biol. Rep. 2012, 39, 2921–2935. [Google Scholar] [CrossRef] [PubMed]
- Maasz, G.; Pirger, Z.; Reglodi, D.; Petrovics, D.; Schmidt, J.; Kiss, P.; Rivnyak, A.; Hashimoto, H.; Avar, P.; Jambor, E.; et al. Comparative protein composition of the brains of PACAP-deficient mice using mass spectrometry-based proteomic analysis. J. Mol. Neurosci. 2014, 54, 310–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maasz, G.; Zrinyi, Z.; Reglodi, D.; Petrovics, D.; Rivnyak, A.; Kiss, T.; Jungling, A.; Tamas, A.; Pirger, Z. Pituitary adenylate cyclase-activating polypeptide (PACAP) has a neuroprotective function in dopamine-based neurodegeneration in rat and snail parkinsonian models. Dis. Model. Mech. 2017, 10, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Llobet, N.; Vidal-Sancho, L.; Masana, M.; Fournier, A.; Albrech, J.; Vaudry, D.; Xifró, X. Pituitary adenylate cyclase-activating polypeptide (PACAP) enhances hippocampal synaptic plasticity and improves memory performance in Huntington’s disease. Mol. Neurobiol. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Nakamachi, T.; Shibato, J.; Rakwal, R.; Tsuchida, M.; Shioda, S.; Numazawa, S. PACAP38 differentially effects genes and CRMP2 protein expression in ischemic core and penumbra regions of permanent middle cerebral artery occlusion model mice brain. Int. J. Mol. Sci. 2014, 15, 17014–17034. [Google Scholar] [CrossRef] [PubMed]
- Fahrenkrug, J.; Hannibal, J.; Honore, B.; Vorum, H. Altered calmodulin response to light in the suprachiasmatic nucleus of PAC1 receptor knockout mice revealed by proteomic analysis. J. Mol. Neurosci. 2005, 25, 251–258. [Google Scholar] [CrossRef]
- Waschek, J.A.; Baca, S.M.; Akerman, S. PACAP and migraine headache: Immunomodulation of neural circuits in autonomic ganglia and brain parenchyma. J. Headache Pain 2018, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Ishido, M.; Masuo, Y. Transcriptome of pituitary adenylate cyclase-activating polypeptide-differentiated PC12 cells. Regul. Pept. 2004, 123, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Nicot, A.; DiCicco-Bloom, E. Regulation of neuroblast mitosis is determined by PACAP receptor isoform expression. Proc. Natl. Acad. Sci. USA 2001, 98, 4758–4763. [Google Scholar] [CrossRef] [PubMed]
- Basille, M.; Gonzalez, B.; Leroux, P.; Jeandel, L.; Fournier, A.; Vaudry, H. Localization and characterization of PACAP receptors in the rat cerebellum during development: Evidence for a stimulatory effect of PACAP on immature cerebellar granule cells. Neuroscience 1993, 57, 329–338. [Google Scholar] [CrossRef]
- Vaudry, D.; Gonzalez, B.; Basille, M.; Pamantung, T.; Fournier, A.; Vaudry, H. PACAP acts as a neurotrophic factor during histogenesis of the rat cerebellar cortex. Ann. N. Y. Acad. Sci. 2000, 921, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Dénes, V.; Czotter, N.; Lakk, M.; Berta, G.; Gábriel, R. PAC1-expressing structures of neural retina alter their PAC1 isoform splicing during postnatal development. Cell Tissue Res. 2014, 355, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Vaczy, A.; Werling, D.; Kiss, P.; Tamas, A.; Kovacs, K.; Fabian, E.; Kvarik, T.; Mammel, B.; Danyadi, B. Protective effects of PACAP in the retina. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer: Cham, Switzerland, 2016; pp. 501–527. [Google Scholar]
- Spengler, D.; Waeber, C.; Pantaloni, C.; Holsboer, F.; Bockaert, J.; Seeburg, P.H.; Journot, L. Differential signal transduction by five splice variants of the PACAP receptor. Nature 1993, 365, 170–175. [Google Scholar] [PubMed]
- Lucero, M.T. Sniffing out a role for PACAP in the olfactory system. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer: Cham, Switzerland, 2016; pp. 483–499. [Google Scholar]
- Yuan, Z.; Guan, Y.; Wang, L.; Wei, W.; Kane, A.B.; Chin, Y.E. Central role of the threonine residue within the P+1 loop of receptor tyrosine kinase in Stat3 constitutive phosphorylation in metastatic cancer cells. Mol. Cell. Biol. 2004, 24, 9390–9400. [Google Scholar] [CrossRef] [PubMed]
- Kisseleva, T.; Bhattacharya, S.; Braunstein, J.; Schindler, C.W. Signaling through the JAK/Stat pathway, recent advances and future challenges. Gene 2002, 285, 1–24. [Google Scholar] [CrossRef]
- Nishimoto, M.; Furuta, A.; Aoki, S.; Kudo, Y.; Miyakawa, H.; Wada, K. PACAP/PAC1 autocrine system promotes proliferation and astrogenesis in neural progenitor cells. Glia 2007, 55, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, N.M.; Sherwood, N.M. PACAP maintains cell cycling and inhibits apoptosis in chick neuroblasts. Mol. Cell. Endocrinol. 2004, 221, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Nicot, A.; Lelievre, V.; Tam, J.; Waschek, J.A.; DiCicco-Bloom, E. Pituitary adenylate cyclase-activating polypeptide and sonic hedgehog interact to control cerebellar granule precursor cell proliferation. J. Neurosci. 2002, 22, 9244–9254. [Google Scholar] [PubMed]
- Yan, Y.; Zhou, X.; Pan, Z.; Ma, J.; Waschek, J.A.; DiCicco-Bloom, E. Pro-and anti-mitogenic actions of pituitary adenylate cyclase-activating polypeptide in developing cerebral cortex: Potential mediation by developmental switch of PAC1 receptor mRNA isoforms. J. Neurosci. 2013, 33, 3865–3878. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.R.; Resnick, D.Z.; Niewiadomski, P.; Dong, H.; Liau, L.M.; Waschek, J.A. Pituitary adenylyl cyclase activating polypeptide inhibits gli1 gene expression and proliferation in primary medulloblastoma derived tumorsphere cultures. BMC Cancer 2010, 10, 676. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszak, J.; Zawilska, J.B. PACAP38 and PACAP6-38 exert cytotoxic activity against human retinoblastoma Y79 cells. J. Mol. Neurosci. 2014, 54, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, S.V.; O’Keeffe, G.W.; Sullivan, A.M. Neurotrophic factors: From neurodevelopmental regulators to novel therapies for Parkinson’s disease. Neural Regen. Res. 2014, 9, 1708–1711. [Google Scholar] [PubMed]
- Harada, T.; Morooka, T.; Ogawa, S.; Nishida, E. ERK induces p35, a neuron-specific activator of Cdk5, through induction of Egr1. Nat. Cell Biol. 2001, 3, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Belluscio, L.; Friedman, B.; Alderson, R.F.; Wiegand, S.J.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron 1990, 5, 501–509. [Google Scholar] [CrossRef]
- Kuroda, M.; Muramatsu, R.; Maedera, N.; Koyama, Y.; Hamaguchi, M.; Fujimura, H.; Yoshida, M.; Konishi, M.; Itoh, N.; Mochizuki, H.; et al. Peripherally derived FGF21 promotes remyelination in the central nervous system. J. Clin. Investig. 2017, 127, 3496–3509. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, Y.; Weiss, S. Insulin-like growth factor-I is a differentiation factor for postmitotic CNS stem cell-derived neuronal precursors: Distinct actions from those of brain-derived neurotrophic factor. J. Neurosci. 1998, 18, 2118–2128. [Google Scholar] [PubMed]
- Bani-Yaghoub, M.; Felker, J.M.; Sans, C.; Naus, C.C. The effects of bone morphogenetic protein 2 and 4 (BMP2 and BMP4) on gap junctions during neurodevelopment. Exp. Neurol. 2000, 162, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Guirland, C.; Buck, K.B.; Gibney, J.A.; DiCicco-Bloom, E.; Zheng, J.Q. Direct cAMP signaling through G-protein-coupled receptors mediates growth cone attraction induced by pituitary adenylate cyclase-activating polypeptide. J. Neurosci. 2003, 23, 2274–2283. [Google Scholar] [PubMed]
- Falluel-Morel, A.; Vaudry, D.; Aubert, N.; Galas, L.; Benard, M.; Basille, M.; Fontaine, M.; Fournier, A.; Vaudry, H.; Gonzalez, B.J. Pituitary adenylate cyclase-activating polypeptide prevents the effects of ceramides on migration, neurite outgrowth, and cytoskeleton remodeling. Proc. Natl. Acad. Sci. USA 2005, 102, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- DiCicco-Bloom, E.; Lu, N.; Pintar, J.E.; Zhang, J. The PACAP ligand/receptor system regulates cerebral cortical neurogenesis. Ann. N. Y. Acad. Sci. 1998, 865, 274–289. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A.; Casillas, R.A.; Nguyen, T.B.; DiCicco-Bloom, E.M.; Carpenter, E.M.; Rodriguez, W.I. Neural tube expression of pituitary adenylate cyclase-activating peptide (PACAP) and receptor: Potential role in patterning and neurogenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 9602–9607. [Google Scholar] [CrossRef] [PubMed]
- Negishi, M.; Oinuma, I.; Katoh, H. Plexins: Axon guidance and signal transduction. Cell. Mol. Life Sci. 2005, 62, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Südhof, T.C. Snares and Munc18 in synaptic vesicle fusion. Nat. Rev. Neurosci. 2002, 3, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, M.; Adolfsen, B.; Galle, K.T.; Littleton, J.T. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science 2005, 310, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.C. The cytoskeleton and neurite initiation. Bioarchitecture 2013, 3, 86–109. [Google Scholar] [CrossRef] [PubMed]
- Plantier, M.; Fattoum, A.; Menn, B.; Ben-Ari, Y.; Der Terrossian, E.; Represa, A. Acidic calponin immunoreactivity in postnatal rat brain and cultures: Subcellular localization in growth cones, under the plasma membrane and along actin and glial filaments. Eur. J. Neurosci. 1999, 11, 2801–2812. [Google Scholar] [CrossRef] [PubMed]
- Giblin, S.P.; Midwood, K.S. Tenascin-C: Form versus function. Cell Adhes. Migr 2015, 9, 48–82. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Murphy-Ullrich, J.E.; Erickson, H.P. Mitogenesis, cell migration, andloss of focal adhesions induced by tenascin-C interacting with its cell surfacereceptor, annexin II. Mol. Biol. Cell 1996, 6, 883–892. [Google Scholar] [CrossRef]
- Galas, L.; Benard, M.; Lebon, A.; Komuro, Y.; Schapman, D.; Vaudry, H.; Vaudry, D.; Komuro, H. Postnatal migration of cerebellar interneurons. Brain Sci. 2017, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Raoult, E.; Benard, M.; Komuro, H.; Lebon, A.; Vivien, D.; Fournier, A.; Vaudry, H.; Vaudry, D.; Galas, L. Cortical-layer-specific effects of PACAP and tPA on interneuron migration during post-natal development of the cerebellum. J. Neurochem. 2014, 130, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Aubert, N.; Basille, M.; Falluel-Morel, A.; Vaudry, D.; Bucharles, C.; Jolivel, V.; Fisch, C.; de Jouffrey, S.; le Bigot, J.F.; Fournier, A.; et al. Molecular, cellular, and functional characterizations of pituitary adenylate cyclase-activating polypeptide and its receptors in the cerebellum of new and old world monkeys. J. Comp. Neurol. 2007, 504, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Chen, X.Q.; Bai, Q.X.; Wang, Z.; Zhang, T.; Yang, L.; Dong, B.X.; Gao, G.X.; Gu, H.T.; Zhu, H.F. Increased 14-3-3ζ expression in the multidrug-resistant leukemia cell line HL-60/VCR as compared to the parental line mediates cell growth and apoptosis in part through modification of gene expression. Acta Haematol. 2014, 132, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Allais, A.; Burel, D.; Roy, V.; Arthaud, S.; Galas, L.; Isaac, E.R.; Desfeux, A.; Parent, B.; Fournier, A.; Chapillon, P.; et al. Balanced effect of PACAP and FasL on granule cell death during cerebellar development: A morphological, functional and behavioural characterization. J. Neurochem. 2010, 113, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Matsuzaki, S.; Hattori, T.; Kuwahara, R.; Taniguchi, M.; Hashimoto, H.; Shintani, N.; Baba, A.; Kumamoto, N.; Yamada, K. Increased stathmin1 expression in the dentate gyrus of mice causes abnormal axonal arborizations. PLoS ONE 2010, 5, e8596. [Google Scholar] [CrossRef] [PubMed]
- Allais, A.; Burel, D.; Isaac, E.R.; Gray, S.L.; Basille, M.; Ravni, A.; Sherwood, N.M.; Vaudry, H.; Gonzalez, B.J. Altered cerebellar development in mice lacking pituitary adenylate cyclase-activating polypeptide. Eur. J. Neurosci. 2007, 25, 2604–2618. [Google Scholar] [CrossRef] [PubMed]
- Sándor, B.; Fintor, K.; Reglodi, D.; Fulop, D.; Helyes, Z.; Szántó, I.; Nagy, P.; Hashimoto, H.; Tamás, A. Structural and morphometric comparison of lower incisors in PACAP-deficient and wild-type mice. J. Mol. Neurosci. 2016, 59, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Sandor, B.; Tamas, A.; Kiss, P.; Hashimoto, H.; Nagy, A.D.; Fulop, B.D.; Juhasz, T.; Manavalan, S.; Reglodi, D. Early neurobehavioral development of mice lacking endogenous PACAP. J. Mol. Neurosci. 2017, 61, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lelievre, V.; Zhao, P.; Torres, M.; Rodriguez, W.; Byun, J.Y.; Doshi, S.; Ioffe, Y.; Gupta, G.; de los Monteros, A.E.; et al. Pituitary adenylyl cyclase-activating polypeptide stimulates DNA synthesis but delays maturation of oligodendrocyte progenitors. J. Neurosci. 2001, 21, 3849–3859. [Google Scholar] [PubMed]
- Vincze, A.; Reglodi, D.; Helyes, Z.; Hashimoto, H.; Shintani, N.; Abraham, H. Role of endogenous pituitary adenylate cyclase activating polypeptide (PACAP) in myelination of the rodent brain: Lessons from PACAP-deficient mice. Int. J. Dev. Neurosci. 2011, 29, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M. PACAP signaling to dream: A camp-dependent pathway that regulates cortical astrogliogenesis. Mol. Neurobiol. 2009, 39, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vertongen, P.; Camby, I.; Darro, F.; Kiss, R.; Robberecht, P. VIP and pituitary adenylate cyclase activating polypeptide (PACAP) have an antiproliferative effect on the T98G human glioblastoma cell line through interaction with VIP2 receptor. Neuropeptides 1996, 30, 491–496. [Google Scholar] [CrossRef]
- Waschek, J.A. VIP and PACAP: Neuropeptide modulators of CNS inflammation, injury, and repair. Br. J. Pharmacol. 2013, 169, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Cseh, S.; Somoskoi, B.; Fulop, B.D.; Szentleleky, E.; Szegeczki, V.; Kovacs, A.; Varga, A.; Kiss, P.; Hashimoto, H.; et al. Disturbed spermatogenic signaling in pituitary adenylate cyclase activating polypeptide-deficient mice. Reproduction 2018, 155, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Hattori, S.; Takao, K.; Tanda, K.; Toyama, K.; Shintani, N.; Baba, A.; Hashimoto, H.; Miyakawa, T. Comprehensive behavioral analysis of pituitary adenylate cyclase-activating polypeptide (PACAP) knockout mice. Front. Behav. Neurosci. 2012, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Farkas, J.; Kovács, L.Á.; Gáspár, L.; Nafz, A.; Gaszner, T.; Ujvári, B.; Kormos, V.; Csernus, V.; Hashimoto, H.; Reglődi, D. Construct and face validity of a new model for the three-hit theory of depression using PACAP mutant mice on CD1 background. Neuroscience 2017, 354, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, Y.; Hayata-Takano, A.; Hazama, K.; Nakazawa, T.; Shintani, N.; Kasai, A.; Nagayasu, K.; Hashimoto, R.; Tanida, M.; Katayama, T.; et al. Atomoxetine reverses locomotor hyperactivity, impaired novel object recognition, and prepulse inhibition impairment in mice lacking pituitary adenylate cyclase-activating polypeptide. Neuroscience 2015, 297, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kormos, V.; Gaspar, L.; Kovacs, L.A.; Farkas, J.; Gaszner, T.; Csernus, V.; Balogh, A.; Hashimoto, H.; Reglodi, D.; Helyes, Z. Reduced response to chronic mild stress in PACAP mutant mice is associated with blunted FosB expression in limbic forebrain and brainstem centers. Neuroscience 2016, 330, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Shintani, N.; Tanaka, K.; Mori, W.; Hirose, M.; Matsuda, T.; Sakaue, M.; Miyazaki, J.-I.; Niwa, H.; Tashiro, F. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP). Proc. Natl. Acad. Sci. USA 2001, 98, 13355–13360. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Maeda, Y.; Ago, Y.; Ishihama, T.; Takemoto, K.; Nakagawa, A.; Shintani, N.; Hashimoto, H.; Baba, A.; Matsuda, T. An enriched environment ameliorates memory impairments in PACAP-deficient mice. Behav. Brain Res. 2014, 272, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Basille, M.; Pamantung, T.F.; Fontaine, M.; Fournier, A.; Vaudry, H.; Gonzalez, B.J. Pituitary adenylate cyclase-activating polypeptide prevents c2-ceramide-induced apoptosis of cerebellar granule cells. J. Neurosci. Res. 2003, 72, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Takei, N.; Skoglösa, Y.; Lindholm, D. Neurotrophic and neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on mesencephalic dopaminergic neurons. J. Neurosci. Res. 1998, 54, 698–706. [Google Scholar] [CrossRef]
- Rozzi, S.J.; Borelli, G.; Ryan, K.; Steiner, J.P.; Reglodi, D.; Mocchetti, I.; Avdoshina, V. PACAP27 is protective against tat-induced neurotoxicity. J. Mol. Neurosci. 2014, 54, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aubert, N.; Vaudry, D.; Falluel-Morel, A.; Desfeux, A.; Fisch, C.; Ancian, P.; de Jouffrey, S.; Le Bigot, J.-F.; Couvineau, A.; Laburthe, M. PACAP prevents toxicity induced by cisplatin in rat and primate neurons but not in proliferating ovary cells: Involvement of the mitochondrial apoptotic pathway. Neurobiol. Dis. 2008, 32, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Babai, N.; Koszegi, Z.; Tamas, A.; Reglodi, D.; Gabriel, R. PACAP-mediated neuroprotection of neurochemically identified cell types in MSG-induced retinal degeneration. J. Mol. Neurosci. 2008, 36, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Tang, Z.; Yin, J.; Maalouf, M.; Beach, T.G.; Reiman, E.M.; Shi, J. Pituitary adenylate cyclase-activating polypeptide protects against β-amyloid toxicity. Neurobiol. Aging 2014, 35, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, H.; Nakamachi, T.; Dohi, K.; Shioda, S. Role of PACAP in ischemic neural death. J. Mol. Neurosci. 2008, 36, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, B.; Abad, C.; Chhith, S.; Cheung-Lau, G.; Hajji, O.; Nobuta, H.; Waschek, J. Impaired nerve regeneration and enhanced neuroinflammatory response in mice lacking pituitary adenylyl cyclase activating peptide. Neuroscience 2008, 151, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.B.; Nobuta, H.; Abad, C.; Lee, S.K.; Bala, N.; Zhu, C.; Richter, F.; Chesselet, M.-F.; Waschek, J.A. PACAP deficiency sensitizes nigrostriatal dopaminergic neurons to paraquat-induced damage and modulates central and peripheral inflammatory activation in mice. Neuroscience 2013, 240, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Arimura, A.; Somogyvari-Vigh, A.; Shioda, S.; Banks, W.A. Prevention of ischemia-induced death of hippocampal neurons by pituitary adenylate cyclase activating polypeptide. Brain Res. 1996, 736, 280–286. [Google Scholar] [CrossRef]
- Reglodi, D.; Somogyvari-Vigh, A.; Vigh, S.; Kozicz, T.; Arimura, A. Delayed systemic administration of PACAP38 is neuroprotective in transient middle cerebral artery occlusion in the rat. Stroke 2000, 31, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Tamas, A.; Somogyvari-Vigh, A.; Szanto, Z.; Kertes, E.; Lenard, L.; Arimura, A.; Lengvari, I. Effects of pretreatment with PACAP on the infarct size and functional outcome in rat permanent focal cerebral ischemia. Peptides 2002, 23, 2227–2234. [Google Scholar] [CrossRef]
- Lamine, A.; Letourneau, M.; Doan, N.D.; Maucotel, J.; Couvineau, A.; Vaudry, H.; Chatenet, D.; Vaudry, D.; Fournier, A. Characterizations of a synthetic pituitary adenylate cyclase-activating polypeptide analog displaying potent neuroprotective activity and reduced in vivo cardiovascular side effects in a Parkinson’s disease model. Neuropharmacology 2016, 108, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Tamas, A.; Lubics, A.; Lengvari, I.; Reglodi, D. Protective effects of PACAP in excitotoxic striatal lesion. Ann. N. Y. Acad. Sci. 2006, 1070, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Polanco, M.J.; Parodi, S.; Piol, D.; Stack, C.; Chivet, M.; Contestabile, A.; Miranda, H.C.; Lievens, P.M.; Espinoza, S.; Jochum, T.; et al. Adenylyl cyclase activating polypeptide reduces phosphorylation and toxicity of the polyglutamine-expanded androgen receptor in spinobulbar muscular atrophy. Sci. Transl. Med. 2016, 8, 370ra181. [Google Scholar] [CrossRef] [PubMed]
- Kovesdi, E.; Tamas, A.; Reglodi, D.; Farkas, O.; Pal, J.; Toth, G.; Bukovics, P.; Doczi, T.; Buki, A. Posttraumatic administration of pituitary adenylate cyclase activating polypeptide in central fluid percussion injury in rats. Neurotox. Res. 2008, 13, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Nakamachi, T.; Rakwal, R.; Shibato, J.; Ogawa, T.; Aiuchi, T.; Tsuruyama, T.; Tamaki, K.; Shioda, S. Transcriptomics and proteomics analyses of the PACAP38 influenced ischemic brain in permanent middle cerebral artery occlusion model mice. J. Neuroinflamm. 2012, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Samal, B.; Hamelink, C.R.; Xiang, C.C.; Chen, Y.; Chen, M.; Vaudry, D.; Brownstein, M.J.; Hallenbeck, J.M.; Eiden, L.E. Neuroprotection by endogenous and exogenous PACAP following stroke. Regul. Pept. 2006, 137, 4–19. [Google Scholar] [CrossRef]
- Araki, T.; Milbrandt, J. Ninjurin, a novel adhesion molecule, is induced by nerve injury and promotes axonal growth. Neuron 1996, 17, 353–361. [Google Scholar] [CrossRef]
- Brifault, C.; Gras, M.; Liot, D.; May, V.; Vaudry, D.; Wurtz, O. Delayed pituitary adenylate cyclase–activating polypeptide delivery after brain stroke improves functional recovery by inducing m2 microglia/macrophage polarization. Stroke 2015, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Rogalla, T.; Ehrnsperger, M.; Preville, X.; Kotlyarov, A.; Lutsch, G.; Ducasse, C.; Paul, C.; Wieske, M.; Arrigo, A.P.; Buchner, J.; et al. Regulation of Hsp27 oligomerization, chaperone function, and protective activity against oxidative stress/tumor necrosis factor alpha by phosphorylation. J. Biol. Chem. 1999, 274, 18947–18956. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Kang, S.W.; Chang, T.S.; Jeong, W.; Kim, K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 2001, 52, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Shendelman, S.; Jonason, A.; Martinat, C.; Leete, T.; Abeliovich, A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004, 11, e362. [Google Scholar]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Brifault, C.; Vaudry, D.; Wurtz, O. The neuropeptide PACAP, a potent disease modifier candidate for brain stroke treatment. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Springer: Cham, Switzerland, 2016; Volume 11, pp. 583–606. [Google Scholar]
- Reglodi, D.; Vaczy, A.; Rubio-Beltran, E.; MaassenVanDenBrink, A. Protective effects of PACAP in ischemia. J. Headache Pain 2018, 19, 19. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivnyak, A.; Kiss, P.; Tamas, A.; Balogh, D.; Reglodi, D. Review on PACAP-Induced Transcriptomic and Proteomic Changes in Neuronal Development and Repair. Int. J. Mol. Sci. 2018, 19, 1020. https://doi.org/10.3390/ijms19041020
Rivnyak A, Kiss P, Tamas A, Balogh D, Reglodi D. Review on PACAP-Induced Transcriptomic and Proteomic Changes in Neuronal Development and Repair. International Journal of Molecular Sciences. 2018; 19(4):1020. https://doi.org/10.3390/ijms19041020
Chicago/Turabian StyleRivnyak, Adam, Peter Kiss, Andrea Tamas, Dorottya Balogh, and Dora Reglodi. 2018. "Review on PACAP-Induced Transcriptomic and Proteomic Changes in Neuronal Development and Repair" International Journal of Molecular Sciences 19, no. 4: 1020. https://doi.org/10.3390/ijms19041020