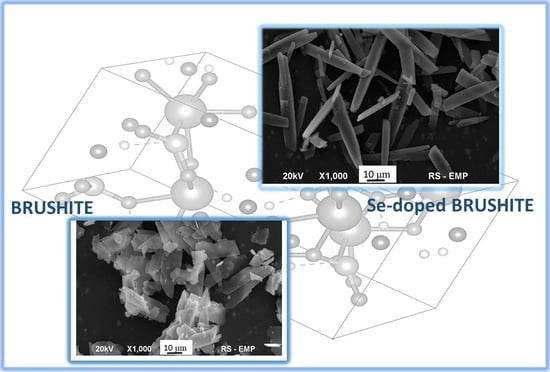
Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering
Abstract
:1. Introduction
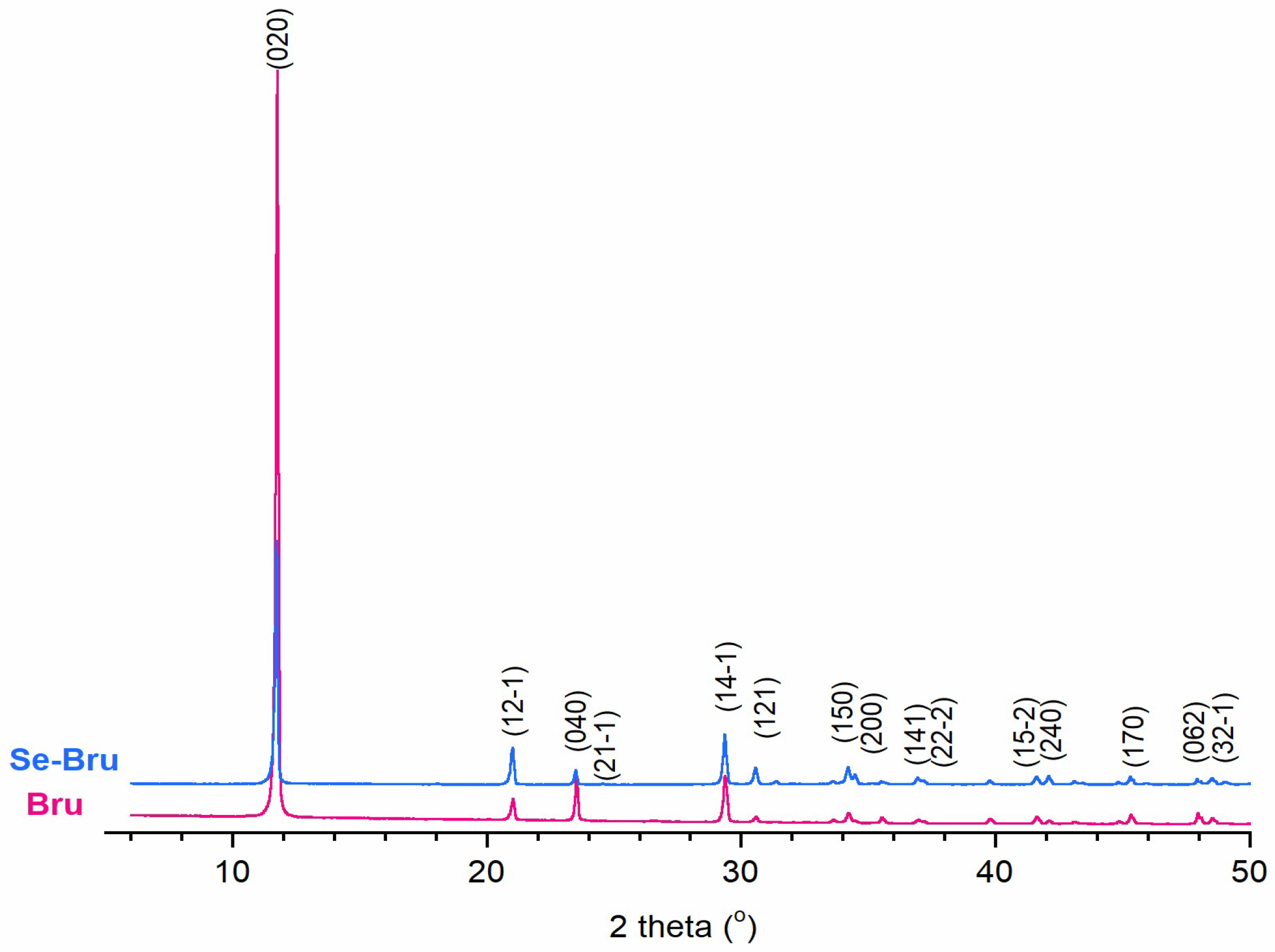
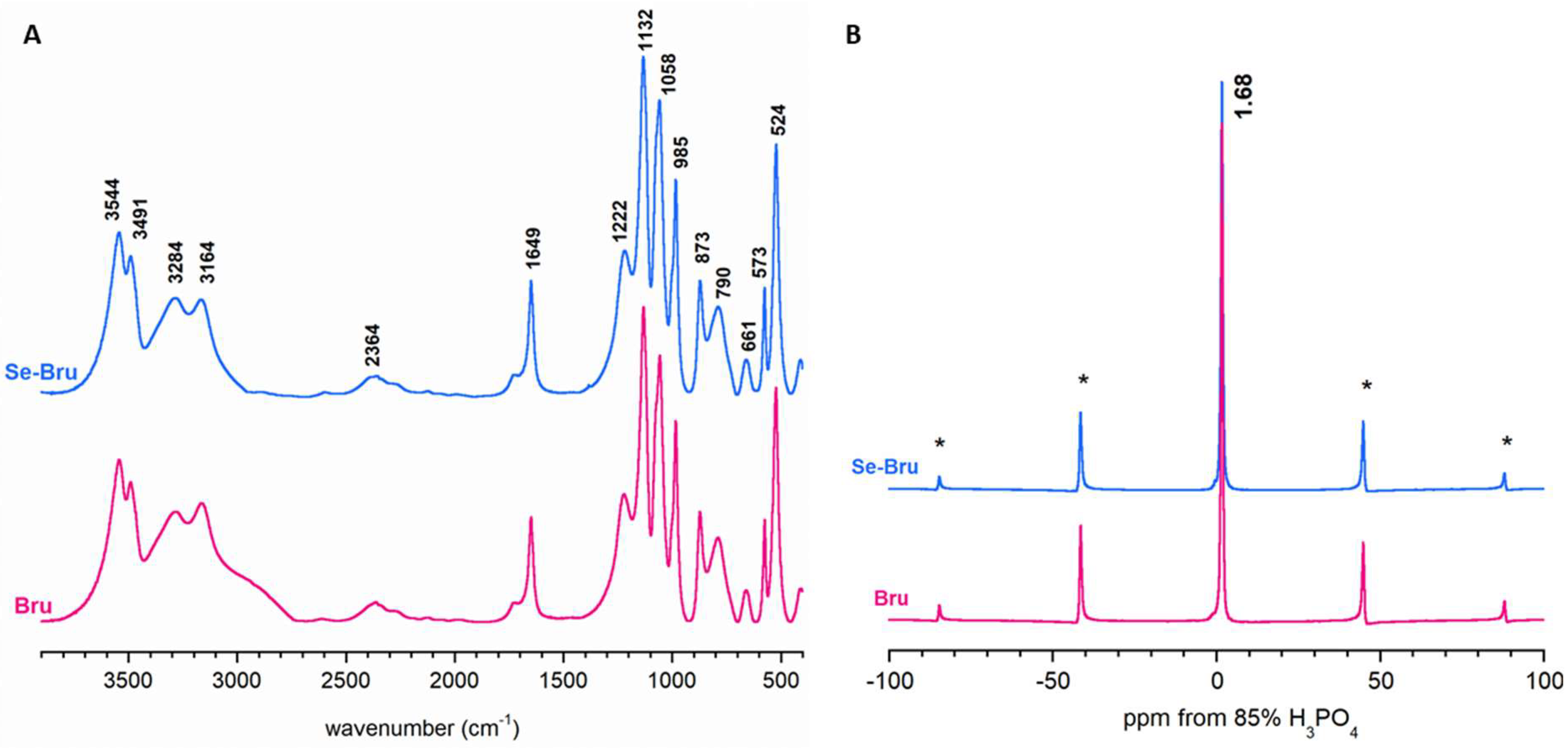
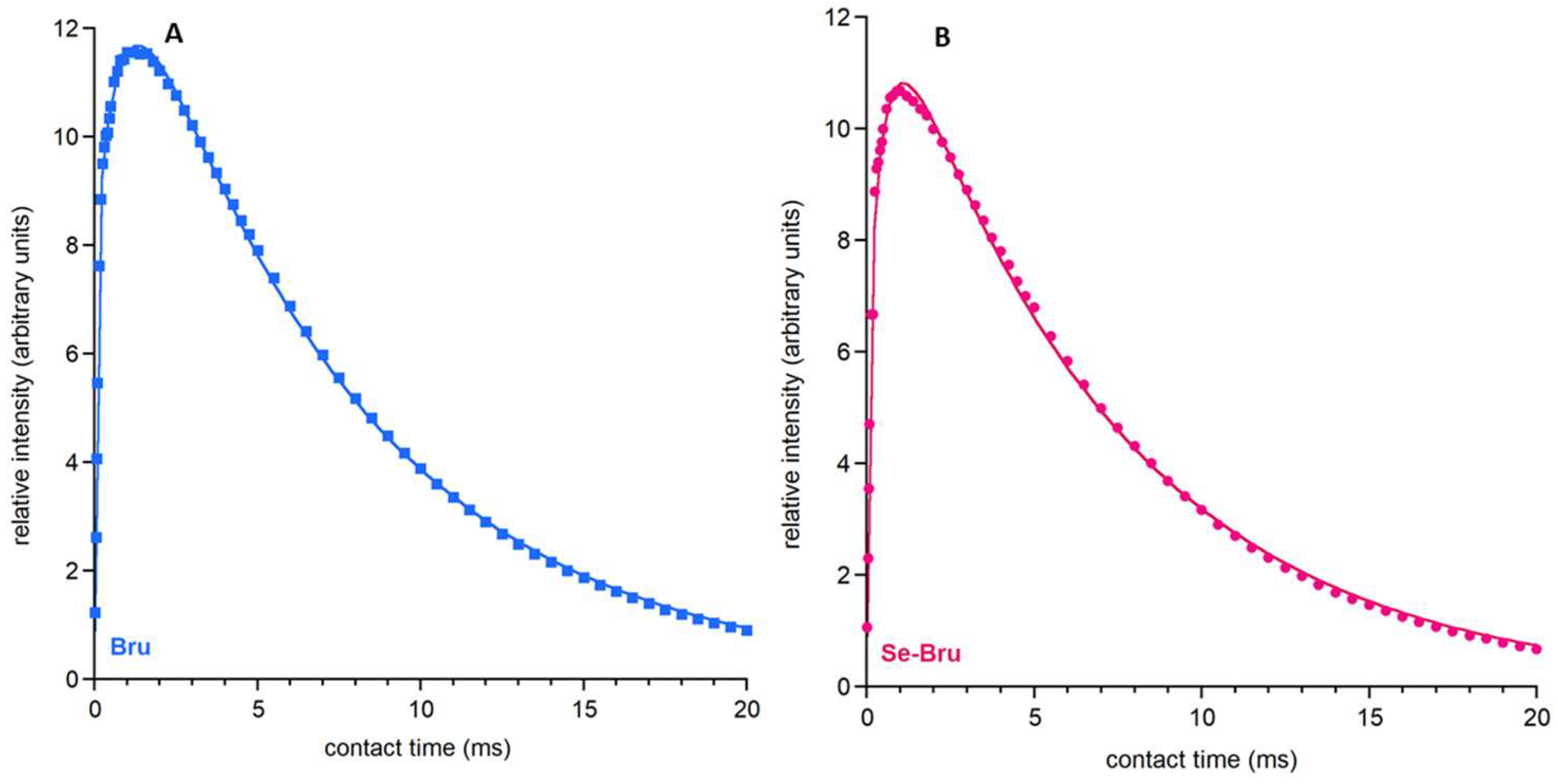
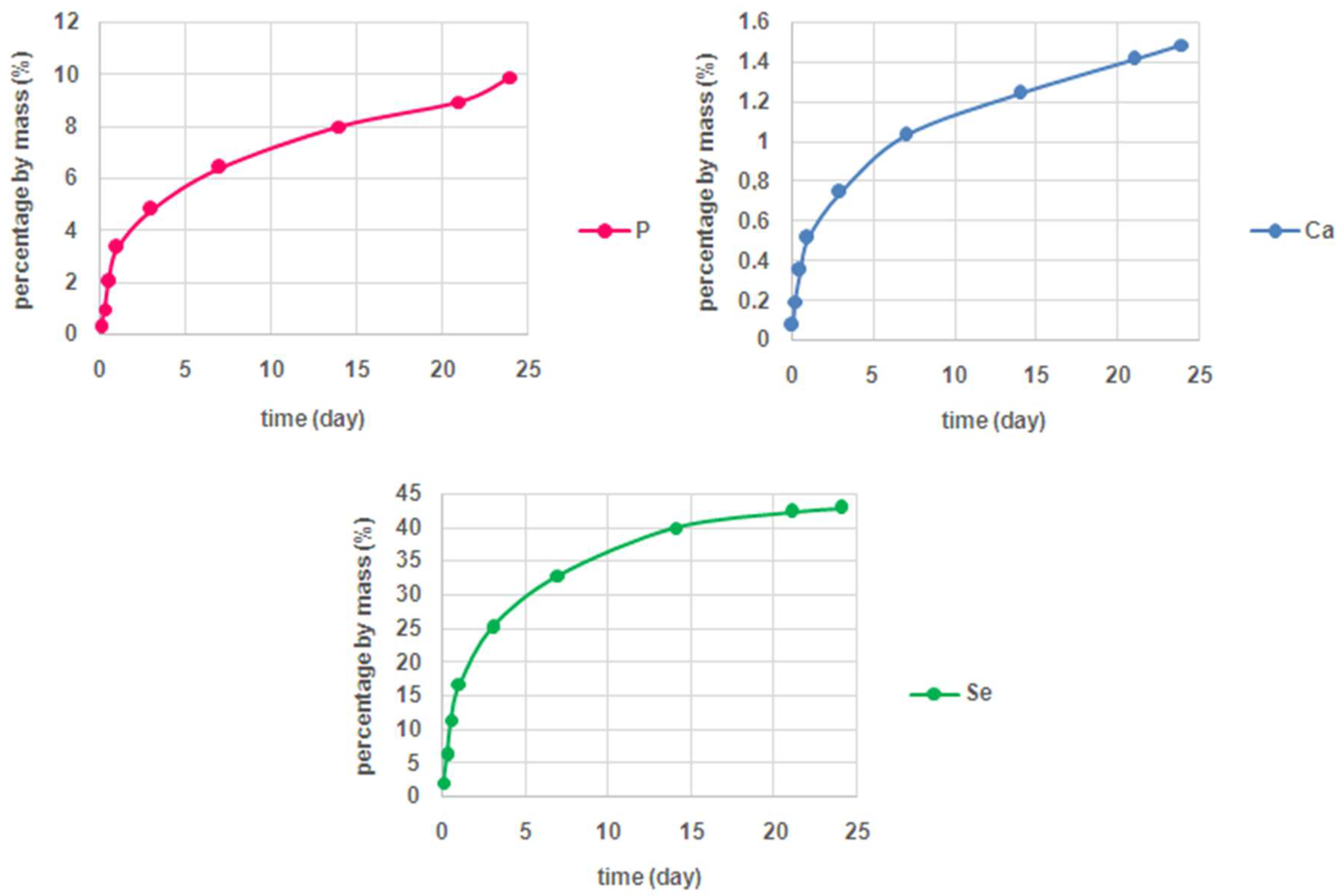
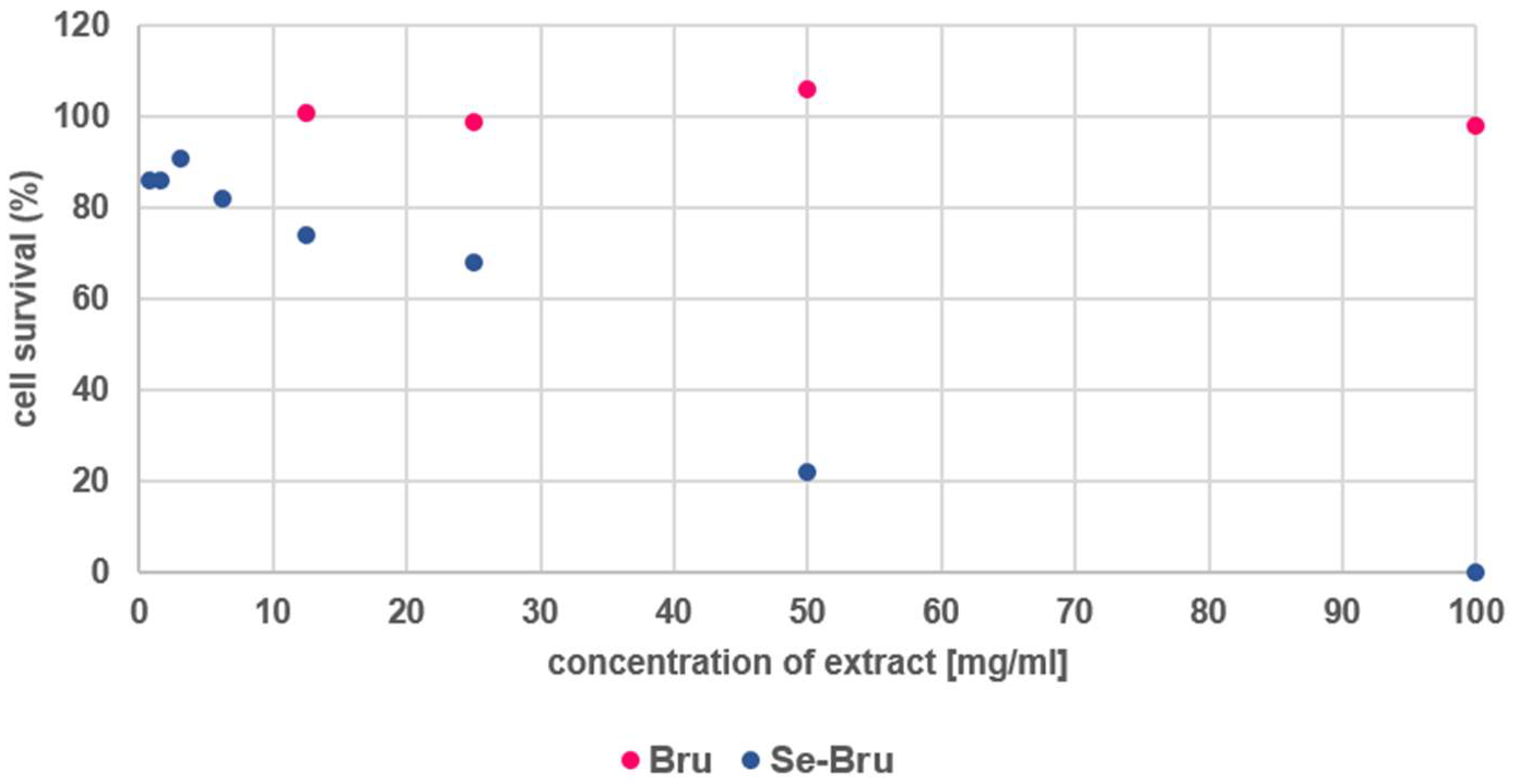
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Peak Position-2 Theta (°) | ||
|---|---|---|
| Peak Index | Bru | Se-Bru |
| 020 | 11.75 | 11.70 |
| 12-1 | 21.00 | 20.99 |
| 040 | 23.50 | 23.47 |
| 21-1 | 24.56 | 24.53 |
| 14-1 | 29.35 | 29.33 |
| 121 | 30.56 | 30.54 |
| 150 | 34.19 | 34.18 |
| 200 | 34.45 | 34.45 |
| 141 | 37.06 | 36.90 |
| 22-2 | 37.17 | 37.14 |
| 15-2 | 41.61 | 41.60 |
| 240 | 42.09 | 42.08 |
| 170 | 45.30 | 45.27 |
| 062 | 47.95 | 47.92 |
| 32-1 | 48.61 | 48.60 |
Appendix B
| Wavenumbers (CM−1) | Vibration Modes | |
|---|---|---|
| Bru | Se-Bru | |
| 3544-3491 | 3544-3491 | ν3 H2O (lattice water molecules) |
| 3283-3163 | 3285-3167 | ν1 H2O (lattice water molecules) |
| 2943 | 2945 | PO-H stretching |
| 2364 | 2359 | |
| 1725 | 1727 | Combination (bending) and rotation of residual free water |
| 1649 | 1650 | H-O-H bending of lattice water molecules |
| 1222 | 1219 | δ (PO-H) |
| 1133 | 1134 | νd(P-OH) |
| 1058 | 1060 | |
| 985 | 985 | νs(P-OH) |
| 874 | 873 | ν(P-O(H)) |
| 790 | 790 | δ(P-O(H)) |
| 660 | 660 | water libration |
| 576 | 577 | δ(O-P-O(H)) |
| 524 | 523 | δ(O-P-O(H)) |
| 410 | 412 | δ(O-P-O(H)) |
References
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef] [Green Version]
- Mandel, S.; Tas, A.C. Brushite (CaHPO4·2H2O) to octacalcium phosphate (Ca8(HPO4)2(PO4)4·5H2O) transformation in DMEM solution at 36.5 °C. Mater. Sci. Eng. C 2010, 30, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Ganzano, M.; Bigi, A. Ionic substitutions in calcium phosphates synthesized at low temperature. Acta Biomater. 2010, 6, 1882–1894. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Shin, M.C.; Kim, Y.N.; Oh, J.M. Brushite ceramics coatings for dental brace brackets fabricated via aerosol deposition. Ceram. Int. 2017, 43, 1044–1051. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Kwiatkowska-Różycka, D. Substituted Hydroxyapatites with Antibacterial Properties. Biomed. Res. Int. 2014, 2014, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of Selenium to Human Health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 64, 527–542. [Google Scholar] [CrossRef]
- Zeng, H.; Cao, J.J.; Combs, G.F., Jr. Selenium in Bone Health: Roles in Antioxidant Protection and Cell Proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.P.; Gandin, V. Selenium compounds as therapeutic agents in cancer. Biochim. Biophys. Acta 2015, 1850, 1642–1660. [Google Scholar] [CrossRef]
- Blackburn, G.; Scott, T.G.; Bayer, I.S. Bionanomaterials for bone tumor engineering and tumor destruction. J. Mater. Chem. B 2013, 1, 1519. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Zhou, L.; Chen, J.; Liu, Y.; Qiu, Z.; Zhang, S. Dual functional selenium-substituted hydroxyapatite. Interface Focus 2012, 2, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hang, H.; Yan, L.; Zhang, S. Selenium-substituted hydroxyapatite nanoparticles and their in vivo antitumor effect on hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2016, 140, 297–306. [Google Scholar]
- Wang, Y.; Wang, J.; Hao, H.; Cai, M.; Wang, S.; Ma, J.; Li, Y.; Mao, C.; Zhang, S. In Vitro and In Vivo Mechanism of Bone Tumor Inhibition by Selenium-Doped Bone Mineral Nanoparticles. ACS Nano. 2018, 10, 9927–9937. [Google Scholar] [CrossRef] [PubMed]
- Uskokovic, V.; Iyer, M.A.; Wu, V.M. One Ion to Rule Them All: Combined Antibacterial, Osteoinductive and Anticancer Properties of Selenite-Incorporated Hydroxyapatite. J. Mater. Chem. B 2017, 5, 1430–1445. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chai, Y.; Cao, N.; Wang, Y. Synthesis and characterization of selenium substituted hydroxyapatite via hydrothermal method. Mater. Lett. 2014, 134, 123–125. [Google Scholar] [CrossRef]
- Kolmas, J.; Groszyk, E.; Piotrowska, U. Nanocrystalline hydroxyapatite enriched in selenite and manganese ions: Physicochemical and antibacterial properties. Nanoscale Res. Lett. 2015, 10, 278. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B. Surface Characterization and Biocompatibility of Selenium-Doped Hydroxyapatite Coating on Titanium Alloy. Int. J. Appl. Ceram. Technol. 2016, 13, 1059–1068. [Google Scholar] [CrossRef]
- Kolmas, J.; Oledzka, E.; Sobczak, M.; Nałęcz-Jawecki, G. Nanocrystalline hydroxyapatite doped with selenium oxyanions: A new material for potential biomedical applications. Mater. Sci. Eng. C 2014, 39, 134–142. [Google Scholar] [CrossRef]
- Trpkovska, M.; Soptrajanov, B.; Malkov, P. FTIR reinvestigation of the spectra of synthetic brushite and its partially deuterated analogues. J. Mol. Struct. 1999, 480–481, 661–666. [Google Scholar] [CrossRef]
- Rey, C.; Marsan, O.; Combes, C.; Drouet, C.; Grossin, D.; Sarda, S. Characterization of Calcium Phosphates using vibrational spectroscopies. In Advances in Calcium Phosphate Biomaterials; Ben-Nissan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 229–267. [Google Scholar]
- Kaflak-Hachulska, A.; Slosarczyk, A.; Kolodziejski, W. Kinetics of NMR cross-polarization from protons to phosphorus-31 in natural brushite. Solid State Nucl. Magn. Reson. 2000, 15, 237–238. [Google Scholar] [CrossRef]
- Pajor, K.; Pajchel, L.; Kolodziejska, B.; Kolmas, J. Selenium-Doped Hydroxyapatite Nanocrystals–Synthesis, Physicochemical Properties and Biological Significance. Crystals 2018, 8, 188. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, P.; Ma, Z.; Zhang, J. Enhanced Healing of Rat Calvarial Critical Size Defect with Selenium-Doped Lamellar Biocomposites. Biol. Trace Elem. Res. 2013, 155, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, X.; Li, H.; Fan, D.; Song, Z.; Ma, H.; Hua, X.; Hui, J. Monodisperse selenium-substituted hydroxyapatite: Controllable synthesis and biocompatibility. Mat. Sci. Eng. C 2017, 73, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hang, H.; Zhang, S. Biomimetic Coprecipitation of Silk Fibrin and Calcium Phosphate: Influence of Selenite Ions. Biol. Trace Elem. Res. 2017, 178, 338–347. [Google Scholar] [CrossRef]
- Hemalatha, T.; Krithiga, G.; Kumar, B.S. Preparation and Characterization of Hydroxyapatite-Coated Selenium Nanoparticles and their Interaction with Osteosarcoma (SaOS−2) Cells. Acta Metall. Sin. (Engl. Lett.) 2014, 27, 1152–1158. [Google Scholar] [CrossRef]
- Wei, l.; Yang, H.; Hong, J.; He, Z.; Deng, C. Synthesis and structure properties of Se and Sr codoped hydroxyapatite and their biocompatibility. J. Mater Sci. 2019, 54, 2514–2525. [Google Scholar] [CrossRef]
- Guerra-Lopez, J.R.; Guida, J.A.; Ramos, M.A.; Punte, G. The influence of Ni(II) on brushite structure stabilization. J. Mol. Str. 2017, 720–724. [Google Scholar] [CrossRef]
- Cabrejos-Azama, J.; Alkhraisat, M.H.; Rueda, C.; Torres, J.; Pintado, C.; Blanco, L.; & López-Cabarcos, E. Magnesium substitution in brushite cements: Efficacy of a new biomaterial loaded with vancomycin for the treatment of Staphylococcus aureus infections. Mat. Sci. Eng. C 2016, 61, 72–78. [Google Scholar] [CrossRef]
- Cummings, H.; Han, W.; Vahabzadeh, S.; Elsawa, S.F. Cobalt-Doped Brushite Cement: Preparation, Characterization, and In Vitro Interaction with Osteosarcoma Cells. JOM 2017, 69, 1348–1353. [Google Scholar] [CrossRef]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef]
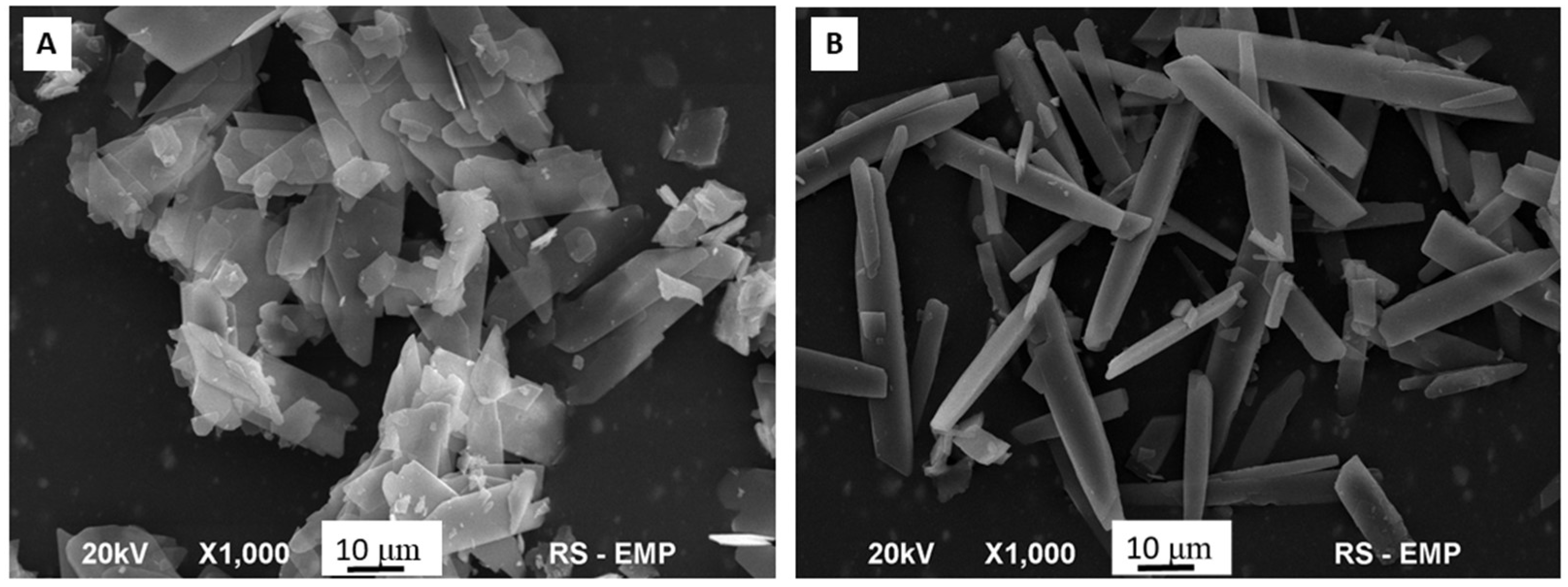
| Parameters | Bru | Se-Bru |
|---|---|---|
| Phase Composition | 100% DCPD | 100% DCPD |
| Unit Cell Parameters | ||
| a (Å) | 5.915 | 6.238 |
| b (Å) | 15.12 | 15.16 |
| c (Å) | 6.242 | 5.806 |
| β (˚) | 116.4 | 116.4 |
| Volume ((Å)3) | 500.2 | 491.7 |
| Se Content (wt%) | -------- | 0.67 ± 0.03% |
| Parameters | Bru | Se-Bru |
|---|---|---|
| T1ρH | 7.09 ± 0.05 | 6.84 ± 0.08 |
| λ | 0.51 ± 0.01 | 0.54 ± 0.02 |
| Tdf | 0.88 ± 0.03 | 0.56 ± 0.04 |
| TCP* | 0.0809 ± 0.001 | 0.101 ± 0.005 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laskus, A.; Zgadzaj, A.; Kolmas, J. Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 4042. https://doi.org/10.3390/ijms19124042
Laskus A, Zgadzaj A, Kolmas J. Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering. International Journal of Molecular Sciences. 2018; 19(12):4042. https://doi.org/10.3390/ijms19124042
Chicago/Turabian StyleLaskus, Aleksandra, Anna Zgadzaj, and Joanna Kolmas. 2018. "Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering" International Journal of Molecular Sciences 19, no. 12: 4042. https://doi.org/10.3390/ijms19124042
APA StyleLaskus, A., Zgadzaj, A., & Kolmas, J. (2018). Selenium-Enriched Brushite: A Novel Biomaterial for Potential Use in Bone Tissue Engineering. International Journal of Molecular Sciences, 19(12), 4042. https://doi.org/10.3390/ijms19124042