Discovery of Cryoprotective Activity in Human Genome-Derived Intrinsically Disordered Proteins
Abstract
1. Introduction
2. Results
2.1. Bioinformatics for Selecting Human Genome-Derived IDPs (Intrinsically Disordered Proteins)
2.2. Disordered State of Predicted IDPs/IDRs
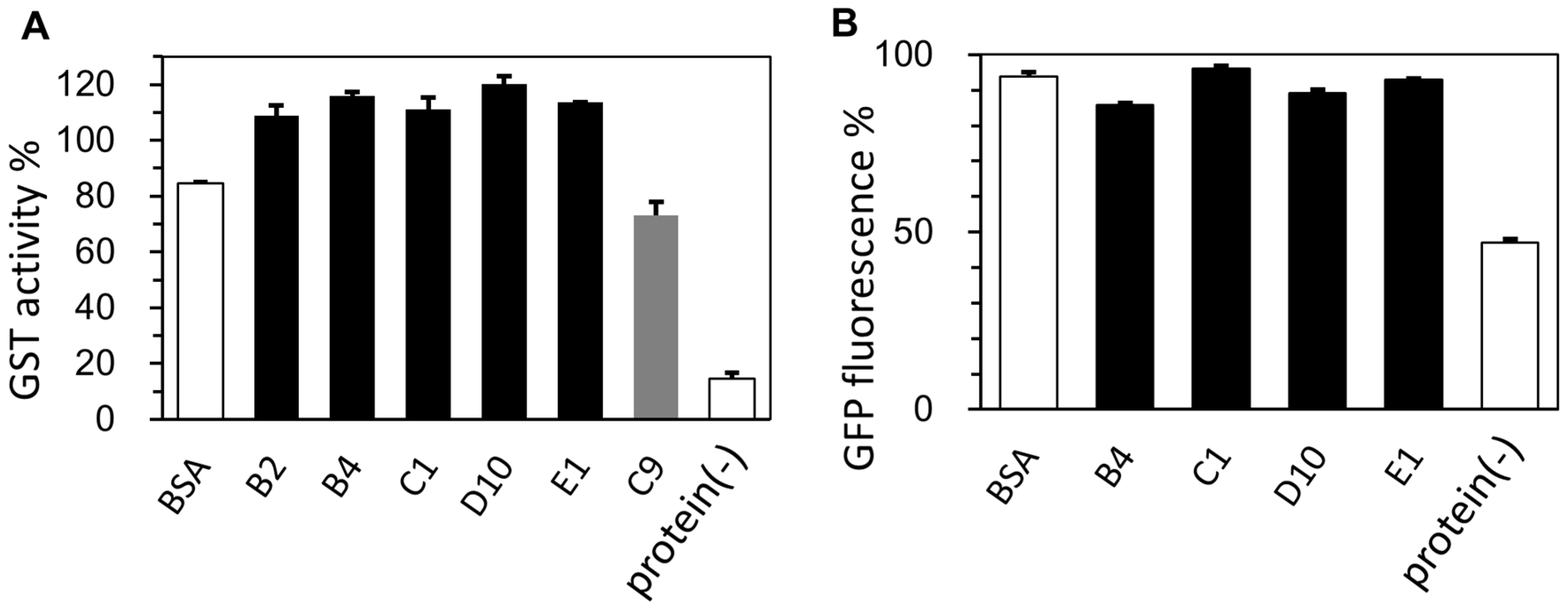
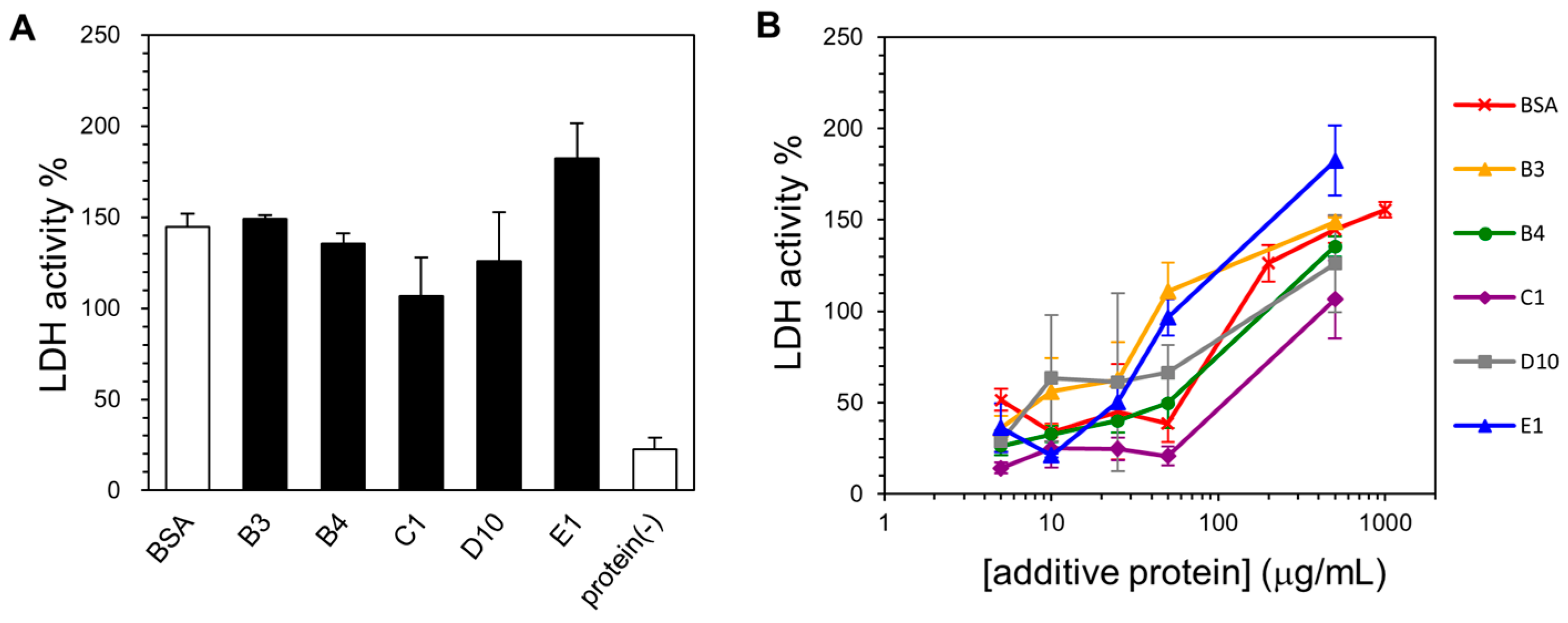
2.3. Cryoprotective Activity of Human Genome-Derived IDPs
2.4. Lyophilization-Protective Activity of Human Genome-Derived IDPs
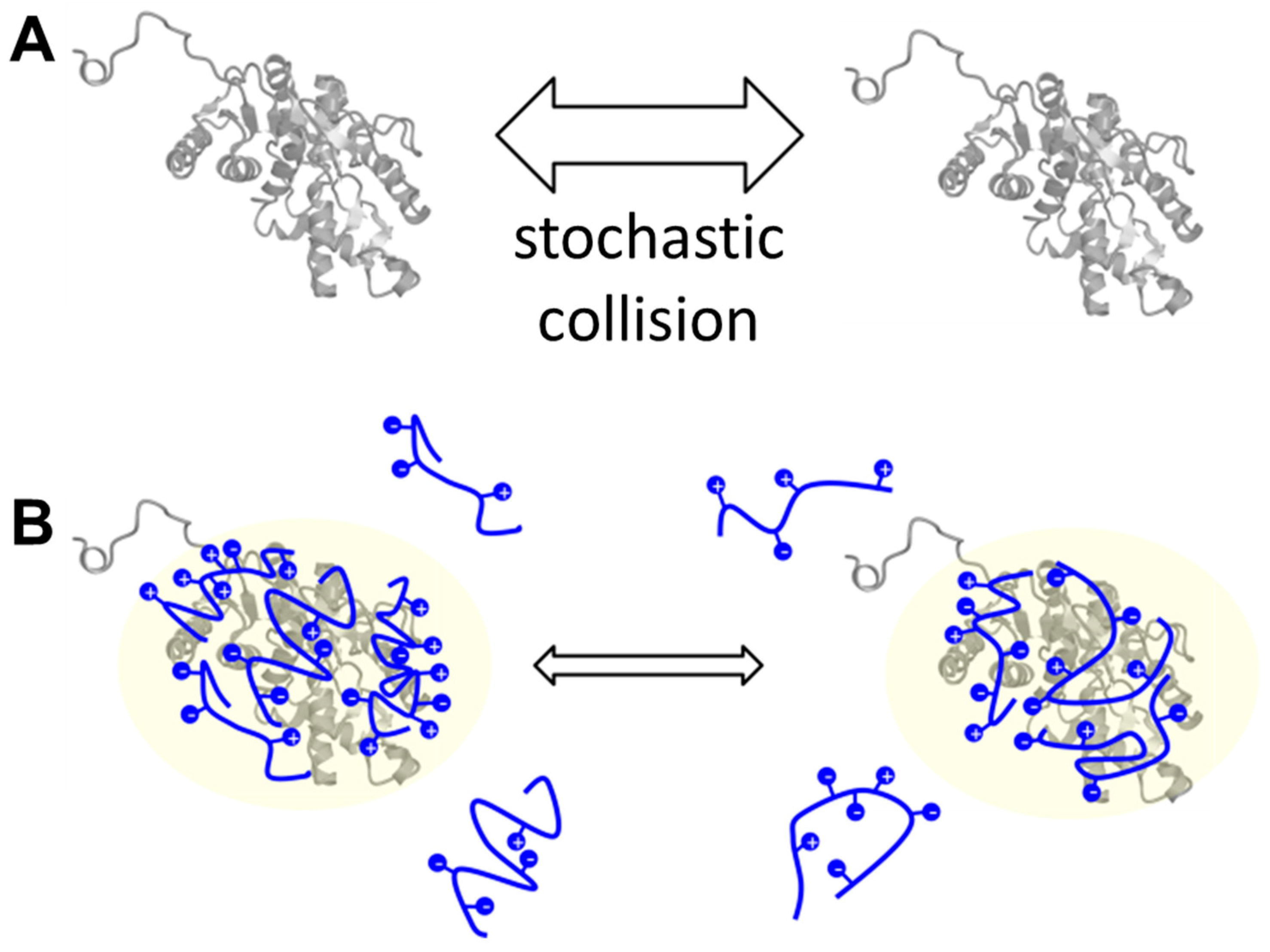
3. Discussion
4. Materials and Methods
4.1. Expression and Preparation of the IDP Samples And Non-IDP Proteins
4.2. Circular Dichroism (CD) Measurements
4.3. Nuclear Magnetic Resonance (NMR) Analysis
4.4. Cryoprotection Assay and Analysis
4.5. Lyophilization Protection Assay and Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wright, P.E.; Dyson, H.J. Intrinsically unstructured proteins: Re-assessing the protein structure-function paradigm. J. Mol. Biol. 1999, 293, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.K.; Lawson, J.D.D.; Brown, C.J.; Williams, R.M.; Romero, P.; Oh, J.S.; Oldfield, C.J.; Campen, A.M.; Ratliff, C.M.; Hipps, K.W.; et al. Intrinsically disordered protein. J. Mol. Graph. Model. 2001, 19, 26–59. [Google Scholar] [CrossRef]
- Dunker, A.K.; Brown, C.J.; Lawson, J.D.; Iakoucheva, L.M.; Obradović, Z. Intrinsic disorder and protein function. Biochemistry 2002, 41, 6573–6582. [Google Scholar] [CrossRef] [PubMed]
- Dyson, H.J.; Wright, P.E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005, 6, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P. The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett. 2005, 579, 3346–3354. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Dunker, A.K. Understanding protein non-folding. Biochim. Biophys. Acta 2010, 1804, 1231–1264. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P.; Fersht, A. Structure and Function of Intrinsically Disordered Proteins; Chapman and Hall/CRC: London, UK, 2009; pp. 1–86. [Google Scholar]
- Garner, E.; Cannon, P.; Romero, P.; Obradovic, Z.; Dunker, A. Predicting Disordered Regions from Amino Acid Sequence: Common Themes Despite Differing Structural Characterization. Genome Inform. Ser. Workshop Genome Inform. 1998, 9, 201–213. [Google Scholar] [PubMed]
- Romero, P.; Obradovic, Z.; Li, X.; Garner, E.C.; Brown, C.J.; Dunker, A.K. Sequence complexity of disordered protein. Proteins 2001, 42, 38–48. [Google Scholar] [CrossRef]
- Oldfield, C.J.; Cheng, Y.; Cortese, M.S.; Brown, C.J.; Uversky, V.N.; Dunker, A.K. Comparing and combining predictors of mostly disordered proteins. Biochemistry 2005, 44, 1989–2000. [Google Scholar] [CrossRef] [PubMed]
- Linding, R.; Jensen, L.J.; Diella, F.; Bork, P.; Gibson, T.J.; Russell, R.B. Protein disorder prediction: Implications for structural proteomics. Structure 2003, 11, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Linding, R.; Russell, R.B.; Neduva, V.; Gibson, T.J. GlobPlot: Exploring protein sequences for globularity and disorder. Nucleic Acids Res. 2003, 31, 3701–3708. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, Z.; Peng, K.; Vucetic, S.; Radivojac, P.; Dunker, A.K. Exploiting heterogeneous sequence properties improves prediction of protein disorder. Proteins 2005, 61, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Ward, J.J. Prediction of disordered regions in proteins from position specific score matrices. Proteins 2003, 53, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Sweredoski, M.J.; Baldi, P. Accurate prediction of protein disordered regions by mining protein structure data. Data Min. Knowl. Discov. 2005, 11, 213–222. [Google Scholar] [CrossRef]
- Peng, K.; Radivojac, P.; Vucetic, S.; Dunker, A.K.; Obradovic, Z. Length-dependent prediction of protein intrinsic disorder. BMC Bioinformatics 2006, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, S.; Homma, K.; Minezaki, Y.; Gojobori, T.; Nishikawa, K. Development of an accurate classification system of proteins into structured and unstructured regions that uncovers novel structural domains: Its application to human transcription factors. BMC Struct. Biol. 2009, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, S.; Hosoda, K.; Homma, K.; Gojobori, T.; Nishikawa, K. Binary classification of protein molecules into intrinsically disordered and ordered segments. BMC Struct. Biol. 2011, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Muraoka, Y.; Hirose, S.; Tomii, K.; Noguchi, T. Predicting mostly disordered proteins by using structure-unknown protein data. BMC Bioinform. 2007, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Shimizu, K.; Kanai, S.; Kuroda, Y.; Noguchi, T. POODLE-L: A two-level SVM prediction system for reliably predicting long disordered regions. Bioinformatics 2007, 23, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Hirose, S.; Shimizu, K.; Noguchi, T. POODLE-I: Disordered region prediction by integrating POODLE series and structural information predictors based on a workflow approach. In Silico Biol. 2010, 10, 185–191. [Google Scholar] [PubMed]
- Dunker, A.K.; Silman, I.; Uversky, V.N.; Sussman, J.L. Function and structure of inherently disordered proteins. Curr. Opin. Struct. Biol. 2008, 18, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Takayama, S.; Campen, A.M.; Vise, P.; Marshall, T.W.; Oldfield, C.J.; Williams, C.J.; Dunker, A.K. Evolutionary rate heterogeneity in proteins with long disordered regions. J. Mol. Evol. 2002, 55, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.J.; Johnson, A.K.; Dunker, A.K.; Daughdrill, G.W. Evolution and disorder. Curr. Opin. Struct. Biol. 2011, 21, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, D.; Kalmar, E.; Torok, Z.; Tompa, P. Chaperone activity of ERD10 and ERD14, two disordered stress-related plant proteins. Plant Physiol. 2008, 147, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P.; Kovacs, D. Intrinsically disordered chaperones in plants and animals. Biochem. Cell Biol. 2010, 88, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Duan, X.; Wang, B.; Hong, B.; Ho, T.; Wu, R. Expression of a Late Embryogenesis Abundant Protein Gene, HVA1, from Barley Confers Tolerance to Water Deficit and Salt Stress in Transgenic Rice. Plant Physiol. 1996, 110, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Goyal, K.; Walton, L.J.; Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 2005, 388, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Honjoh, K.I.; Matsumoto, H.; Shimizu, H.; Ooyama, K.; Tanaka, K.; Oda, Y.; Takata, R.; Joh, T.; Suga, K.; Miyamoto, T.; et al. Cryoprotective activities of group 3 late embryogenesis abundant proteins from Chlorella vulgaris C-27. Biosci. Biotechnol. Biochem. 2000, 64, 1656–1663. [Google Scholar] [CrossRef] [PubMed]
- Nylander, M.; Svensson, J.; Palva, E.T.; Welin, B.V. Stress-induced accumulation and tissue-specific localization of dehydrins in Arabidopsis thaliana. Plant Mol. Biol. 2001, 45, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Terashima, S.; Fukaya, T.; Kuboi, T. Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. Planta 2003, 217, 290–298. [Google Scholar] [PubMed]
- Puhakainen, T.; Hess, M.W.; Mäkelä, P.; Svensson, J.; Heino, P.; Palva, E.T. Overexpression of multiple dehydrin genes enhances tolerance to freezing stress in Arabidopsis. Plant Mol. Biol. 2004, 54, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Hughes, S.; Graether, S.P. Cryoprotective mechanism of a small intrinsically disordered dehydrin protein. Protein Sci. 2011, 20, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, K.; Takagi, H.; Takahashi, M.; Yamada, H.; Nakamori, S.; Sericin, P. Cryoprotective effect of the serine-rich repetitive sequence in silk protein sericin. J. Biochem. 2001, 129, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Keshava Prasad, T.S.; Goel, R.; Kandasamy, K.; Keerthikumar, S.; Kumar, S.; Mathivanan, S.; Telikicherla, D.; Raju, R.; Shafreen, B.; Venugopal, A.; et al. Human Protein Reference Database—2009 update. Nucleic Acids Res. 2009, 37, D767–D772. [Google Scholar] [CrossRef] [PubMed]
- Vucetic, S.; Obradovic, Z.; Vacic, V.; Radivojac, P.; Peng, K.; Iakoucheva, L.M.; Cortese, M.S.; Lawson, J.D.; Brown, C.J.; Sikes, J.G.; et al. DisProt: A database of protein disorder. Bioinformatics 2005, 21, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Goda, N.; Shimizu, K.; Kuwahara, Y.; Tenno, T.; Noguchi, T.; Ikegami, T.; Ota, M.; Hiroaki, H. A Method for Systematic Assessment of Intrinsically Disordered Protein Regions by NMR. Int. J. Mol. Sci. 2015, 16, 15743–15760. [Google Scholar] [CrossRef] [PubMed]
- Goda, N.; Matsuo, N.; Tenno, T.; Ishino, S.; Ishino, Y.; Fukuchi, S.; Ota, M.; Hiroaki, H. An optimized N(pro)-based method for the expression and purification of intrinsically disordered proteins for an NMR study. Intrinsically Disord. Proteins 2015, 3, e1011004. [Google Scholar] [CrossRef] [PubMed]
- Kriwacki, R.W.; Hengst, L.; Tennant, L.; Reed, S.I.; Wright, P.E. Structural studies of p21Waf1/Cip1/Sdi1 in the free and Cdk2-bound state: Conformational disorder mediates binding diversity. Proc. Natl. Acad. Sci. USA 1996, 93, 11504–11509. [Google Scholar] [CrossRef] [PubMed]
- Umetsu, Y.; Goda, N.; Taniguchi, R.; Satomura, K.; Ikegami, T.; Furuse, M.; Hiroaki, H. 1H, 13C, and 15N resonance assignment of the first PDZ domain of mouse ZO-1. Biomol. NMR Assign. 2011, 5, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Markert, C.L. Lactate Dehydrogenase Isozymes: Dissociation and Recombination of Subunits. Science 1963, 140, 1329–1330. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, T.; Okahashi, N.; Sakuma, R.; Aoyama, T.; Akahane, T.; Matsumoto, J.J. Freeze denaturation of enzymes and its prevention with additives. Cryobiology 1985, 22, 446–456. [Google Scholar] [CrossRef]
- Momma, M.; Kaneko, S.; Haraguchi, K.; Matsukura, U. Peptide mapping and assessment of cryoprotective activity of 26/27-kDa dehydrin from soybean seeds. Biosci. Biotechnol. Biochem. 2003, 67, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, N.; Goda, N.; Hiroaki, H. Nagoya University: Nagoya, Japan, Unpublished work. 2018.
- Hughes, S.L.; Schart, V.; Malcolmson, J.; Hogarth, K.A.; Martynowicz, D.M.; Tralman-Baker, E.; Patel, S.N.; Graether, S.P. The importance of size and disorder in the cryoprotective effects of dehydrins. Plant Physiol. 2013, 163, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.A.; Croy, C.H.; Vasanwala, F.H.; Uversky, V.N.; Van, Y.-Y.Y.J.; Dunker, A.K. Sweeping away protein aggregation with entropic bristles: Intrinsically disordered protein fusions enhance soluble expression. Biochemistry 2012, 51, 7250–7262. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. The most important thing is the tail: Multitudinous functionalities of intrinsically disordered protein termini. FEBS Lett. 2013, 587, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortee, S.; Tripathi, R.; Watson, M.; Schierle, G.S.K.; Kurniawan, D.P.; Kaminski, C.F.; Wise, M.J.; Tunnacliffe, A. Intrinsically disordered proteins as molecular shields. Mol. Biosyst. 2012, 8, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Van der Lee, R.; Lang, B.; Kruse, K.; Gsponer, J.; Sánchez de Groot, N.; Huynen, M.A.; Matouschek, A.; Fuxreiter, M.; Babu, M.M. Intrinsically Disordered Segments Affect Protein Half-Life in the Cell and during Evolution. Cell Rep. 2014, 8, 1832–1844. [Google Scholar] [CrossRef] [PubMed]
- Gsponer, J.; Futschik, M.E.; Teichmann, S.A.; Babu, M.M. Tight regulation of unstructured proteins: From transcript synthesis to protein degradation. Science 2008, 322, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef] [PubMed]

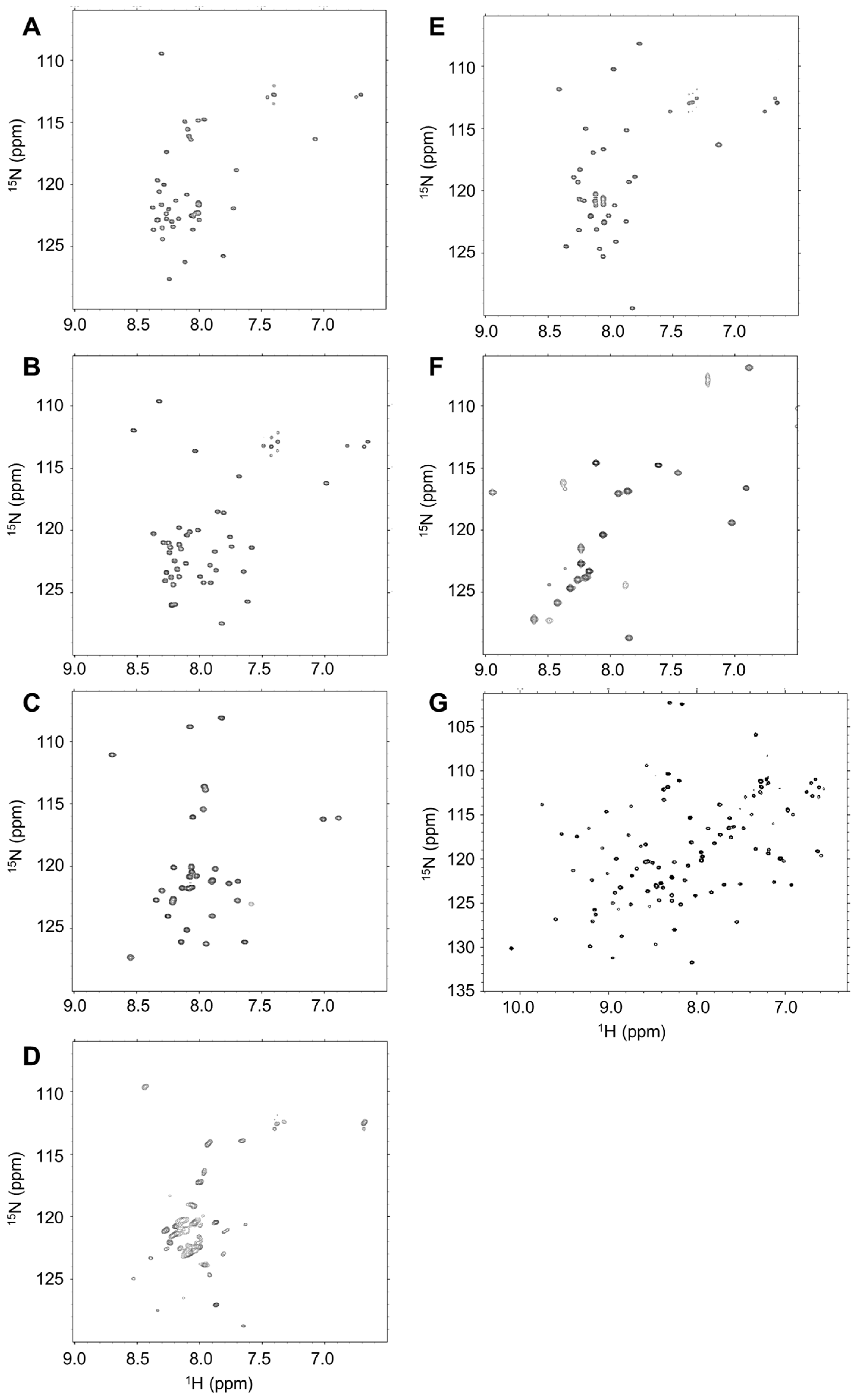

| IDP Name | RefSeq ID | Start | End | Length/Total Res. | Sequence | M.W. |
|---|---|---|---|---|---|---|
| B3 | NP_066926 | 1 | 44 | 44/44 | MADKPDMGEIASFDKAKLKKTETQEKNTLPTKETIEQEKRSEIS | 5025 |
| B4 | NP_001317 | 1 | 44 | 44/44 | MSGDGATEQAAEYVPEKVKKAEKKLEENPYDLDAWSILIREAQV | 4951 |
| C1 | NP_570859 | 1 | 36 | 36/36 | MAALRYAGLDDTDSEDELPPGWEERTTKDGWVYYAK | 4150 |
| D10 | NP_002537 | 24 | 62 | 39/401 | FPPKYLHYDEETSHQLLCDKCPPGTYLKQHCTAKWKTVC | 4610 |
| E1 | NP_005222 | 305 | 342 | 37/550 | GFGGKYGVQKDRMDKNASTFEDVTQVSSAYQKTVPVE | 4068 |
| C9* | NP_001035095 | 132 | 164 | 34*/1077 | S*PTEEIWVENKTPDGKVYYYNARTRESAWTKPDG | 3989 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, N.; Goda, N.; Shimizu, K.; Fukuchi, S.; Ota, M.; Hiroaki, H. Discovery of Cryoprotective Activity in Human Genome-Derived Intrinsically Disordered Proteins. Int. J. Mol. Sci. 2018, 19, 401. https://doi.org/10.3390/ijms19020401
Matsuo N, Goda N, Shimizu K, Fukuchi S, Ota M, Hiroaki H. Discovery of Cryoprotective Activity in Human Genome-Derived Intrinsically Disordered Proteins. International Journal of Molecular Sciences. 2018; 19(2):401. https://doi.org/10.3390/ijms19020401
Chicago/Turabian StyleMatsuo, Naoki, Natsuko Goda, Kana Shimizu, Satoshi Fukuchi, Motonori Ota, and Hidekazu Hiroaki. 2018. "Discovery of Cryoprotective Activity in Human Genome-Derived Intrinsically Disordered Proteins" International Journal of Molecular Sciences 19, no. 2: 401. https://doi.org/10.3390/ijms19020401
APA StyleMatsuo, N., Goda, N., Shimizu, K., Fukuchi, S., Ota, M., & Hiroaki, H. (2018). Discovery of Cryoprotective Activity in Human Genome-Derived Intrinsically Disordered Proteins. International Journal of Molecular Sciences, 19(2), 401. https://doi.org/10.3390/ijms19020401







