Calorie Restriction Effect of Heat-Processed Onion Extract (ONI) Using In Vitro and In Vivo Animal Models
Abstract
:1. Introduction
2. Results
2.1. Sample Preparation
2.2. α-Glucosidase Inhibitory Activity
2.3. Blood Glucose Lowering Effect of Onion Extracts In Vivo
2.4. Pharmacodynamics Parameters
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Sample Preparation
4.3. Total Amadori Compounds Analysis
4.4. α-Glucosidase Inhibition Assay
4.5. Sugar Loading Test
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ARCs | Amadori rearrangement compounds |
| SD rat | Sprague Dawley rat |
| MRPs | Maillard reaction products |
| AF | Arginyl-fructose |
| AFG | Arginyl-fructosyl-glucose |
| ONI_H | Heat-treated onion extract containing high ARCs |
| ONI_L | Heat-treated onion extract containing low ARCs |
References
- Kim, S.H.; Jo, S.H.; Kwon, Y.I.; Hwang, J.K. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int. J. Mol. Sci. 2011, 12, 3757–3769. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.; Singh, S.; Kochhar, A. Therapeutic potential of antidiabetic neutraceuticals. Phytopharmacol 2012, 2, 144–169. [Google Scholar]
- Kwon, Y.I.; Apostolidis, E.; Kim, Y.C.; Shetty, K. Health benefits of traditional corn, beans, and pumpkin: In vitro studies for hyperglycemia and hypertension management. J. Med. Food 2007, 10, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Vattem, D.A.; Shetty, K. Evaluation of clonal herbs of Lamiaceae species for management of diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 107–118. [Google Scholar] [PubMed]
- Rasheed, D.; Porzel, A.; Frolov, A.; El Saedi, H.R.; Wessjohann, L.A.; Farag, M.A. Comparative analysis of Hibiscus sabdariffa (roselle) hot and cold extracts in respect to their potential for α-glucosidase inhibition. Food Chem. 2018, 250, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Vessal, M.; Hemmati, M.; Vasei, M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 135, 357–364. [Google Scholar] [CrossRef]
- Coskun, O.; Kanter, M.; Korkmaz, A.; Oter, S. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and β-cell damage in rat pancreas. Pharmacol. Res. 2005, 51, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Kumari, K.; Augusti, K.T. Antidiabetic and antioxidant effects of S-methyl cysteine sulfoxide isolated from onions (Allium cepa Linn) as compared to standard drugs in alloxan diabetic rats. Indian J. Exp. Biol. 2002, 40, 1005–1009. [Google Scholar] [PubMed]
- Kumari, K.; Augusti, K.T. Lipid lowering effect of S-methyl cysteine sulfoxide from Allium cepa Linn in high cholesterol diet fed rats. J. Ethnopharmacol. 2007, 109, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Breene, W.M. Healthfulness and nutritional quality of fresh versus processed fruits and vegetables: A review. J. Foodserv. 1994, 8, 1–45. [Google Scholar] [CrossRef]
- Lombard, K.; Peffley, E.; Geoffriau, E.; Thompson, L.; Herring, A. Quercetin in onion (Allium cepa L.) after heat-treatment simulating home preparation. J. Food Compost. Anal. 2005, 18, 571–581. [Google Scholar] [CrossRef]
- Moreno, F.J.; Corzo-Martı, M.; Del Castillo, M.D.; Villamiel, M. Changes in antioxidant activity of dehydrated onion and garlic during storage. Food Res. Int. 2006, 39, 891–897. [Google Scholar] [CrossRef]
- Woo, K.S.; Hwang, I.G.; Kim, T.M.; Kim, D.J.; Hong, J.T.; Jeong, H.S. Changes in the antioxidant activity of onion (Allium cepa) extracts with heat treatment. Food Sci. Biotechnol. 2007, 16, 828–831. [Google Scholar]
- Hwang, I.G.; Kim, H.Y.; Lee, S.H.; Hwang, C.R.; Oh, S.H.; Woo, K.S.; Kim, D.J.; Lee, J.S.; Jeong, H.S. Isolation and identification of an antioxidant substance from heated onion (Allium cepa L.). J. Korean Soc. Food Sci. Nutr. 2011, 40, 470–474. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, G.N.; Lee, S.H.; Kim, E.S.; Ha, K.S.; Kwon, Y.I.; Jeong, H.S.; Jang, H.D. In vitro and cellular antioxidant activity of arginyl-fructose and arginyl-fructosyl-glucose. Food Sci. Biotechnol. 2009, 18, 1505–1510. [Google Scholar]
- Ha, K.S.; Jo, S.H.; Kang, B.H.; Apostolidis, E.; Lee, M.S.; Jang, H.D.; Kwon, Y.I. In Vitro and In Vivo Antihyperglycemic Effect of 2 Amadori Rearrangement Compounds, Arginyl-Fructose and Arginyl-Fructosyl-Glucose. J. Food Sci. 2011, 76, H188–H193. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-H.; Ha, K.S.; Jo, S.H.; Lee, C.-M.; Kim, Y.-C.; Chung, K.-H.; Kwon, Y.-I. Effect of long-term dietary arginyl-fructose (AF) on hyperglycemia and HbA1c in diabetic db/db mice. Int. J. Mol. Sci. 2014, 15, 8352–8359. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Kin, O.-H.; Kwak, J.H.; Lee, K.H.; Kwon, Y.-I.; Chung, K.H.; Lee, J.H. Antihyperglycemic effect of short-term arginyl-fructose supplementation in subjects with prediabetes and newly diagnosed type 2 diabetes: Randomized, double-blinded, placebo-controlled trial. Trials 2015, 16, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Yukinaga, M.; Yinan, Z.; Takeshi, T.; Kenji, K.; Hiromichi, O. Isolation and Physiological Activites of a New Amino Acid Derivative from Korean Red Ginseng. J. Ginseng Res. 1994, 18, 204–211. [Google Scholar]
- Takaku, T.; Han, L.K.; Kameda, K.; Ninomiya, H.; Okuda, H. Production of arginyl-fructosyl-glucose during processing of red ginseng. J. Tradit. Med. 1996, 13, 118–123. [Google Scholar]
- Suzuki, Y.; Choi, K.J.; Uchida, K.; Ko, S.R.; Sohn, H.J.; Park, J.D. Arginyl-fructosyl-glucose and arginyl-fructose, compounds related to browning reaction in the model system of steaming and heat-drying processes for the preparation of red ginseng. J. Ginseng Res. 2004, 28, 143–148. [Google Scholar]
- Jariyapamornkoon, N.; Yibchok-anun, S.; Adisakwattana, S. Inhibition of advanced glycation end products by red grape skin extract and its antioxidant activity. BMC Complement. Altern. Med. 2013, 13, 171. [Google Scholar] [CrossRef] [PubMed]
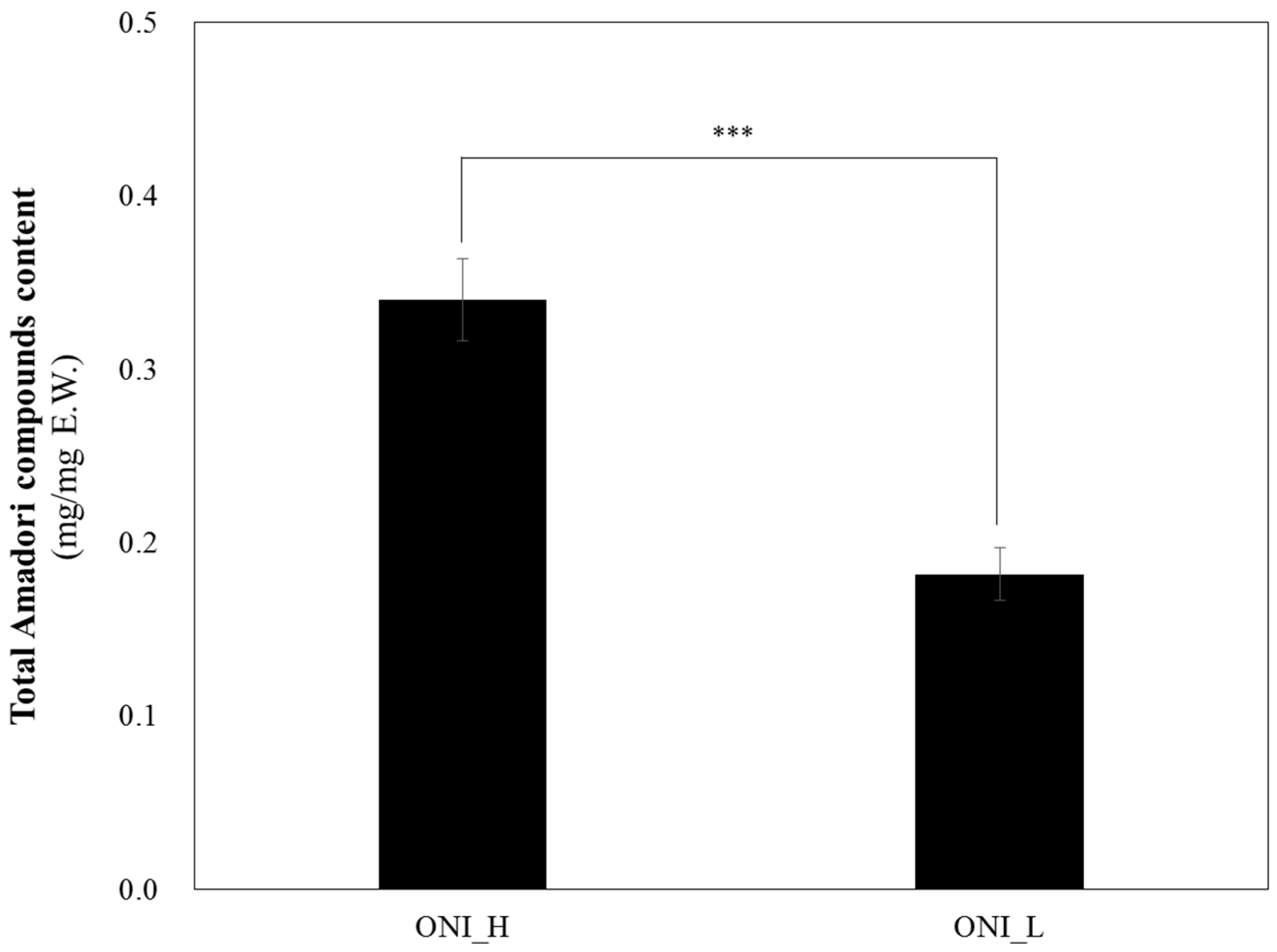




| Samples | pH | Brix | AF (%) | Arg (%) | Glu (%) | Fru (%) | Que (ug/g) | Total 1 (%) |
|---|---|---|---|---|---|---|---|---|
| ONI_H | 6.97 | 30.1 | 13.3 | 8.56 | 6.08 | 10.08 | 200 | 38.02 |
| ONI_L | 7.59 | 28.3 | 5.55 | 9.46 | 6.86 | 6.99 | 103 | 28.90 |
| IC50 (mg/mL) | ||
|---|---|---|
| Enzymes | ONI_H | ONI_L |
| Sucrase | 0.34 ± 0.03 | ND 1 |
| α-glucosidase | 5.87 ± 0.60 | >12.59 ± 0.27 |
| Groups | PD Parameters | ||
|---|---|---|---|
| Cmax (mg/dL) | Tmax (h) | AUCt (h∙mg/dL) | |
| Sucrose 2.0 g/kg | 188.60 ± 5.37 a | 0.50 ± 0.00 b | 442.22 ± 18.45 a |
| Acarbose 5.0 mg/kg | 129.47 ± 15.84 c | 1.10 ± 0.55 a | 353.65 ± 34.41 b |
| ONI_H 0.5 g/kg | 172.27 ± 3.96 b | 0.50 ± 0.00 b | 418.11 ± 13.83 a |
| ONI_L 0.5 g/kg | 187.00 ± 1.90 a | 0.50 ± 0.00 b | 422.33 ± 14.38 a |
| Starch 2.0 g/kg | 204.04 ± 8.73 a | 0.75 ± 0.29 a | 451.90 ± 3.94 a |
| Acarbose 5.0 mg/kg | 133.43 ± 10.28 c | 1.00 ± 0.61 a | 350.48 ± 19.40 c |
| ONI_H 0.5 g/kg | 175.13 ± 14.09 b | 0.50 ± 0.00 a | 406.69 ± 22.62 b |
| ONI_L 0.5 g/kg | 193.77 ± 11.48 a | 0.50 ± 0.00 a | 434.95 ± 19.47 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y.-R.; Choi, H.-Y.; Lee, J.-Y.; Jang, S.I.; Kang, H.; Oh, J.-B.; Jang, H.-D.; Kwon, Y.-I. Calorie Restriction Effect of Heat-Processed Onion Extract (ONI) Using In Vitro and In Vivo Animal Models. Int. J. Mol. Sci. 2018, 19, 874. https://doi.org/10.3390/ijms19030874
Kang Y-R, Choi H-Y, Lee J-Y, Jang SI, Kang H, Oh J-B, Jang H-D, Kwon Y-I. Calorie Restriction Effect of Heat-Processed Onion Extract (ONI) Using In Vitro and In Vivo Animal Models. International Journal of Molecular Sciences. 2018; 19(3):874. https://doi.org/10.3390/ijms19030874
Chicago/Turabian StyleKang, Yu-Ri, Hwang-Yong Choi, Jung-Yun Lee, Soo In Jang, Hanna Kang, Jung-Bae Oh, Hae-Dong Jang, and Young-In Kwon. 2018. "Calorie Restriction Effect of Heat-Processed Onion Extract (ONI) Using In Vitro and In Vivo Animal Models" International Journal of Molecular Sciences 19, no. 3: 874. https://doi.org/10.3390/ijms19030874





