The Protective Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside in the OVA-Induced Asthma Mice Model
Abstract
:1. Introduction
2. Results
2.1. Effects of TSG on Airway Hyperresponsiveness (AHR)
2.2. Effects of TSG on the Inflammatory Cell Population in the BAFL and Blood
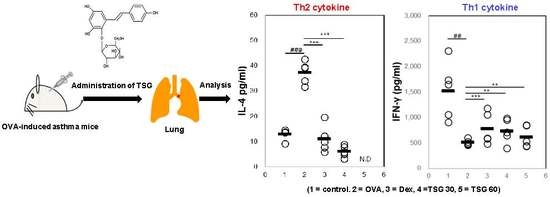
2.3. Effects of TSG on the Production of Cytokines in OVA-Induced Asthmatic Mice
2.4. Effects of TSG on the Release of Immunoglobulin Class Switching in Serum
2.5. Effects of TSG on Histological Changes in Asthmatic Mice
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Sensitization and Provocation of Airway Inflammation with OVA
4.4. Assessment of Airway Hyperresponsiveness on the TSG in OVA-Induced Asthmatic Mice
4.5. Measurement of Inflammatory Cytokine and Immunoglobulin Production in OVA or TSG Exposed Mice
4.6. Histological Analysis of Lung Tissue in OVA or TSG Exposed Mice
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patadia, M.O.; Murrill, L.L.; Corey, J. Asthma: Symptoms and presentation. Otolaryngol. Clin. N. Am. 2014, 47, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Braman, S.S. The global burden of asthma. Chest 2006, 130, 4S–12S. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Mantzouranis, E.; Cruz, A.A.; Aït-Khaled, N.; Baena-Cagnani, C.E.; Bleecker, E.R.; Brightling, C.E.; Burney, P.; Bush, A.; Busse, W.W.; et al. Uniform definition of asthma severity, control, and exacerbations: Document presented for the World Health Organization Consultation on Severe Asthma. J. Allergy Clin. Immunol. 2010, 126, 926–938. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.S.; Bailey, C.M.; Qin, X.; Moorman, J.E. Assessing asthma severity among children and adults with current asthma. J. Asthma 2014, 51, 610–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodge, R.R.; Burrows, B. The prevalence and incidence of asthma and asthma-like symptoms in a general population sample. Am. Rev. Respir. Dis. 1980, 122, 567–575. [Google Scholar]
- Scichilone, N.; Pedone, C.; Battaglia, S.; Sorino, C.; Bellia, V. Diagnosis and management of asthma in the elderly. Eur. J. Intern. Med. 2014, 25, 336–342. [Google Scholar] [CrossRef]
- To, T.; Stanojevic, S.; Moores, G.; Gershon, A.S.; Bateman, E.D.; Cruz, A.A.; Boulet, L.P. Global asthma prevalence in adults: Findings from the cross-sectional world health survey. BMC Public Health 2012, 12, 204. [Google Scholar] [CrossRef]
- Adcock, I.M.; Ito, K. Steroid resistance in asthma: A major problem requiring novel solutions or a non-issue? Curr. Opin. Pharmacol. 2004, 4, 257–262. [Google Scholar] [CrossRef]
- Agertoft, L.; Pedersen, S. Effects of long-term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respir. Med. 1994, 88, 373–381. [Google Scholar] [CrossRef]
- Xu, W.; Hu, M.; Zhang, Q.; Yu, J.; Su, W. Effects of anthraquinones from Cassia occidentalis L. on ovalbumin-induced airways inflammation in a mouse model of allergic asthma. J. Ethnopharmacol. 2018, 221, 1–9. [Google Scholar] [CrossRef]
- Bounda, G.A.; Feng, Y.U. Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacogn. Res. 2015, 7, 225–236. [Google Scholar]
- Lv, L.; Shao, X.; Wang, L.; Huang, D.; Ho, C.T.; Sang, S. Stilbene glucoside from Polygonum multiflorum Thunb.: A novel natural inhibitor of advanced glycation end product formation by trapping of methylglyoxal. J. Agric. Food Chem. 2010, 58, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Hwang, Y.H.; Mun, S.K.; Hong, S.G.; Kim, K.J.; Kang, K.Y.; Son, Y.J.; Yee, S.T. Protective Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside on Ovariectomy Induced Osteoporosis Mouse Model. Int. J. Mol. Sci. 2018, 19, 2554. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, R.; Zhou, S.; Ma, J.; Xie, Y.; Xu, B.; Long, H.; Luo, K.; Fang, K. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside inhibits septic serum-induced inflammatory injury via interfering with the ROS-MAPK-NF-κB signaling pathway in pulmonary aortic endothelial cells. Int. J. Mol. Med. 2018, 41, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.Y.; Bayarsengee, U.; Wang, C.C.; Chiang, Y.H.; Cheng, C.W. The natural compound 2,3,5,4′-tetrahydroxystilbene-2-O-β-d glucoside protects against adriamycin-induced nephropathy through activating the Nrf2-Keap1 antioxidant pathway. Environ. Toxicol. 2018, 33, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, C.; Zhu, M.; Wang, X.; Zhang, L.; Zhao, J. 2,3,5,4-tetrahydroxy diphenylethylene-2-O-glucoside inhibits the adhesion and invasion of A549 human lung cancer cells. Mol. Med. Rep. 2017, 16, 8900–8906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Jiang, L.L.; Luo, N.; Fan, Y.X.; Ma, J.; Li, P.; Li, H.J. Enhanced absorption and inhibited metabolism of emodin by 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucopyranoside: Possible mechanisms for Polygoni Multiflori Radix-induced liver injury. Chin. J. Nat. Med. 2017, 15, 451–457. [Google Scholar] [CrossRef]
- Lei, X.; Chen, J.; Ren, J.; Li, Y.; Zhai, J.; Mu, W.; Zhang, L.; Zheng, W.; Tian, G.; Shang, H. Liver Damage Associated with Polygonum multiflorum Thunb.: A Systematic Review of Case Reports and Case Series. Evid. Based Complement. Altern. Med. 2015, 2015, 459749. [Google Scholar] [CrossRef]
- Li, C.; Niu, M.; Bai, Z.; Zhang, C.; Zhao, Y.; Li, R.; Tu, C.; Li, H.; Jing, J.; Meng, Y.; et al. Screening for main components associated with the idiosyncratic hepatotoxicity of a tonic herb, Polygonum multiflorum. Front. Med. 2017, 2, 253–265. [Google Scholar] [CrossRef]
- Bateman, E.D.; Hurd, S.S.; Barnes, P.J.; Bousquet, J.; Drazen, J.M.; FitzGerald, J.M.; Gibson, P.; Ohta, K.; O’Byrne, P.; Pedersen, S.E.; et al. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2008, 31, 143–178. [Google Scholar] [CrossRef] [Green Version]
- Ziment, I.; Tashkin, D.P. Alternative medicine for allergy and asthma. J. Allergy Clin. Immunol. 2000, 106, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, T.; Uchikawa, R.; Yamada, M.; Arizono, N.; Oikawa, S.; Kawanishi, S.; Nishio, A.; Nakase, H.; Kuribayashi, K. Environmental pollutant tributyltin promotes Th2 polarization and exacerbates airway inflammation. Eur. J. Immunol. 2004, 34, 1312–1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinke, J.W.; Borish, L. Th2 cytokines and asthma. Interleukin-4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir. Res. 2001, 2, 66–70. [Google Scholar] [PubMed]
- Greenfeder, S.; Umland, S.P.; Cuss, F.M.; Chapman, R.W.; Egan, R.W. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir. Res. 2001, 2, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B. The role of eosinophils in the pathogenesis of asthma. Trends Mol. Med. 2005, 11, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, L.K.; Fonseca, B.P.; Barboza, B.A.; Viola, J.P. The role of interferon-gamma on immune and allergic responses. Mem. Inst. Oswaldo Cruz. 2005, 100, 137–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, C.; Hettiaratchi, A.; Webb, D.C.; Thomas, P.S.; Foster, P.S.; Kumar, R.K. Suppression of cytokine expression by roflumilast and dexamethasone in a model of chronic asthma. Clin. Exp. Allergy 2008, 38, 847–856. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, C.; He, H.; Ding, W. 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-glucoside prevention of lipopolysaccharide-induced depressive-like behaviors in mice involves neuroinflammation and oxido-nitrosative stress inhibition. Behav. Pharmacol. 2017, 28, 365–374. [Google Scholar] [CrossRef]
- Xu, S.; Liu, J.; Shi, J.; Wang, Z.; Ji, L. 2,3,4′,5-tetrahydroxystilbene-2-O-β-d-glucoside exacerbates acetaminophen-induced hepatotoxicity by inducing hepatic expression of CYP2E1, CYP3A4 and CYP1A2. Sci. Rep. 2017, 7, 16511. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Zhang, Y.; Zhao, P.; Zhang, X. Regulatory effect of baicalin on the imbalance of Th17/Treg responses in mice with allergic asthma. J. Ethnopharmacol. 2017, 208, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Ma, X.; Xun, Y.; Pan, L. Protective effect of forsythiaside A on OVA-induced asthma in mice. Eur. J. Pharmacol. 2017, 812, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Wang, Y.; Gu, Y.; Wang, J.; Zhang, H.; Gao, H.; Jin, Q.; Zhao, L. Polydatin attenuates reactive oxygen species-induced airway remodeling by promoting Nrf2-mediated antioxidant signaling in asthma mouse model. Life Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Choi, Y.H.; Xian, Z.; Zheng, M.; Piao, H.; Yan, G. Aloperine suppresses allergic airway inflammation through NF-κB, MAPK, and Nrf2/HO-1 signaling pathways in mice. Int. Immunopharmacol. 2018, 65, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, F.; Roshan, N.M.; Kaveh, M.; Eftekhar, N.; Boskabady, M.H. Curcumin affects tracheal responsiveness and lung pathology in asthmatic rats. Pharmacol. Rep. 2018, 70, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.F. The effectiveness and side effects of dexamethasone in preterm infants with bronchopulmonary dysplasia. Arch. Dis. Child. 1993, 69, 331. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Hong, S.G.; Mun, S.K.; Kim, S.J.; Lee, S.J.; Kim, J.J.; Kang, K.Y.; Yee, S.T. The Protective Effects of Astaxanthin on the OVA-Induced Asthma Mice Model. Molecules 2017, 22, 2019. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Paik, M.J.; Yee, S.T. Diisononyl phthalate induces asthma via modulation of Th1/Th2 equilibrium. Toxicol. Lett. 2017, 272, 49–59. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, Y.-H.; Kim, S.-J.; Kim, H.; Yee, S.-T. The Protective Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside in the OVA-Induced Asthma Mice Model. Int. J. Mol. Sci. 2018, 19, 4013. https://doi.org/10.3390/ijms19124013
Hwang Y-H, Kim S-J, Kim H, Yee S-T. The Protective Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside in the OVA-Induced Asthma Mice Model. International Journal of Molecular Sciences. 2018; 19(12):4013. https://doi.org/10.3390/ijms19124013
Chicago/Turabian StyleHwang, Yun-Ho, Su-Jin Kim, Hangun Kim, and Sung-Tae Yee. 2018. "The Protective Effects of 2,3,5,4′-Tetrahydroxystilbene-2-O-β-d-Glucoside in the OVA-Induced Asthma Mice Model" International Journal of Molecular Sciences 19, no. 12: 4013. https://doi.org/10.3390/ijms19124013