Presence of TERT Promoter Mutations is a Secondary Event and Associates with Elongated Telomere Length in Myxoid Liposarcomas
Abstract
:1. Introduction
2. Results
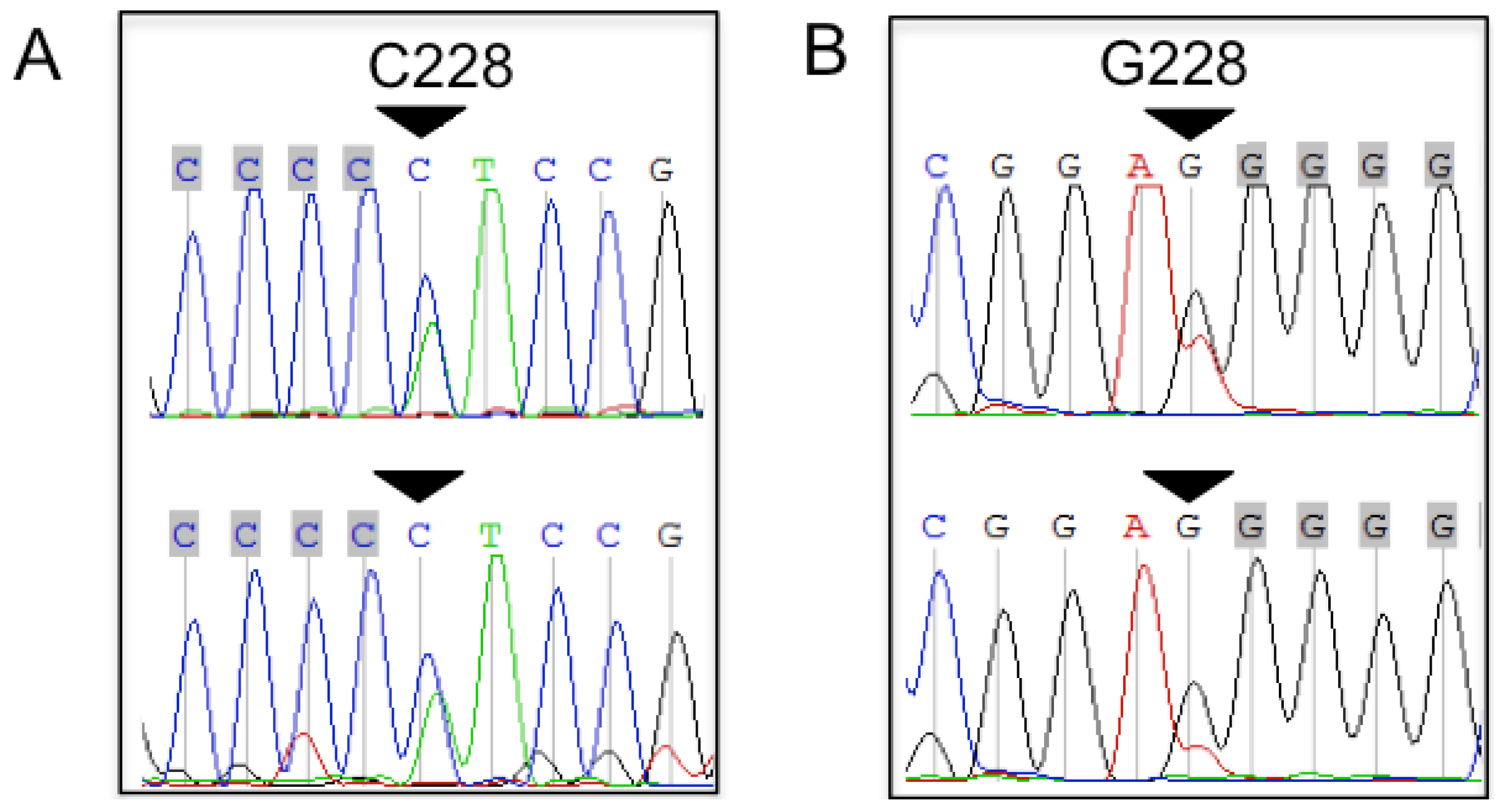
2.1. TERT Promoter Mutations in Bone and Soft Tissue Sarcomas (STS)
2.2. Tumor Grading Correlates with the Presence of TERT Promoter Mutations
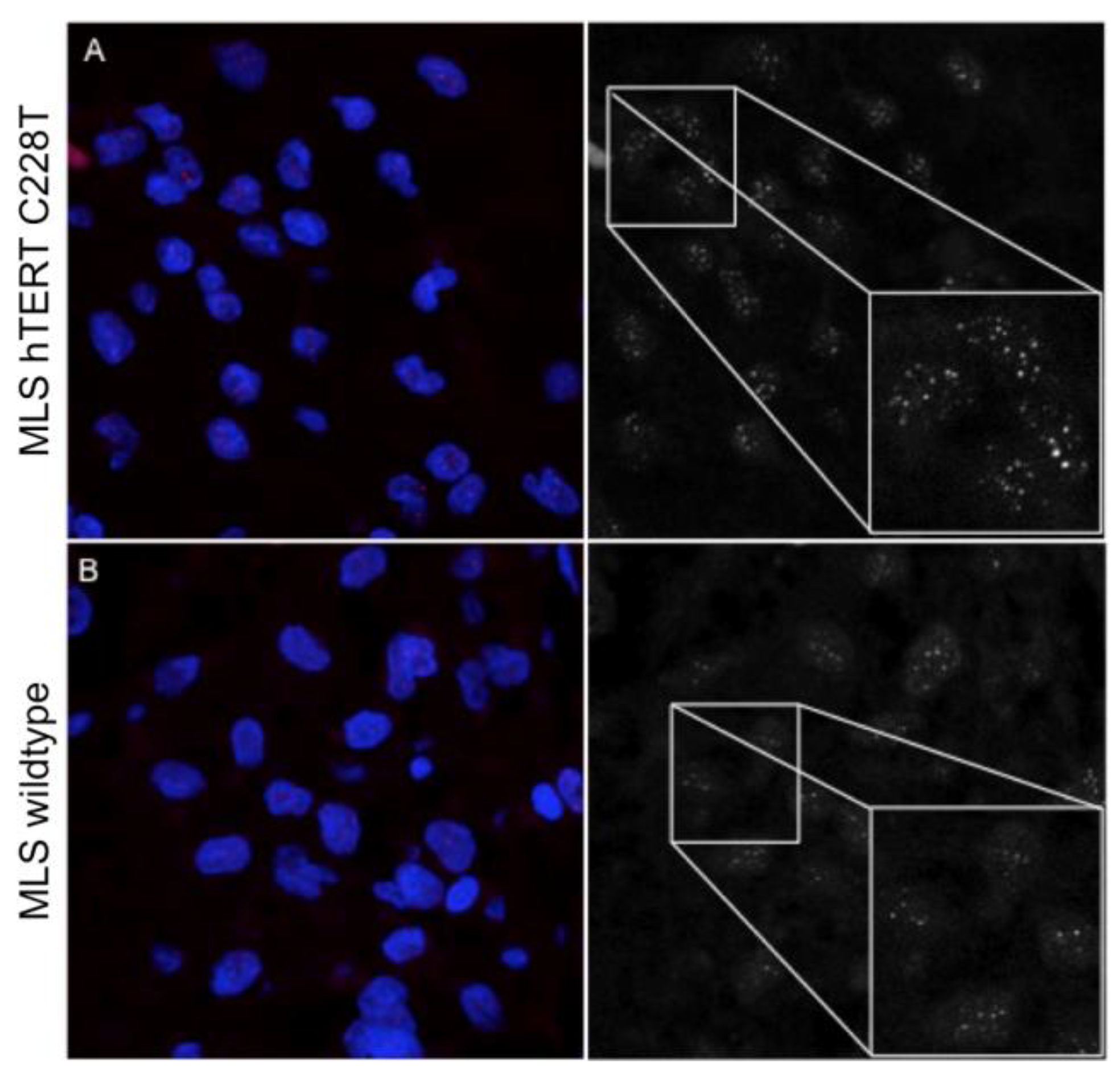
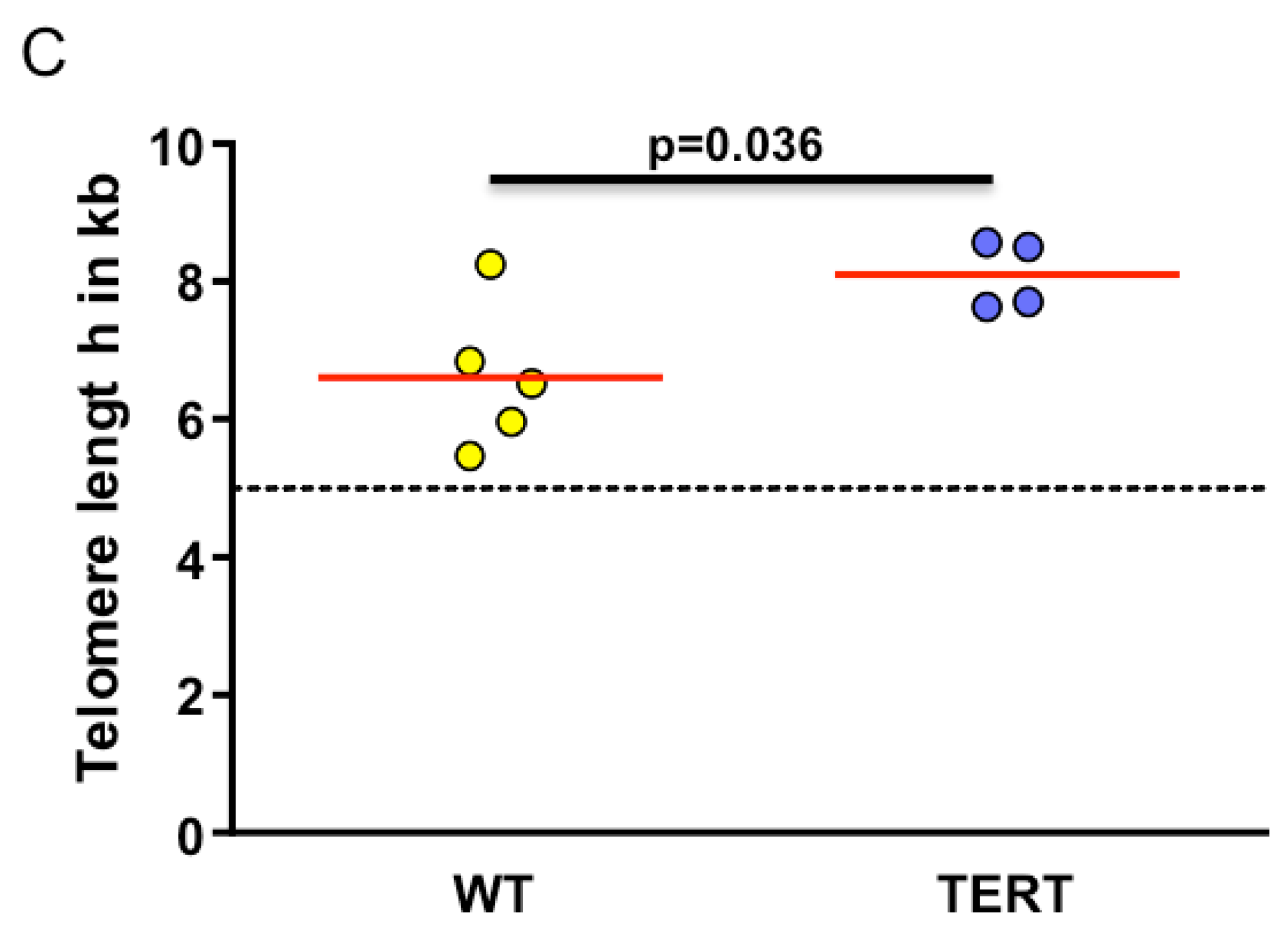
2.3. Myxoid Liposarcomas (MLSs) Exhibit Longer Telomeres than Wildtype Tumors
3. Discussion
4. Material and Methods
4.1. Sarcoma Samples
4.2. Polymerase Chain Reaction (PCR) Amplification and Sanger Sequencing
4.3. Telomere Length Analysis by Quantitative In Situ Hybridization (Q-FISH)
4.4. Alternative Lengthening of Telomeres (ALT) Assessment
4.5. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De Lange, T. Protection of mammalian telomeres. Oncogene 2002, 21, 532–540. [Google Scholar] [PubMed]
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Morales, C.P.; Holt, S.E.; Ouellette, M.; Kaur, K.J.; Yan, Y.; Wilson, K.S.; White, M.A.; Wright, W.E.; Shay, J.W. Absence of cancer-associated changes in human fibroblasts immortalized with telomerase. Nat. Genet. 1999, 21, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, P.; Schrezenmeier, H.; Akkad, J.; Brassat, U.; Vankann, L.; Panse, J.; Wilop, S.; Balabanov, S.; Schwarz, K.; Martens, U.M.; et al. Telomere elongation and clinical response to androgen treatment in a patient with aplastic anemia and a heterozygous htert gene mutation. Ann. Hematol. 2012, 91, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Bryan, T.M.; Englezou, A.; Dalla-Pozza, L.; Dunham, M.A.; Reddel, R.R. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat. Med. 1997, 3, 1271–1274. [Google Scholar] [PubMed]
- Ropio, J.; Merlio, J.P.; Soares, P.; Chevret, E. Telomerase activation in hematological malignancies. Genes 2016, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. Tert promoter mutations in familial and sporadic melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly recurrent tert promoter mutations in human melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.; Almeida, A.; Populo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of tert promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Killela, P.J.; Reitman, Z.J.; Jiao, Y.; Bettegowda, C.; Agrawal, N.; Diaz, L.A., Jr.; Friedman, A.H.; Friedman, H.; Gallia, G.L.; Giovanella, B.C.; et al. Tert promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. USA 2013, 110, 6021–6026. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, B.; Rachakonda, P.S.; Hemminki, K.; Kumar, R. Tert promoter mutations in cancer development. Curr. Opin. Genet. Dev. 2014, 24, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yuan, X.; Xu, D. Cancer-specific telomerase reverse transcriptase (tert) promoter mutations: Biological and clinical implications. Genes 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.J.; Rube, H.T.; Xavier-Magalhaes, A.; Costa, B.M.; Mancini, A.; Song, J.S.; Costello, J.F. Understanding tert promoter mutations: A common path to immortality. Mol. Cancer Res. MCR 2016, 14, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Chibon, F.; Lagarde, P.; Salas, S.; Perot, G.; Brouste, V.; Tirode, F.; Lucchesi, C.; de Reynies, A.; Kauffmann, A.; Bui, B.; et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat. Med. 2010, 16, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Rieker, R.J.; Weitz, J.; Lehner, B.; Egerer, G.; Mueller, A.; Kasper, B.; Schirmacher, P.; Joos, S.; Mechtersheimer, G. Genomic profiling reveals subsets of dedifferentiated liposarcoma to follow separate molecular pathways. Virchows Arch. Int. J. Pathol. 2010, 456, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Segal, N.H.; Pavlidis, P.; Antonescu, C.R.; Maki, R.G.; Noble, W.S.; DeSantis, D.; Woodruff, J.M.; Lewis, J.J.; Brennan, M.F.; Houghton, A.N.; et al. Classification and subtype prediction of adult soft tissue sarcoma by functional genomics. Am. J. Pathol. 2003, 163, 691–700. [Google Scholar] [CrossRef]
- De Alava, E. Molecular pathology in sarcomas. Clin. Transl. Oncol. 2007, 9, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Habener, J.F. Chop, a novel developmentally regulated nuclear protein that dimerizes with transcription factors c/ebp and lap and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992, 6, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Fornace, A.J., Jr.; Nebert, D.W.; Hollander, M.C.; Luethy, J.D.; Papathanasiou, M.; Fargnoli, J.; Holbrook, N.J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol. Cell. Biol. 1989, 9, 4196–4203. [Google Scholar] [CrossRef] [PubMed]
- Goransson, M.; Andersson, M.K.; Forni, C.; Stahlberg, A.; Andersson, C.; Olofsson, A.; Mantovani, R.; Aman, P. The myxoid liposarcoma fus-ddit3 fusion oncoprotein deregulates nf-kappab target genes by interaction with nfkbiz. Oncogene 2009, 28, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Riggi, N.; Cironi, L.; Provero, P.; Suva, M.L.; Stehle, J.C.; Baumer, K.; Guillou, L.; Stamenkovic, I. Expression of the fus-chop fusion protein in primary mesenchymal progenitor cells gives rise to a model of myxoid liposarcoma. Cancer Res. 2006, 66, 7016–7023. [Google Scholar] [CrossRef] [PubMed]
- Perez-Losada, J.; Pintado, B.; Gutierrez-Adan, A.; Flores, T.; Banares-Gonzalez, B.; del Campo, J.C.; Martin-Martin, J.F.; Battaner, E.; Sanchez-Garcia, I. The chimeric fus/tls-chop fusion protein specifically induces liposarcomas in transgenic mice. Oncogene 2000, 19, 2413–2422. [Google Scholar] [CrossRef] [PubMed]
- Perez-Losada, J.; Sanchez-Martin, M.; Rodriguez-Garcia, M.A.; Perez-Mancera, P.A.; Pintado, B.; Flores, T.; Battaner, E.; Sanchez-Garcia, I. Liposarcoma initiated by fus/tls-chop: The fus/tls domain plays a critical role in the pathogenesis of liposarcoma. Oncogene 2000, 19, 6015–6022. [Google Scholar] [CrossRef] [PubMed]
- Avigad, S.; Naumov, I.; Ohali, A.; Jeison, M.; Berco, G.H.; Mardoukh, J.; Stark, B.; Ash, S.; Cohen, I.J.; Meller, I.; et al. Short telomeres: A novel potential predictor of relapse in ewing sarcoma. Clin. Cancer Res. 2007, 13, 5777–5783. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wu, X.; Wang, S.; Chang, D.; Pollock, R.E.; Lev, D.; Gu, J. Long telomeres in peripheral blood leukocytes are associated with an increased risk of soft tissue sarcoma. Cancer 2013, 119, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.; Argani, P.; Hicks, J.L.; DeMarzo, A.M.; Meeker, A.K. Telomere lengths of translocation-associated and nontranslocation-associated sarcomas differ dramatically. Am. J. Pathol. 2004, 164, 1523–1529. [Google Scholar] [CrossRef]
- Jeyapalan, J.N.; Mendez-Bermudez, A.; Zaffaroni, N.; Dubrova, Y.E.; Royle, N.J. Evidence for alternative lengthening of telomeres in liposarcomas in the absence of alt-associated pml bodies. Int. J. Cancer 2008, 122, 2414–2421. [Google Scholar] [CrossRef] [PubMed]
- Henson, J.D.; Hannay, J.A.; McCarthy, S.W.; Royds, J.A.; Yeager, T.R.; Robinson, R.A.; Wharton, S.B.; Jellinek, D.A.; Arbuckle, S.M.; Yoo, J.; et al. A robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomas. Clin. Cancer Res. 2005, 11, 217–225. [Google Scholar] [PubMed]
- Liau, J.Y.; Tsai, J.H.; Jeng, Y.M.; Lee, J.C.; Hsu, H.H.; Yang, C.Y. Leiomyosarcoma with alternative lengthening of telomeres is associated with aggressive histologic features, loss of atrx expression, and poor clinical outcome. Am. J. Surg. Pathol. 2015, 39, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Jeng, Y.M.; Liau, J.Y.; Tsai, J.H.; Hsu, H.H.; Yang, C.Y. Alternative lengthening of telomeres and loss of atrx are frequent events in pleomorphic and dedifferentiated liposarcomas. Mod. Pathol. 2015, 28, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Daidone, M.G.; Daprai, L.; Villa, R.; Cantu, S.; Pilotti, S.; Mariani, L.; Gronchi, A.; Henson, J.D.; Reddel, R.R.; et al. Telomere maintenance mechanisms in liposarcomas: Association with histologic subtypes and disease progression. Cancer Res. 2006, 66, 8918–8924. [Google Scholar] [CrossRef] [PubMed]
- Gocha, A.R.; Nuovo, G.; Iwenofu, O.H.; Groden, J. Human sarcomas are mosaic for telomerase-dependent and telomerase-independent telomere maintenance mechanisms: Implications for telomere-based therapies. Am. J. Pathol. 2013, 182, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.E.; Varkonyi, R.J.; Schwalm, J.; Cragle, R.; Klein-Szanto, A.; Patchefsky, A.; Cukierman, E.; von Mehren, M.; Broccoli, D. Multiple mechanisms of telomere maintenance exist in liposarcomas. Clin. Cancer Res. 2005, 11, 5347–5355. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Akaike, K.; Kurisaki-Arakawa, A.; Toda-Ishii, M.; Mukaihara, K.; Suehara, Y.; Takagi, T.; Kaneko, K.; Yao, T. Tert promoter mutations are rare in bone and soft tissue sarcomas of japanese patients. Mol. Clin. Oncol. 2016, 4, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Koelsche, C.; Renner, M.; Hartmann, W.; Brandt, R.; Lehner, B.; Waldburger, N.; Alldinger, I.; Schmitt, T.; Egerer, G.; Penzel, R.; et al. Tert promoter hotspot mutations are recurrent in myxoid liposarcomas but rare in other soft tissue sarcoma entities. J. Exp. Clin. Cancer Res. CR 2014, 33, 33. [Google Scholar] [CrossRef] [PubMed]
- Campanella, N.C.; Penna, V.; Abrahao-Machado, L.F.; Cruvinel-Carloni, A.; Ribeiro, G.; Soares, P.; Scapulatempo-Neto, C.; Reis, R.M. Tert promoter mutations in soft tissue sarcomas. Int. J. Biol. Markers 2016, 31, e62–e67. [Google Scholar] [CrossRef] [PubMed]
- Chiba, K.; Johnson, J.Z.; Vogan, J.M.; Wagner, T.; Boyle, J.M.; Hockemeyer, D. Cancer-associated tert promoter mutations abrogate telomerase silencing. eLife 2015, 4, e07918. [Google Scholar] [CrossRef] [PubMed]
- Kinde, I.; Munari, E.; Faraj, S.F.; Hruban, R.H.; Schoenberg, M.; Bivalacqua, T.; Allaf, M.; Springer, S.; Wang, Y.; Diaz, L.A., Jr.; et al. Tert promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res. 2013, 73, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Liu, T.; Sofiadis, A.; Juhlin, C.C.; Zedenius, J.; Hoog, A.; Larsson, C.; Xu, D. Tert promoter mutation as an early genetic event activating telomerase in follicular thyroid adenoma (fta) and atypical fta. Cancer 2014, 120, 2965–2979. [Google Scholar] [CrossRef] [PubMed]
- Hosler, G.A.; Davoli, T.; Mender, I.; Litzner, B.; Choi, J.; Kapur, P.; Shay, J.W.; Wang, R.C. A primary melanoma and its asynchronous metastasis highlight the role of braf, cdkn2a, and tert. J. Cutan. Pathol. 2015, 42, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.; Pincus, L.; Ruben, B.; et al. The genetic evolution of melanoma from precursor lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.A.; Laughlin, T.S.; Rothberg, P.G. Mutations of the tert promoter are common in basal cell carcinoma and squamous cell carcinoma. Mod. Pathol. 2014, 27, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Arita, H.; Narita, Y.; Fukushima, S.; Tateishi, K.; Matsushita, Y.; Yoshida, A.; Miyakita, Y.; Ohno, M.; Collins, V.P.; Kawahara, N.; et al. Upregulating mutations in the tert promoter commonly occur in adult malignant gliomas and are strongly associated with total 1p19q loss. Acta Neuropathol. 2013, 126, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Nault, J.C.; Mallet, M.; Pilati, C.; Calderaro, J.; Bioulac-Sage, P.; Laurent, C.; Laurent, A.; Cherqui, D.; Balabaud, C.; Zucman-Rossi, J. High frequency of telomerase reverse-transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat. Commun. 2013, 4, 2218. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.C.; Renwick, P.J.; Dal Cin, P.; Van den Berghe, H.; Fletcher, C.D. Translocation t(12;16)(q13;p11) in myxoid liposarcoma and round cell liposarcoma: Molecular and cytogenetic analysis. Cancer Res. 1995, 55, 24–27. [Google Scholar] [PubMed]
- Hummel, S.; Ventura Ferreira, M.S.; Heudobler, D.; Huber, E.; Fahrenkamp, D.; Gremse, F.; Schmid, K.; Muller-Newen, G.; Ziegler, P.; Jost, E.; et al. Telomere shortening in enterocytes of patients with uncontrolled acute intestinal graft-versus-host disease. Blood 2015, 126, 2518–2521. [Google Scholar] [CrossRef] [PubMed]
- Ventura Ferreira, M.S.; Crysandt, M.; Ziegler, P.; Hummel, S.; Wilop, S.; Kirschner, M.; Schemionek, M.; Jost, E.; Wagner, W.; Brummendorf, T.H.; et al. Evidence for a pre-existing telomere deficit in non-clonal hematopoietic stem cells in patients with acute myeloid leukemia. Ann. Hematol. 2017, 96, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Collado, M.; Blasco, M.A.; Serrano, M. Cellular senescence in cancer and aging. Cell 2007, 130, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chan, S.S.; Chang, S. Telomere dysfunction and tumour suppression: The senescence connection. Nat. Rev. Cancer 2008, 8, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.R.; Wood, E.; Collins, K. A telomerase component is defective in the human disease dyskeratosis congenita. Nature 1999, 402, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Kabjorn Gustafsson, C.; Stahlberg, A.; Engtrom, K.; Danielsson, A.; Turesson, I.; Aman, P. Cell senescence in myxoid/round cell liposarcoma. Sarcoma 2014, 2014, 208786. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Rubio, R.; Menendez, P. Modeling sarcomagenesis using multipotent mesenchymal stem cells. Cell Res. 2012, 22, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Mohseny, A.B.; Hogendoorn, P.C.; Cleton-Jansen, A.M. Mesenchymal stem cell transformation and sarcoma genesis. Clin. Sarcoma Res. 2013, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Scheel, C.; Schaefer, K.L.; Jauch, A.; Keller, M.; Wai, D.; Brinkschmidt, C.; van Valen, F.; Boecker, W.; Dockhorn-Dworniczak, B.; Poremba, C. Alternative lengthening of telomeres is associated with chromosomal instability in osteosarcomas. Oncogene 2001, 20, 3835–3844. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.S.; Wang, Z.; He, X.J.; Diplas, B.H.; Yang, R.; Killela, P.J.; Meng, Q.; Ye, Z.Y.; Wang, W.; Jiang, X.T.; et al. Recurrent tert promoter mutations identified in a large-scale study of multiple tumour types are associated with increased tert expression and telomerase activation. Eur. J. Cancer 2015, 51, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Borah, S.; Xi, L.; Zaug, A.J.; Powell, N.M.; Dancik, G.M.; Cohen, S.B.; Costello, J.C.; Theodorescu, D.; Cech, T.R. Cancer. Tert promoter mutations and telomerase reactivation in urothelial cancer. Science 2015, 347, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Murali, R.; Puig-Butille, J.A.; Schilling, B.; Livingstone, E.; Potrony, M.; Carrera, C.; Schimming, T.; Moller, I.; Schwamborn, M.; et al. Tert promoter mutation status as an independent prognostic factor in cutaneous melanoma. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Populo, H.; Boaventura, P.; Vinagre, J.; Batista, R.; Mendes, A.; Caldas, R.; Pardal, J.; Azevedo, F.; Honavar, M.; Guimaraes, I.; et al. Tert promoter mutations in skin cancer: The effects of sun exposure and x-irradiation. J. Investig. Dermatol. 2014, 134, 2251–2257. [Google Scholar] [CrossRef] [PubMed]
- Remke, M.; Ramaswamy, V.; Peacock, J.; Shih, D.J.; Koelsche, C.; Northcott, P.A.; Hill, N.; Cavalli, F.M.; Kool, M.; Wang, X.; et al. Tert promoter mutations are highly recurrent in shh subgroup medulloblastoma. Acta Neuropathol. 2013, 126, 917–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachakonda, P.S.; Hosen, I.; de Verdier, P.J.; Fallah, M.; Heidenreich, B.; Ryk, C.; Wiklund, N.P.; Steineck, G.; Schadendorf, D.; Hemminki, K.; et al. Tert promoter mutations in bladder cancer affect patient survival and disease recurrence through modification by a common polymorphism. Proc. Natl. Acad. Sci. USA 2013, 110, 17426–17431. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, P.; Li, C.; Huang, Y.; Li, X.; Wang, Y.; Chen, C.; Lv, Z.; Tang, A.; Sun, X.; et al. Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: A genomic and molecular study. Eur. Urol. 2014, 65, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Dang, S.; Wu, K.; Shao, Y.; Yang, Q.; Ji, M.; Shi, B.; Hou, P. Tert promoter mutations predict worse survival in laryngeal cancer patients. Int. J. Cancer 2014, 135, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. Tert promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, N.; Cao, J.; Sofiadis, A.; Dinets, A.; Zedenius, J.; Larsson, C.; Xu, D. The age- and shorter telomere-dependent tert promoter mutation in follicular thyroid cell-derived carcinomas. Oncogene 2014, 33, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- George, J.R.; Henderson, Y.C.; Williams, M.D.; Roberts, D.B.; Hei, H.; Lai, S.Y.; Clayman, G.L. Association of tert promoter mutation, but not braf mutation, with increased mortality in ptc. J. Clin. Endocrinol. Metab. 2015, 100, E1550–E1559. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, G.; Qu, Y.; Wang, M.; Cui, B.; Ji, M.; Shi, B.; Hou, P. Tert promoter mutations and long telomere length predict poor survival and radiotherapy resistance in gliomas. Oncotarget 2016, 7, 8712–8725. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.K.; Schenone, M.; Ferreira, M.V.; Kramann, R.; Joyce, C.E.; Hartigan, C.; Beier, F.; Brummendorf, T.H.; Germing, U.; Platzbecker, U.; et al. Rps14 haploinsufficiency causes a block in erythroid differentiation mediated by s100a8 and s100a9. Nat. Med. 2016, 22, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.; Beier, F.; Hummel, S.; Balabanov, S.; Lassay, L.; Orlikowsky, T.; Dingli, D.; Brummendorf, T.H.; Traulsen, A. Reconstructing the in vivo dynamics of hematopoietic stem cells from telomere length distributions. eLife 2015, 4, e08687. [Google Scholar] [CrossRef] [PubMed]
- Beier, F.; Masouleh, B.K.; Buesche, G.; Ventura Ferreira, M.S.; Schneider, R.K.; Ziegler, P.; Wilop, S.; Vankann, L.; Gattermann, N.; Platzbecker, U.; et al. Telomere dynamics in patients with del (5q) mds before and under treatment with lenalidomide. Leuk. Res. 2015, 39, 1292–1298. [Google Scholar] [CrossRef] [PubMed]
- Beier, F.; Foronda, M.; Martinez, P.; Blasco, M.A. Conditional trf1 knockout in the hematopoietic compartment leads to bone marrow failure and recapitulates clinical features of dyskeratosis congenita. Blood 2012, 120, 2990–3000. [Google Scholar] [CrossRef] [PubMed]
- Heaphy, C.M.; Subhawong, A.P.; Hong, S.M.; Goggins, M.G.; Montgomery, E.A.; Gabrielson, E.; Netto, G.J.; Epstein, J.I.; Lotan, T.L.; Westra, W.H.; et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am. J. Pathol. 2011, 179, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
| Present Study (n = 116) 22 Subtypes | Saito 2016 (n = 180) 19 Subtypes | Campanella 2016 (n = 68) 15 Subtypes | Koelsche 2014 (n = 341) 16 Subtypes | Killela 2013 (n = 95) 6 Subtypes | |
|---|---|---|---|---|---|
| Soft tissue sarcomas | |||||
| WDLS | 0/5 (0%) | 0/18 (0%) | 0/1 (0%) | NA | NA |
| Myxoid liposarcoma | 4/9 (44%) | 3/13 (23%) | 2/9 (22.2%) | 29/39 (74%) | 19/24 (79.1%) |
| Pleomorphic liposarcoma | 1/4 (25%) | NA | 1/1 (100%) | 0/15 (0%) | NA |
| Dedifferentiated liposarcoma | 0/12 (0%) | NA | 1/1 (100%) | 0/61 (0%) | NA |
| Liposarcoma | 0/7 (0%) | NA | NA | NA | NA |
| Leiomyosarcoma | 0/9 (0%) | 0/24 (0%) | 0/13 (0%) | 0/27 (0%) | NA |
| Cytosarcoma Phyllodes | 0/1 (0%) | NA | NA | NA | NA |
| Angiosarcoma | 0/3 (0%) | NA | NA | 0/9 (0%) | NA |
| Rabdomyosarcoma | 0/8 (0%) | 0/5 (0%) | NA | NA | NA |
| Haemangiosarcoma | 0/1 (0%) | NA | NA | NA | NA |
| MPNST | 0/8 (0%) | 0/1 (0%) | 0/7 (0%) | 2/35 (6%) | NA |
| UPS (previous MFH) | 2/9 (22.2%) | 1/22 (4.5%) | 0/12 (0%) | 0/40 (0%) | NA |
| Fibrosarcoma | NA | NA | 0/1 (0%) | NA | 1/3 (33.3%) |
| Myofibroblastic sarcoma | 0/3 (0%) | NA | 0/3 (0%) | NA | NA |
| Myxofibrosarcoma | 0/1 (0%) | 0/6 (0%) | 0/5 (0%) | 0/17 (0%) | 1/10 (10%) |
| NOS | 0/11 (0%) | NA | NA | NA | NA |
| NOS, poorly differentiated | 1/8 (12.5%) | NA | NA | NA | NA |
| Clear cell sarcoma | 0/1 (0%) | 0/1 (0%) | 0/1 (0%) | 0/5 (0%) | NA |
| Synovial sarcoma | 0/9 (0%) | 0/7 (0%) | 0/9 (0%) | 1/25 (4%) | NA |
| Ewing sarcoma | 0/1 (0%) | 0/6 (0%) | NA | NA | NA |
| SFT | 1/1 (100%) | 5/40 (12.5%) | 0/1 (0%) | 4/31 (13%) | 2/10 (20%) |
| Bone sarcomas | |||||
| Chondrosarcoma | 2/8 (25%) | 0/10 (0%) | NA | 0/8 (0%) | 1/2 (50%) |
| Osteosarcoma | 0/3 (0%) | 0/14 (0%) | NA | NA | 1/23 (4.3%) |
| Overall | 11/116 (9.5%) | 9/167 (5.4%) | 4/68 (5.9%) | 36/341 (10.5%) | 25/72 (34.7%) |
| Characteristics (n = 116) | WT (n) | TERTp (n) |
|---|---|---|
| Age (in years) | ||
| ≤51 | 39 | 4 |
| >51 | 66 | 7 |
| Gender | ||
| Male | 57 | 7 |
| Female | 48 | 4 |
| Race | ||
| Caucasian | 99 | 11 |
| Non Caucasian | 6 | 0 |
| Tumor location | ||
| Abdominal | 16 | 0 |
| Arm/forearm | 7 | 0 |
| Cervical | 5 | 0 |
| Femoral | 11 | 3 |
| Head | 3 | 0 |
| Hip | 5 | 1 |
| Jaw | 2 | 1 |
| Knee | 1 | 1 |
| Leg | 4 | 0 |
| Lung | 8 | 1 |
| Retroperitoneal | 18 | 0 |
| Spine | 2 | 1 |
| Testis | 4 | 0 |
| Thigh | 4 | 1 |
| Others | 15 | 2 |
| Grade | ||
| G1/well differentiated | 16 | 5 |
| G2-G3 | 86 | 6 |
| NA | 3 | 0 |
| Disease progression | ||
| No | 53 | 5 |
| Yes | 45 | 3 |
| NA | 7 | 3 |
| Metastasis | ||
| Absent | 55 | 6 |
| Present | 42 | 2 |
| NA | 8 | 3 |
| Diagnosis | Age/Gender | Location | TERTp Mutation | Tumor Grade | Metastasis | Progression |
|---|---|---|---|---|---|---|
| MLS | 61/F | thigh | C228T | G1 | NA | NA |
| MLS | 71/M | femoral | C228T | G1 | yes | yes |
| MLS | 23/M | lung | C228T | G1 | NA | NA |
| MLS | 43/F | spine | C228T | G2 | yes | yes |
| PS | 58/F | femoral | C228T | G2 | no | no |
| MFH | 68/M | skin | C228T | G2 | no | no |
| MFH | 78/M | hip | C228T | G3 | yes | no |
| CS | 37/M | femoral | C228T | G2 | no | no |
| CS | 46/F | jaw | C250T | G1 | no | no |
| SFT | 64/F | NA | C228T | well diff. | yes | yes |
| NOS | 68/M | ear | C228T | G2 | no | no |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ventura Ferreira, M.S.; Crysandt, M.; Braunschweig, T.; Jost, E.; Voss, B.; Bouillon, A.-S.; Knuechel, R.; Brümmendorf, T.H.; Beier, F. Presence of TERT Promoter Mutations is a Secondary Event and Associates with Elongated Telomere Length in Myxoid Liposarcomas. Int. J. Mol. Sci. 2018, 19, 608. https://doi.org/10.3390/ijms19020608
Ventura Ferreira MS, Crysandt M, Braunschweig T, Jost E, Voss B, Bouillon A-S, Knuechel R, Brümmendorf TH, Beier F. Presence of TERT Promoter Mutations is a Secondary Event and Associates with Elongated Telomere Length in Myxoid Liposarcomas. International Journal of Molecular Sciences. 2018; 19(2):608. https://doi.org/10.3390/ijms19020608
Chicago/Turabian StyleVentura Ferreira, Monica S., Martina Crysandt, Till Braunschweig, Edgar Jost, Barbara Voss, Anne-Sophie Bouillon, Ruth Knuechel, Tim H. Brümmendorf, and Fabian Beier. 2018. "Presence of TERT Promoter Mutations is a Secondary Event and Associates with Elongated Telomere Length in Myxoid Liposarcomas" International Journal of Molecular Sciences 19, no. 2: 608. https://doi.org/10.3390/ijms19020608





