Chemoprevention of Colorectal Cancer by Dietary Compounds
Abstract
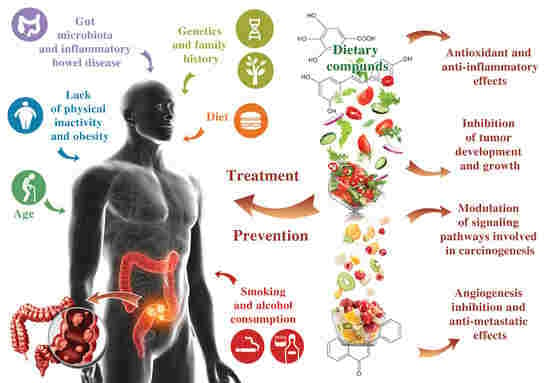
:1. Introduction
2. General Aspects of CRC
3. General Aspects Regarding the Dietary Compounds
3.1. Phenolic Compounds
3.2. Carotenoids
3.3. Iridoids
3.4. Nitrogen Compounds
3.5. Organosulfur Compounds
3.6. Phytosterols
3.7. Essential Oil Compounds
3.8. Polyunsaturated Fatty Acids (PUFA)
3.9. Dietary Fiber
4. In Vitro Studies
4.1. Polyphenols
4.1.1. Flavones
4.1.2. Isoflavones
4.1.3. Phenocarboxilic Acids
4.1.4. Stilbens
4.1.5. Other Compounds
4.2. Non-polyphenolic Compounds—In Vitro Mechanism of Action
4.2.1. Carotenoids
4.2.2. Nitrogen Compounds
5. In Vivo Studies in CRC Animal Models
5.1. The Effect of Phenolic Compounds
5.1.1. Isoflavones
5.1.2. Anthocyanidins
5.1.3. Phenocarboxilic Acids
5.1.4. Lignans
5.2. The Effect of Non-Phenolic Compounds
5.2.1. Carotenoids
5.2.2. Iridoids
5.2.3. Nitrogen Compounds
5.2.4. Phytosterols
5.2.5. Organosulfur Compounds
5.2.6. Essential Oils
5.2.7. Polyunsaturated Fatty Acids
5.2.8. Dietary Fiber
6. Chemoprevention of CRC by Dietary Compounds in Humans
6.1. Phenolic Compounds
6.1.1. Isoflavones
6.1.2. Lignans
6.1.3. Anthocyanidins
6.2. Non-phenolic Compounds
6.2.1. Organosulfur Compounds
6.2.2. Carotenoids
6.2.3. Polyunsaturated Fatty Acids
6.2.4. Dietary Fiber
7. Bioavailability of the Natural Dietary Compounds
7.1. Bioavailability of Phenolic Compounds
7.2. Bioavailability of Non-Phenolic Compounds
7.3. Encapsulation Strategies for Increased Bioavailability
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Van der Stok, E.P.; Spaander, M.C.W.; Grunhagen, D.J.; Verhoef, C.; Kuipers, E.J. Surveillance after curative treatment for colorectal cancer. Nat. Rev. Clin. Oncol. 2017, 14, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.S.; Seymour, M.T. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Ther. Adv. Med. Oncol. 2011, 3, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Redondo-Blanco, S.; Gutierrez-del-Rio, I.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Colon microbiota fermentation of dietary prebiotics towards short—Chain fatty acids and their roles as anti-inflammatory and antitumor agents: A review. J. Funct. Foods 2016, 25, 511–522. [Google Scholar] [CrossRef]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the Effectiveness of Natural Compound Mixtures in Cancer through Their Molecular Mode of Action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yang, H.L.; Yang, Y.J.; Wang, L.; Lee, S.C. Overcome Cancer Cell Drug Resistance Using Natural Products. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.; To, K.K.; Lin, G. Circumvention of multi-drug resistance of cancer cells by Chinese herbal medicines. Chin. Med. 2010, 5, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, X.Q.; Bi, H.; Feng, J.Q.; Cao, J.G. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol. Sin. 2005, 26, 1009–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganta, S.; Amiji, M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm. 2009, 6, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witold, K.; Anna, K.; Maciej, T.; Jakub, J. Adenomas-Genetic factors in colorectal cancer prevention. Rep. Pract. Oncol. Radiother. 2018, 23, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Sakai, E.; Nakajima, A.; Kaneda, A. Accumulation of aberrant DNA methylation during colorectal cancer development. World J. Gastroenterol. 2014, 20, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Oines, M.; Helsingen, L.M.; Bretthauer, M.; Emilsson, L. Epidemiology and risk factors of colorectal polyps. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Colussi, D.; Brandi, G.; Bazzoli, F.; Ricciardiello, L. Molecular pathways involved in colorectal cancer: Implications for disease behavior and prevention. Int. J. Mol. Sci. 2013, 14, 16365–16385. [Google Scholar] [CrossRef] [PubMed]
- Nazemalhosseini Mojarad, E.; Kuppen, P.J.; Aghdaei, H.A.; Zali, M.R. The CpG island methylator phenotype (CIMP) in colorectal cancer. Gastroenterol. Hepatol. Bed Bench 2013, 6, 120–128. [Google Scholar] [PubMed]
- Worthley, D.L.; Leggett, B.A. Colorectal cancer: Molecular features and clinical opportunities. Clin. Biochem. Rev. 2010, 31, 31–38. [Google Scholar] [PubMed]
- Dos Reis, S.A.; da Conceicao, L.L.; Siqueira, N.P.; Rosa, D.D.; da Silva, L.L.; Peluzio, M.D. Review of the mechanisms of probiotic actions in the prevention of colorectal cancer. Nutr. Res. 2017, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Li, L.; Shen, J.; Zhang, L.; Cho, C. Chronic Inflammation and Colorectal Cancer: The Role of Vascular Endothelial Growth Factor. Curr. Pharm. Des. 2015, 21, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Chang, D.K. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 2014, 20, 9872–9881. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cao, A.T.; Cong, Y. Microbiota regulation of inflammatory bowel disease and colorectal cancer. Semin. Cancer Biol. 2013, 23, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, U.; Jiao, S.; Schumacher, F.R.; Hutter, C.M.; Aragaki, A.K.; Baron, J.A.; Berndt, S.I.; Bezieau, S.; Brenner, H.; Butterbach, K.; et al. Identification of Genetic Susceptibility Loci for Colorectal Tumors in a Genome-Wide Meta-analysis. Gastroenterology 2013, 144, 799–807.e724. [Google Scholar] [CrossRef] [PubMed]
- Coppede, F.; Lopomo, A.; Spisni, R.; Migliore, L. Genetic and epigenetic biomarkers for diagnosis, prognosis and treatment of colorectal cancer. World J. Gastroenterol. 2014, 20, 943–956. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, J.; Han, X.; Yang, H.; Wang, S.; Lin, D.; Shi, Y. Effectors of epidermal growth factor receptor pathway: The genetic profiling ofKRAS, BRAF, PIK3CA, NRAS mutations in colorectal cancer characteristics and personalized medicine. PLoS ONE 2013, 8, e81628. [Google Scholar] [CrossRef] [PubMed]
- Bogaert, J.; Prenen, H. Molecular genetics of colorectal cancer. Ann. Gastroenterol. 2014, 27, 9–14. [Google Scholar] [PubMed]
- Gagniere, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.A.; Pezet, D.; Bonnet, M. Gut microbiota imbalance and colorectal cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Nistal, E.; Fernandez-Fernandez, N.; Vivas, S.; Olcoz, J.L. Factors Determining Colorectal Cancer: The Role of the Intestinal Microbiota. Front. Oncol. 2015, 5, 220. [Google Scholar] [CrossRef] [PubMed]
- Rezasoltani, S.; Asadzadeh Aghdaei, H.; Dabiri, H.; Akhavan Sepahi, A.; Modarressi, M.H.; Nazemalhosseini Mojarad, E. The association between fecal microbiota and different types of colorectal polyp as precursors of colorectal cancer. Microb. Pathog. 2018, 124, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Niederreiter, L.; Adolph, T.E.; Tilg, H. Food, microbiome and colorectal cancer. Dig. Liver Dis. 2018, 50, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Zackular, J.P.; Rogers, M.A.; Ruffin, M.T.T.; Schloss, P.D. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 2014, 7, 1112–1121. [Google Scholar] [CrossRef] [PubMed]
- Fagunwa, I.O.; Loughrey, M.B.; Coleman, H.G. Alcohol, smoking and the risk of premalignant and malignant colorectal neoplasms. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Kruger, C.; Zhou, Y. Red meat and colon cancer: A review of mechanistic evidence for heme in the context of risk assessment methodology. Food Chem. Toxicol. 2018, 118, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. Obesity and diabetes as prognostic factors in patients with colorectal cancer. Diabetes Metab. Syndr. 2017, 11 (Suppl. 1), S109–S114. [Google Scholar] [CrossRef] [PubMed]
- Talibov, M.; Sormunen, J.; Hansen, J.; Kjaerheim, K.; Martinsen, J.-I.; Sparen, P.; Tryggvadottir, L.; Weiderpass, E.; Pukkala, E. Benzene exposure at workplace and risk of colorectal cancer in four Nordic countries. Cancer Epidemiol. 2018, 55, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; De, P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15–49-year-olds in Canada, 1969–2010. Cancer Epidemiol. 2016, 42, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Grimmett, C.; Simon, A.; Lawson, V.; Wardle, J. Diet and physical activity intervention in colorectal cancer survivors: A feasibility study. Eur. J. Oncol. Nurs. 2015, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tayyem, R.F.; Bawadi, H.A.; Shehadah, I.; Agraib, L.M.; AbuMweis, S.S.; Al-Jaberi, T.; Al-Nusairr, M.; Bani-Hani, K.E.; Heath, D.D. Dietary patterns and colorectal cancer. Clin. Nutr. 2017, 36, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, R.L.; Pierre, F.; Corpet, D.E. Processed meat and colorectal cancer: A review of epidemiologic and experimental evidence. Nutr. Cancer 2008, 60, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- Pericleous, M.; Mandair, D.; Caplin, M.E. Diet and supplements and their impact on colorectal cancer. J. Gastrointest. Oncol. 2013, 4, 409–423. [Google Scholar] [PubMed]
- Barzi, A.; Lenz, A.M.; Labonte, M.J.; Lenz, H.J. Molecular pathways: Estrogen pathway in colorectal cancer. Clin. Cancer Res. 2013, 19, 5842–5848. [Google Scholar] [CrossRef] [PubMed]
- Wild, N.; Andres, H.; Rollinger, W.; Krause, F.; Dilba, P.; Tacke, M.; Karl, J. A combination of serum markers for the early detection of colorectal cancer. Clin. Cancer Res. 2010, 16, 6111–6121. [Google Scholar] [CrossRef] [PubMed]
- Berretta, M.; Alessandrini, L.; De Divitiis, C.; Nasti, G.; Lleshi, A.; Di Francia, R.; Facchini, G.; Cavaliere, C.; Buonerba, C.; Canzonieri, V. Serum and tissue markers in colorectal cancer: State of art. Crit. Rev. Oncol. Hematol. 2017, 111, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzialo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Mussatto, S.I.; Martinez-Avila, G.; Montanez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Donovan, J.L. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free Radic. Res. 2004, 38, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Reis Giada, M.D.L. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. In Oxidative Stress and Chronic Degenerative Diseases—A Role for Antioxidants; Croatia InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef] [Green Version]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Mezzomo, N.; Ferreira, S.R.S. Carotenoids Functionality, Sources, and Processing by Supercritical Technology: A Review. J. Chem. 2016, 2016, 1–16. [Google Scholar] [CrossRef]
- Wang, Y.; Chung, S.J.; McCullough, M.L.; Song, W.O.; Fernandez, M.L.; Koo, S.I.; Chun, O.K. Dietary carotenoids are associated with cardiovascular disease risk biomarkers mediated by serum carotenoid concentrations. J. Nutr. 2014, 144, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal el, S.M.; Akhtar, H.; Zaheer, K.; Ali, R. Dietary sources of lutein and zeaxanthin carotenoids and their role in eye health. Nutrients 2013, 5, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed]
- Irchhaiya, R.; Kumar, A.; Yadav, A.; Gupta, N.; Kumar, S.; Gupta, N.; Kumar, S.; Yadav, V.; Prakash, A.; Gurjar, H. Metabolites in plants and its classification. World J. Pharm. Pharm. Sci. 2014, 4, 287–305. [Google Scholar]
- Kabera, J.N.; Semana, E.; Mussa, A.R.; Xin, H. Plant Secondary Metabolites: Biosynthesis, Classification, Function and Pharmacological Properties. J. Pharm. Pharmacol. 2014, 2, 377–392. [Google Scholar]
- Gonzalez-Vallinas, M.; Gonzalez-Castejon, M.; Rodriguez-Casado, A.; Ramirez de Molina, A. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran. J. Basic Med. Sci. 2013, 16, 1031–1048. [Google Scholar] [PubMed]
- Ogbe, R.J.; Ochalefu, D.O.; Mafulul, S.G.; Olaniru, O.B. A review on dietary phytosterols: Their occurrence, metabolism and health benefits. Asian J. Plant Sci. Res. 2015, 5, 10–21. [Google Scholar]
- Bayala, B.; Bassole, I.H.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.B.; Lobaccaro, J.M.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Slavin, J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Zarate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Perez, J.A.; Rodriguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Ibarguren, M.; Lopez, D.J.; Escriba, P.V. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta 2014, 1838, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Duan, Y.; Li, Y.; Tang, Y.; Geng, M.; Oladele, O.A.; Kim, S.W.; Yin, Y. Effects of dietary n-6:n-3 PUFA ratio on fatty acid composition, free amino acid profile and gene expression of transporters in finishing pigs. Br. J. Nutr. 2015, 113, 739–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Shieh, C.H.; Wu, Y.S.; Kalueff, A.; Gaikwad, S.; Su, K.P. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: Acting separately or synergistically? Prog. Lipid Res. 2016, 62, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J. Acad. Nutr. Diet. 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Otles, S.; Ozgoz, S. Health effects of dietary fiber. Acta Sci. Pol. Technol. Aliment. 2014, 13, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Sarker, M.Z.; Ferdosh, S.; Akanda, M.J.; Easmin, M.S.; Bt Shamsudin, S.H.; Bin Yunus, K. Phytosterols and their extraction from various plant matrices using supercritical carbon dioxide: A review. J. Sci. Food Agric. 2015, 95, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Bradford, C. Omega-3, Omega-6 and Omega-9 Fatty Acids: Implications for Cardiovascular and Other Diseases. J. Glycomics Lipidomics 2014, 4. [Google Scholar] [CrossRef]
- Villares, A.; Mateo-Vivaracho, L.; Garcia-Lafuente, A.; Guillamon, E. Storage temperature and UV-irradiation influence on the ergosterol content in edible mushrooms. Food Chem. 2014, 147, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Naumoska, K.; Vovk, I. Analysis of triterpenoids and phytosterols in vegetables by thin-layer chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1381, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Mudannayake, D.C.; Wimalasiri, K.M.S.; Silva, K.; Ajlouni, S. Selected Sri Lankan food plants and other herbs as potential sources of inulin-type fructans. J. Natl. Sci. Found. Sri Lanka 2015, 43. [Google Scholar] [CrossRef]
- Pan, M.H.; Lai, C.S.; Wu, J.C.; Ho, C.T. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol. Nutr. Food Res. 2011, 55, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; George, J.; Ahmad, N. Resveratrol-based combinatorial strategies for cancer management. Ann. N. Y. Acad. Sci. 2013, 1290, 113–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranelletti, F.O.; Maggiano, N.; Serra, F.G.; Ricci, R.; Larocca, L.M.; Lanza, P.; Scambia, G.; Fattorossi, A.; Capelli, A.; Piantelli, M. Quercetin inhibits p21-RAS expression in human colon cancer cell lines and in primary colorectal tumors. Int. J. Cancer 2000, 85, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Psahoulia, F.H.; Drosopoulos, K.G.; Doubravska, L.; Andera, L.; Pintzas, A. Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol. Cancer Ther. 2007, 6, 2591–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier, C.P.; Lima, C.F.; Rohde, M.; Pereira-Wilson, C. Quercetin enhances 5-fluorouracil-induced apoptosis in MSI colorectal cancer cells through p53 modulation. Cancer Chemother. Pharmacol. 2011, 68, 1449–1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-del-Río, I.; Villar, C.J.; Lombó, F. Therapeutic Uses of Kaempferol: Anticancer and Anti-Inflammatory Activity; Nova Science Publishers, Inc: New York, NY, USA, 2016. [Google Scholar]
- Li, W.; Du, B.; Wang, T.; Wang, S.; Zhang, J. Kaempferol induces apoptosis in human HCT116 colon cancer cells via the Ataxia-Telangiectasia Mutated-p53 pathway with the involvement of p53 Upregulated Modulator of Apoptosis. Chem. Biol. Interact. 2009, 177, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Cho, H.J.; Yu, R.; Lee, K.W.; Chun, H.S.; Park, J.H. Mechanisms underlying apoptosis-inducing effects of Kaempferol in HT-29 human colon cancer cells. Int. J. Mol. Sci. 2014, 15, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Park, J.H. Kaempferol Induces Cell Cycle Arrest in HT-29 Human Colon Cancer Cells. J. Cancer Prev. 2013, 18, 257–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.S.; Cho, H.J.; Kwon, G.T.; Park, J.H. Kaempferol Downregulates Insulin-like Growth Factor-I Receptor and ErbB3 Signaling in HT-29 Human Colon Cancer Cells. J. Cancer Prev. 2014, 19, 161–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Konishi, M.; Horinaka, M.; Yasuda, T.; Goda, A.E.; Taniguchi, H.; Yano, K.; Wakada, M.; Sakai, T. Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem. Biophys. Res. Commun. 2008, 375, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Krisanapun, C.; Lee, S.H.; Nualsanit, T.; Sams, C.; Peungvicha, P.; Baek, S.J. Molecular targets of apigenin in colorectal cancer cells: Involvement of p21, NAG-1 and p53. Eur. J. Cancer 2010, 46, 3365–3374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.; Sung, B.; Kang, Y.J.; Kim, D.H.; Jang, J.Y.; Hwang, S.Y.; Kim, M.; Lim, H.S.; Yoon, J.H.; Chung, H.Y.; et al. Apigenin-induced apoptosis is enhanced by inhibition of autophagy formation in HCT116 human colon cancer cells. Int. J. Oncol. 2014, 44, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Allred, K.F.; Dykes, L.; Allred, C.D.; Awika, J.M. Enhanced action of apigenin and naringenin combination on estrogen receptor activation in non-malignant colonocytes: Implications on sorghum-derived phytoestrogens. Food Funct. 2015, 6, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Spector, D.; Anthony, M.; Alexander, D.; Arab, L. Soy consumption and colorectal cancer. Nutr. Cancer 2003, 47, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Negri, E.; Talamini, R.; Bosetti, C.; Parpinel, M.; Gnagnarella, P.; Franceschi, S.; Dal Maso, L.; Montella, M.; Giacosa, A.; et al. Flavonoids and colorectal cancer in Italy. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. Biomed. Res. Int. 2015, 2015, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, W.; Liu, F. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 2004, 215, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, S.X.; Zhou, Z.Q.; Wang, Z.; Zhang, Y.G.; Zhang, Y.; Zhao, P. Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-kappaB) pathway. Tumour Biol. 2014, 35, 11483–11488. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Shiomi, K.; Kuriyama, I.; Takahashi, Y.; Yoshida, H. Inhibitory effects of a major soy isoflavone, genistein, on human DNA topoisomerase II activity and cancer cell proliferation. Int. J. Oncol. 2013, 43, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, B.; Palmini, G.; Mavilia, C.; Zonefrati, R.; Tanini, A.; Brandi, M.L. In vitro effects of polyphenols on colorectal cancer cells. World J. Gastrointest. Oncol. 2014, 6, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Zhou, D.; Chen, H. Genistein, a soya isoflavone, prevents azoxymethane-induced up-regulation of WNT/beta-catenin signalling and reduces colon pre-neoplasia in rats. Br. J. Nutr. 2013, 109, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Ha, J.; Park, O.J. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem. Biophys. Res. Commun. 2005, 332, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Nasr Bouzaiene, N.; Kilani Jaziri, S.; Kovacic, H.; Chekir-Ghedira, L.; Ghedira, K.; Luis, J. The effects of caffeic, coumaric and ferulic acids on proliferation, superoxide production, adhesion and migration of human tumor cells in vitro. Eur. J. Pharmacol. 2015, 766, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Murad, L.D.; Soares Nda, C.; Brand, C.; Monteiro, M.C.; Teodoro, A.J. Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr. Cancer 2015, 67, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W.; Lim, M.S.; Lee, J.W.; Chun, W.; Lee, S.H.; Nam, Y.H.; Park, J.M.; Choi, D.H.; Kang, C.D.; Lee, S.J.; et al. The Cytotoxicity of Kahweol in HT-29 Human Colorectal Cancer Cells Is Mediated by Apoptosis and Suppression of Heat Shock Protein 70 Expression. Biomol. Ther. 2015, 23, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Q.; Hirose, Y.; Yoshimi, N.; Murakami, A.; Koshimizu, K.; Ohigashi, H.; Sakata, K.; Matsumoto, Y.; Sayama, Y.; Mori, H. Further investigation of the modifying effect of various chemopreventive agents on apoptosis and cell proliferation in human colon cancer cells. J. Cancer Res. Clin. Oncol. 2002, 128, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Faried, A.; Kurnia, D.; Faried, L.S.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giftson, J.S.; Jayanthi, S.; Nalini, N. Chemopreventive efficacy of gallic acid, an antioxidant and anticarcinogenic polyphenol, against 1,2-dimethyl hydrazine induced rat colon carcinogenesis. Investig. New Drugs 2010, 28, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Blanco, S.; Fernandez, J.; Gutierrez-Del-Rio, I.; Villar, C.J.; Lombo, F. New Insights toward Colorectal Cancer Chemotherapy Using Natural Bioactive Compounds. Front. Pharmacol. 2017, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Del Follo-Martinez, A.; Banerjee, N.; Li, X.; Safe, S.; Mertens-Talcott, S. Resveratrol and quercetin in combination have anticancer activity in colon cancer cells and repress oncogenic microRNA-27a. Nutr. Cancer 2013, 65, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Reddivari, L.; Charepalli, V.; Radhakrishnan, S.; Vadde, R.; Elias, R.J.; Lambert, J.D.; Vanamala, J.K. Grape compounds suppress colon cancer stem cells in vitro and in a rodent model of colon carcinogenesis. BMC Complement. Altern. Med. 2016, 16, 278. [Google Scholar] [CrossRef] [PubMed]
- Santandreu, F.M.; Valle, A.; Oliver, J.; Roca, P. Resveratrol potentiates the cytotoxic oxidative stress induced by chemotherapy in human colon cancer cells. Cell. Physiol. Biochem. 2011, 28, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, P.; Preet, R.; Choudhuri, M.; Choudhuri, T.; Kundu, C.N. 5-fluorouracil increases the chemopreventive potentials of resveratrol through DNA damage and MAPK signaling pathway in human colorectal cancer cells. Oncol. Res. 2011, 19, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, W.; Sun, H.; Wu, B.; Ji, F.; Sun, T.; Chang, H.; Shen, P.; Wang, Y.; Zhou, D. Resveratrol elicits anti-colorectal cancer effect by activating miR-34c-KITLG in vitro and in vivo. BMC Cancer 2015, 15, 969. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, B.M.; Weigert, A.; Scherzberg, M.C.; Ley, S.; Gilbert, B.; Brecht, K.; Brune, B.; Steinhilber, D.; Stein, J.; Ulrich-Ruckert, S. Resveratrol-induced potentiation of the antitumor effects of oxaliplatin is accompanied by an altered cytokine profile of human monocyte-derived macrophages. Apoptosis 2014, 19, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Braun, D.P. Resveratrol enhances mitomycin C-mediated suppression of human colorectal cancer cell proliferation by up-regulation of p21WAF1/CIP1. Anticancer Res. 2014, 34, 5439–5446. [Google Scholar] [PubMed]
- Chauhan, D.P. Chemotherapeutic potential of curcumin for colorectal cancer. Curr. Pharm. Des. 2002, 8, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008, 75, 787–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: The golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Jiang, L.; Xia, Q.; Zhong, L. Synergistic inhibitory effects of curcumin and 5-fluorouracil on the growth of the human colon cancer cell line HT-29. Chemotherapy 2006, 52, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Srimuangwong, K.; Tocharus, C.; Yoysungnoen Chintana, P.; Suksamrarn, A.; Tocharus, J. Hexahydrocurcumin enhances inhibitory effect of 5-fluorouracil on HT-29 human colon cancer cells. World J. Gastroenterol. 2012, 18, 2383–2389. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Diagaradjane, P.; Anand, P.; Harikumar, K.B.; Deorukhkar, A.; Gelovani, J.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer 2009, 125, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, H.H.; Liu, Y.; Zhou, Q.; Chen, Z.H. Lycopene Consumption and Risk of Colorectal Cancer: A Meta-Analysis of Observational Studies. Nutr. Cancer 2016, 68, 1083–1096. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solis, C.; Pedraza-Chaverri, J.; Torres-Ramos, M.; Jimenez-Farfan, D.; Cruz Salgado, A.; Serrano-Garcia, N.; Osorio-Rico, L.; Sotelo, J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid.-Based Complement. Altern. Med. ECAM 2013, 2013, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Shih, C.J.; Cheng, L.H.; Ho, H.J.; Chen, H.J. Lycopene inhibits growth of human colon cancer cells via suppression of the Akt signaling pathway. Mol. Nutr. Food Res. 2008, 52, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Wang, F.Y.; Kuo, Y.H.; Tang, F.Y. Cancer chemopreventive effects of lycopene: Suppression of MMP-7 expression and cell invasion in human colon cancer cells. J. Agric. Food Chem. 2011, 59, 11304–11318. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Cho, H.J.; Pai, M.H.; Chen, Y.H. Concomitant supplementation of lycopene and eicosapentaenoic acid inhibits the proliferation of human colon cancer cells. J. Nutr. Biochem. 2009, 20, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Mantovani, A. Inflammatory bowel disease and intestinal cancer: A paradigm of the Yin-Yang interplay between inflammation and cancer. Oncogene 2010, 29, 3313–3323. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, S.; Lietz, H.; Mobarhan, S.; Frommel, T.O. In vitro beta-carotene toxicity for human colon cancer cells. Nutr. Cancer 1996, 25, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Serini, S.; Torsello, A.; Di Nicuolo, F.; Piccioni, E.; Ubaldi, V.; Pioli, C.; Wolf, F.I.; Calviello, G. Beta-carotene regulates NF-kappaB DNA-binding activity by a redox mechanism in human leukemia and colon adenocarcinoma cells. J. Nutr. 2003, 133, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Briviba, K.; Schnabele, K.; Schwertle, E.; Blockhaus, M.; Rechkemmer, G. Beta-carotene inhibits growth of human colon carcinoma cells in vitro by induction of apoptosis. Biol. Chem. 2001, 382, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Serini, S.; Maggiano, N.; Angelini, M.; Boninsegna, A.; Di Nicuolo, F.; Ranelletti, F.O.; Calviello, G. Induction of cell cycle arrest and apoptosis in human colon adenocarcinoma cell lines by beta-carotene through down-regulation of cyclin A and Bcl-2 family proteins. Carcinogenesis 2002, 23, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palozza, P.; Calviello, G.; Serini, S.; Maggiano, N.; Lanza, P.; Ranelletti, F.O.; Bartoli, G.M. Beta-Carotene at high concentrations induces apoptosis by enhancing oxy-radical production in human adenocarcinoma cells. Free Radic. Biol. Med. 2001, 30, 1000–1007. [Google Scholar] [CrossRef]
- Nam, K.N.; Park, Y.M.; Jung, H.J.; Lee, J.Y.; Min, B.D.; Park, S.U.; Jung, W.S.; Cho, K.H.; Park, J.H.; Kang, I.; et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur. J. Pharmacol. 2010, 648, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Aung, H.H.; Wang, C.Z.; Ni, M.; Fishbein, A.; Mehendale, S.R.; Xie, J.T.; Shoyama, C.Y.; Yuan, C.S. Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp. Oncol. 2007, 29, 175–180. [Google Scholar] [PubMed]
- Dhar, A.; Mehta, S.; Dhar, G.; Dhar, K.; Banerjee, S.; Van Veldhuizen, P.; Campbell, D.R.; Banerjee, S.K. Crocetin inhibits pancreatic cancer cell proliferation and tumor progression in a xenograft mouse model. Mol. Cancer Ther. 2009, 8, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.H.; Tavakkol-Afshari, J.; Brook, A.; Jafari-Anarkooli, I. Role of caspases and Bax protein in saffron-induced apoptosis in MCF-7 cells. Food Chem. Toxicol. 2009, 47, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.; Guha, D.; Chakraborty, J.; Banerjee, S.; Adhikary, A.; Chakraborty, S.; Das, T.; Sa, G. Crocetin exploits p53-induced death domain (PIDD) and FAS-associated death domain (FADD) proteins to induce apoptosis in colorectal cancer. Sci. Rep. 2016, 6, 32979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bajbouj, K.; Schulze-Luehrmann, J.; Diermeier, S.; Amin, A.; Schneider-Stock, R. The anticancer effect of saffron in two p53 isogenic colorectal cancer cell lines. BMC Complement. Altern. Med. 2012, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Dong, A.; Wang, R.; Shi, A. Crocetin treatment inhibits proliferation of colon cancer cells through down-regulation of genes involved in the inflammation. Saudi J. Biol. Sci. 2017. [Google Scholar] [CrossRef]
- Yaffe, P.B.; Power Coombs, M.R.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine, an alkaloid from black pepper, inhibits growth of human colon cancer cells via G1 arrest and apoptosis triggered by endoplasmic reticulum stress. Mol. Carcinog. 2015, 54, 1070–1085. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, P.B.; Doucette, C.D.; Walsh, M.; Hoskin, D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013, 94, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; He, B.; Zhang, C.; Rodriguez, E.; Hage, D.S.; Moreau, R. Piperine potentiates curcumin-mediated repression of mTORC1 signaling in human intestinal epithelial cells: Implications for the inhibition of protein synthesis and TNFalpha signaling. J. Nutr. Biochem. 2018, 57, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Ginghina, O.; Negrei, C.; Hudita, A.; Ioana-Lavric, V.; Galateanu, B.; Dragomir, S. In vitro impact of some natural compounds on HT-29 colorectal adenocarcinoma cells. Farmacia 2017, 65, 947–953. [Google Scholar]
- Lee, S.H.; Clark, R. Anti-Tumorigenic Effects of Capsaicin in Colon Cancer. J. Food Chem. Nanotechnol. 2016, 2, 162–167. [Google Scholar] [CrossRef]
- Lu, H.F.; Chen, Y.L.; Yang, J.S.; Yang, Y.Y.; Liu, J.Y.; Hsu, S.C.; Lai, K.C.; Chung, J.G. Antitumor activity of capsaicin on human colon cancer cells in vitro and colo 205 tumor xenografts in vivo. J. Agric. Food Chem. 2010, 58, 12999–13005. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.M.; Pyo, J.O.; Kim, G.Y.; Yu, R.; Han, I.S.; Ju, S.A.; Kim, W.H.; Kim, B.S. Capsaicin induces apoptosis by generating reactive oxygen species and disrupting mitochondrial transmembrane potential in human colon cancer cell lines. Cell. Mol. Biol. Lett. 2009, 14, 497–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Li, T.Z.; Xu, G.H.; Luo, B.B.; Chen, Y.X.; Zhang, T. Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways. Neoplasma 2013, 60, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Lin, G.; Huang, H.; Xu, D.; Yu, H.; Ma, X.; Zhu, L.; Ma, D.; Jiang, H. Capsaicin mediates cell cycle arrest and apoptosis in human colon cancer cells via stabilizing and activating p53. Int. J. Biol. Sci. 2014, 10, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Hwang, J.T.; Kwak, D.W.; Lee, Y.K.; Park, O.J. Involvement of AMPK signaling cascade in capsaicin-induced apoptosis of HT-29 colon cancer cells. Ann. N. Y. Acad. Sci. 2007, 1095, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Richardson, R.L.; Dashwood, R.H.; Baek, S.J. Capsaicin represses transcriptional activity of beta-catenin in human colorectal cancer cells. J. Nutr. Biochem. 2012, 23, 646–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.Y.; Trudel, L.J.; Wogan, G.N. Apoptosis induced by capsaicin and resveratrol in colon carcinoma cells requires nitric oxide production and caspase activation. Anticancer Res. 2009, 29, 3733–3740. [Google Scholar] [PubMed]
- Bessler, H.; Djaldetti, M. Capsaicin Modulates the Immune Cross Talk Between Human Mononuclears and Cells from Two Colon Carcinoma Lines. Nutr. Cancer 2017, 69, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.; Lee, J.; Lee, S.H. Synergistic anticancer activity of capsaicin and 3,3′-diindolylmethane in human colorectal cancer. J. Agric. Food Chem. 2015, 63, 4297–4304. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cheng, D.; Wei, S.; Wang, X.; Niu, Y.; Qi, W.; Wang, C. Preventive effect of genistein on AOM/DSS-induced colonic neoplasm by modulating the PI3K/AKT/FOXO3 signaling pathway in mice fed a high-fat diet. J. Funct. Foods 2018, 46, 237–242. [Google Scholar] [CrossRef]
- Son, T.G.; Gong, E.J.; Bae, M.J.; Kim, S.D.; Heo, K.; Moon, C.; Yang, K.; Kim, J.S. Protective effect of genistein on radiation-induced intestinal injury in tumor bearing mice. BMC Complement. Altern. Med. 2013, 13, 103. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-Y.; Seeram, N.P.; Nair, M.G.; Bourquin, L.D. Tart cherry anthocyanins inhibit tumor development in ApcMin mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003, 194, 13–19. [Google Scholar] [CrossRef]
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients 2015, 7, 1696–1715. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.M.; Campanholo, V.M.; Paiotti, A.P.; Artigiani Neto, R.; Oshima, C.T.; Ribeiro, D.A.; Forones, N.M. Chemopreventive activity of grape juice concentrate (G8000TM) on rat colon carcinogenesis induced by azoxymethane. Environ. Toxicol. Pharmacol. 2015, 40, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Garcia, L.; Monte, J.; Villar, C.J.; Lombo, F. Functional Anthocyanin-Rich Sausages Diminish Colorectal Cancer in an Animal Model and Reduce Pro-Inflammatory Bacteria in the Intestinal Microbiota. Genes 2018, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Katayama, M.; Sakata, K.; Kuno, T.; Yoshida, K.; Yamada, Y.; Hirose, Y.; Yoshimi, N.; Mori, H. Inhibitory Effects of Chlorogenic Acid on Azoxymethane-induced Colon Carcinogenesis in Male F344 Rats. Asian Pac. J. Cancer Prev. 2002, 3, 163–166. [Google Scholar] [PubMed]
- Banerjee, N.; Kim, H.; Talcott, S.T.; Turner, N.D.; Byrne, D.H.; Mertens-Talcott, S.U. Plum polyphenols inhibit colorectal aberrant crypt foci formation in rats: Potential role of the miR-143/protein kinase B/mammalian target of rapamycin axis. Nutr. Res. 2016, 36, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Shang, B.; Li, Y.; Zhen, Y. Inhibition of histone deacetylases by trans-cinnamic acid and its antitumor effect against colon cancer xenografts in athymic mice. Mol. Med. Rep. 2016, 13, 4159–4166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.H.; Chellappan, D.R.; Chinnaswamy, P.; Nagarajan, S. Protective effect of p-coumaric acid against 1,2 dimethylhydrazine induced colonic preneoplastic lesions in experimental rats. Biomed. Pharmacother. 2017, 94, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Gunasekaran, S.; Jesudoss, V.A.; Namasivayam, N. The effect of rosmarinic acid on 1,2-dimethylhydrazine induced colon carcinogenesis. Exp. Toxicol. Pathol. 2013, 65, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Giftson Senapathy, J.; Jayanthi, S.; Viswanathan, P.; Umadevi, P.; Nalini, N. Effect of gallic acid on xenobiotic metabolizing enzymes in 1,2-dimethyl hydrazine induced colon carcinogenesis in Wistar rats—A chemopreventive approach. Food Chem. Toxicol. 2011, 49, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.R.; Patel, B.M. Secoisolariciresinol diglucoside rich extract of L. usitatissimum prevents diabetic colon cancer through inhibition of CDK4. Biomed. Pharmacother. 2016, 83, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Gomides, A.F.; Paula, S.O.; Rosa, D.D.; Oliveira, L.L.; Comastri, D.S.; Peluzio Mdo, C. Use of defatted flaxseed meal reduces precancerous colon lesions in C57BL/6 mice. Acta Cir. Bras. 2013, 28, 607–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, S.; Fujii, G.; Takahashi, M.; Nakanishi, R.; Komiya, M.; Shimura, M.; Noma, N.; Onuma, W.; Terasaki, M.; Yano, T.; et al. Sesamol suppresses cyclooxygenase-2 transcriptional activity in colon cancer cells and modifies intestinal polyp development in Apc (Min/+) mice. J. Clin. Biochem. Nutr. 2014, 54, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C. Radioprotection and radiosensitization by curcumin. Adv. Exp. Med. Biol. 2007, 595, 301–320. [Google Scholar] [PubMed]
- Tang, F.Y.; Pai, M.H.; Wang, X.D. Consumption of lycopene inhibits the growth and progression of colon cancer in a mouse xenograft model. J. Agric. Food Chem. 2011, 59, 9011–9021. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Vieiralves, N.F.; Gomes, M.I.; Salvadori, D.M.; Rodrigues, M.A.; Barbisan, L.F. Effects of lycopene, synbiotic and their association on early biomarkers of rat colon carcinogenesis. Food Chem. Toxicol. 2010, 48, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Tung, N.H.; Shoyama, Y.; Sugie, S.; Mori, T.; Tanaka, T. Dietary Crocin Inhibits Colitis and Colitis-Associated Colorectal Carcinogenesis in Male ICR Mice. Evid.-Based Complement. Altern. Med. ECAM 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Giner, E.; Recio, M.C.; Rios, J.L.; Cerda-Nicolas, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Caetano, B.F.R.; Tablas, M.B.; Pereira, N.E.F.; de Moura, N.A.; Carvalho, R.F.; Rodrigues, M.A.M.; Barbisan, L.F. Capsaicin reduces genotoxicity, colonic cell proliferation and preneoplastic lesions induced by 1,2-dimethylhydrazine in rats. Toxicol. Appl. Pharmacol. 2018, 338, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Dashwood, W.M.; Li, L.; Kang, Y.; Kim, E.; Johnson, G.; Fischer, K.A.; Lohr, C.V.; Williams, D.E.; Ho, E.; et al. Nrf2 status affects tumor growth, HDAC3 gene promoter associations, and the response to sulforaphane in the colon. Clin. Epigenetics 2015, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.A.; Al Numair, K.S.; Gabriel Paulraj, M.; Alsaif, M.A.; Muamar, M.A.; Ignacimuthu, S. beta-sitosterol prevents lipid peroxidation and improves antioxidant status and histoarchitecture in rats with 1,2-dimethylhydrazine-induced colon cancer. J. Med. Food 2012, 15, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Asfour, W.; Almadi, S.; Haffar, L. Thymoquinone Suppresses Cellular Proliferation, Inhibits VEGF Production and Obstructs Tumor Progression and Invasion in the Rat Model of DMH-Induced Colon Carcinogenesis. Pharmacol. Pharm. 2013, 4, 7–17. [Google Scholar] [CrossRef]
- Kortum, B.; Campregher, C.; Lang, M.; Khare, V.; Pinter, M.; Evstatiev, R.; Schmid, G.; Mittlbock, M.; Scharl, T.; Kucherlapati, M.H.; et al. Mesalazine and thymoquinone attenuate intestinal tumour development in Msh2(loxP/loxP) Villin-Cre mice. Gut 2015, 64, 1905–1912. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Tao, S.; Rojo de la Vega, M.; Jiang, T.; Wen, Q.; Park, S.L.; Zhang, D.D.; Wondrak, G.T. Nrf2-dependent suppression of azoxymethane/dextran sulfate sodium-induced colon carcinogenesis by the cinnamon-derived dietary factor cinnamaldehyde. Cancer Prev. Res. 2015, 8, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, A.; Sivagami, G.; Nalini, N. Chemopreventive effect of carvacrol on 1,2-dimethylhydrazine induced experimental colon carcinogenesis. J. Cancer Res. Ther. 2016, 12, 755–762. [Google Scholar] [PubMed]
- Wang, W.; Yang, J.; Nimiya, Y.; Lee, K.S.S.; Sanidad, K.; Qi, W.; Sukamtoh, E.; Park, Y.; Liu, Z.; Zhang, G. omega-3 Polyunsaturated fatty acids and their cytochrome P450-derived metabolites suppress colorectal tumor development in mice. J. Nutr. Biochem. 2017, 48, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hao, G.W.; Chen, Y.S.; He, D.M.; Wang, H.Y.; Wu, G.H.; Zhang, B. Growth of Human Colon Cancer Cells in Nude Mice is Delayed by Ketogenic Diet With or Without Omega-3 Fatty Acids and Medium-chain Triglycerides. Asian Pac. J. Cancer Prev. 2015, 16, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Piazzi, G.; D’Argenio, G.; Prossomariti, A.; Lembo, V.; Mazzone, G.; Candela, M.; Biagi, E.; Brigidi, P.; Vitaglione, P.; Fogliano, V.; et al. Eicosapentaenoic acid free fatty acid prevents and suppresses colonic neoplasia in colitis-associated colorectal cancer acting on Notch signaling and gut microbiota. Int. J. Cancer 2014, 135, 2004–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barone, M.; Notarnicola, M.; Caruso, M.G.; Scavo, M.P.; Viggiani, M.T.; Tutino, V.; Polimeno, L.; Pesetti, B.; Di Leo, A.; Francavilla, A. Olive oil and omega-3 polyunsaturated fatty acids suppress intestinal polyp growth by modulating the apoptotic process in ApcMin/+ mice. Carcinogenesis 2014, 35, 1613–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, W.; Huang, X.; Liu, Y.; Li, Q.; Zheng, Z.; Wang, K. A polysaccharide from Lentinus edodes inhibits human colon cancer cell proliferation and suppresses tumor growth in athymic nude mice. Oncotarget 2017, 8, 610–623. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Inoue, H.; Ohta, H.; Miyake, A.; Konishi, M.; Nanba, H. Oral administration of soluble beta-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int. J. Cancer 2013, 133, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Pattananandecha, T.; Sirilun, S.; Duangjitcharoen, Y.; Sivamaruthi, B.S.; Suwannalert, P.; Peerajan, S.; Chaiyasut, C. Hydrolysed inulin alleviates the azoxymethane-induced preneoplastic aberrant crypt foci by altering selected intestinal microbiota in Sprague-Dawley rats. Pharm. Biol. 2016, 54, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Qamar, T.R.; Syed, F.; Nasir, M.; Rehman, H.; Zahid, M.N.; Liu, R.H.; Iqbal, S. Novel Combination of Prebiotics Galacto-Oligosaccharides and Inulin-Inhibited Aberrant Crypt Foci Formation and Biomarkers of Colon Cancer in Wistar Rats. Nutrients 2016, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Hijová, E.; Szabadosova, V.; Štofilová, J.; Hrčková, G. Chemopreventive and metabolic effects of inulin on colon cancer development. J. Vet. Sci. 2013, 14, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Shukla, G. Administration of prebiotic inulin suppresses 1,2 dimethylhydrazine dihydrochloride induced procarcinogenic biomarkers fecal enzymes and preneoplastic lesions in early colon carcinogenesis in Sprague Dawley rats. J. Funct. Foods 2013, 5, 991–996. [Google Scholar] [CrossRef]
- Stofilova, J.; Szabadosova, V.; Hrckova, G.; Salaj, R.; Bertkova, I.; Hijova, E.; Strojny, L.; Bomba, A. Co-administration of a probiotic strain Lactobacillus plantarum LS/07 CCM7766 with prebiotic inulin alleviates the intestinal inflammation in rats exposed to N,N-dimethylhydrazine. Int. Immunopharmacol. 2015, 24, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Yang, L.C.; Chen, H.L. Effects of konjac glucomannan, inulin and cellulose on acute colonic responses to genotoxic azoxymethane. Food Chem. 2014, 155, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, S.R.; Sarbu, M.I.; Matei, C.; Ilie, M.A.; Caruntu, C.; Constantin, C.; Neagu, M.; Tampa, M. Capsaicin: Friend or Foe in Skin Cancer and Other Related Malignancies? Nutrients 2017, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Erin, N.; Boyer, P.J.; Bonneau, R.H.; Clawson, G.A.; Welch, D.R. Capsaicin-mediated denervation of sensory neurons promotes mammary tumor metastasis to lung and heart. Anticancer Res. 2004, 24, 1003–1009. [Google Scholar] [PubMed]
- Ververis, K.; Hiong, A.; Karagiannis, T.C.; Licciardi, P.V. Histone deacetylase inhibitors (HDACIs): Multitargeted anticancer agents. Biologics 2013, 7, 47–60. [Google Scholar] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Arivalagan, S.; Thomas, N.S.; Chandrasekaran, B.; Mani, V.; Siddique, A.I.; Kuppsamy, T.; Namasivayam, N. Combined therapeutic efficacy of carvacrol and X-radiation against 1,2-dimethyl hydrazine-induced experimental rat colon carcinogenesis. Mol. Cell. Biochem. 2015, 410, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Vogtmann, E.; Xiang, Y.B.; Li, H.L.; Levitan, E.B.; Yang, G.; Waterbor, J.W.; Gao, J.; Cai, H.; Xie, L.; Wu, Q.J.; et al. Fruit and vegetable intake and the risk of colorectal cancer: Results from the Shanghai Men’s Health Study. Cancer Causes Control 2013, 24, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Akhter, M.; Iwasaki, M.; Yamaji, T.; Sasazuki, S.; Tsugane, S. Dietary isoflavone and the risk of colorectal adenoma: A case-control study in Japan. Br. J. Cancer 2009, 100, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Joo, J.; Yang, H.R.; Bak, J.; Park, Y.; Kim, J.; Oh, J.H.; Nam, B.H. Risk prediction model for colorectal cancer: National Health Insurance Corporation study, Korea. PLoS ONE 2014, 9, e88079. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ros, R.; Not, C.; Guino, E.; Lujan-Barroso, L.; Garcia, R.M.; Biondo, S.; Salazar, R.; Moreno, V. Association between habitual dietary flavonoid and lignan intake and colorectal cancer in a Spanish case-control study (the Bellvitge Colorectal Cancer Study). Cancer Causes Control 2013, 24, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.P.; Yeo, Y.; Yoon, J.H.; Kim, C.S.; Tokudome, S.; Ngoan, L.T.; Koriyama, C.; Lim, Y.K.; Chang, S.H.; Shin, H.R.; et al. Plasma phytoestrogens concentration and risk of colorectal cancer in two different Asian populations. Clin. Nutr. 2018, 37, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Ganai, A.A.; Farooqi, H. Bioactivity of genistein: A review of in vitro and in vivo studies. Biomed. Pharmacother. 2015, 76, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, Z.; Wang, R.; Wang, J.; Zhang, S.; Cai, X.; Wu, K.; Bergan, R.C.; Xu, L.; Fan, D. Genistein suppresses FLT4 and inhibits human colorectal cancer metastasis. Oncotarget 2015, 6, 3225–3239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Principi, M.; Di Leo, A.; Pricci, M.; Scavo, M.P.; Guido, R.; Tanzi, S.; Piscitelli, D.; Pisani, A.; Ierardi, E.; Comelli, M.C.; et al. Phytoestrogens/insoluble fibers and colonic estrogen receptor beta: Randomized, double-blind, placebo-controlled study. World J. Gastroenterol. 2013, 19, 4325–4333. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Pratico, C.; Calafiore, A.; Coscia, M.; Gentilini, L.; Poggioli, G.; Gionchetti, P.; Campieri, M.; Rizzello, F. Eviendep(R) reduces number and size of duodenal polyps in familial adenomatous polyposis patients with ileal pouch-anal anastomosis. World J. Gastroenterol. 2013, 19, 5671–5677. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.; Lee, J.; Lee, J.; Park, M.S.; Park, J.W.; Park, S.C.; Oh, J.H.; Kim, J. Isoflavone and Soyfood Intake and Colorectal Cancer Risk: A Case-Control Study in Korea. PLoS ONE 2015, 10, e0143228. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shu, X.O.; Li, H.; Chow, W.H.; Cai, H.; Zhang, X.; Gao, Y.T.; Zheng, W. Prospective cohort study of soy food intake and colorectal cancer risk in women. Am. J. Clin. Nutr. 2009, 89, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Thomasset, S.; Berry, D.P.; Cai, H.; West, K.; Marczylo, T.H.; Marsden, D.; Brown, K.; Dennison, A.; Garcea, G.; Miller, A.; et al. Pilot study of oral anthocyanins for colorectal cancer chemoprevention. Cancer Prev. Res. 2009, 2, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Burke, C.A.; Hasson, H.; Kuo, C.T.; Molmenti, C.L.; Seguin, C.; Liu, P.; Huang, T.H.; Frankel, W.L.; Stoner, G.D. A phase Ib study of the effects of black raspberries on rectal polyps in patients with familial adenomatous polyposis. Cancer Prev. Res. 2014, 7, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Haruma, K.; Yoshihara, M.; Kajiyama, G.; Kira, K.; Amagase, H.; Chayama, K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J. Nutr. 2006, 136, 821s–826s. [Google Scholar] [CrossRef] [PubMed]
- McCullough, M.L.; Jacobs, E.J.; Shah, R.; Campbell, P.T.; Gapstur, S.M. Garlic consumption and colorectal cancer risk in the CPS-II Nutrition Cohort. Cancer Causes Control 2012, 23, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhang, X.; Giovannucci, E.L.; Ma, J.; Fuchs, C.S.; Cho, E. No association between garlic intake and risk of colorectal cancer. Cancer Epidemiol. 2013, 37, 152–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.S.; Fang, Y.J.; Chen, Y.M.; Luo, W.P.; Pan, Z.Z.; Zhong, X.; Zhang, C.X. Higher intake of carotenoid is associated with a lower risk of colorectal cancer in Chinese adults: A case-control study. Eur. J. Nutr. 2015, 54, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Leufkens, A.M.; Siersema, P.D.; van Duijnhoven, F.J.; Vrieling, A.; Hulshof, P.J.; van Gils, C.H.; Overvad, K.; Roswall, N.; Kyro, C.; et al. Plasma and dietary carotenoids and vitamins A, C and E and risk of colon and rectal cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2014, 135, 2930–2939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabat, G.C.; Kim, M.Y.; Sarto, G.E.; Shikany, J.M.; Rohan, T.E. Repeated measurements of serum carotenoid, retinol and tocopherol levels in relation to colorectal cancer risk in the Women’s Health Initiative. Eur. J. Clin. Nutr. 2012, 66, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Cockbain, A.J.; Volpato, M.; Race, A.D.; Munarini, A.; Fazio, C.; Belluzzi, A.; Loadman, P.M.; Toogood, G.J.; Hull, M.A. Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut 2014, 63, 1760–1768. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Chan, A.T.; Fuchs, C.S.; Ogino, S.; Hu, F.B.; Mozaffarian, D.; Ma, J.; Willett, W.C.; Giovannucci, E.L.; Wu, K. Dietary intake of fish, omega-3 and omega-6 fatty acids and risk of colorectal cancer: A prospective study in U.S. men and women. Int. J. Cancer 2014, 135, 2413–2423. [Google Scholar] [CrossRef] [PubMed]
- Sasazuki, S.; Inoue, M.; Iwasaki, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Takachi, R.; Tsugane, S.; Japan Public Health Center-Based Prospective Study Group. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int. J. Cancer 2011, 129, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, M.C.; Pastore e Silva Jde, A.; Camargo Cde, Q.; Fabre, M.E.; Gevaerd, S.; Naliwaiko, K.; Moreno, Y.M.; Nunes, E.A.; Trindade, E.B. Fish oil decreases C-reactive protein/albumin ratio improving nutritional prognosis and plasma fatty acid profile in colorectal cancer patients. Lipids 2013, 48, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, M.C.; Camargo, C.Q.; Nunes, E.A.; Fiates, G.M.R.; Trindade, E. A systematic review and meta-analysis of the n-3 polyunsaturated fatty acids effects on inflammatory markers in colorectal cancer. Clin. Nutr. 2016, 35, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, L.S.; Thorlacius-Ussing, O.; Schmidt, E.B.; Rasmussen, H.H.; Lundbye-Christensen, S.; Calder, P.C.; Lindorff-Larsen, K. Randomized clinical trial of perioperative omega-3 fatty acid supplements in elective colorectal cancer surgery. Br. J. Surg. 2014, 101, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.J.; Wu, J.M.; Tsai, H.L.; Huang, C.W.; Lu, C.Y.; Sun, L.C.; Shih, Y.L.; Chen, C.W.; Chuang, J.F.; Wu, M.H.; et al. Prospective double-blind randomized study on the efficacy and safety of an n-3 fatty acid enriched intravenous fat emulsion in postsurgical gastric and colorectal cancer patients. Nutr. J. 2015, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D.; Bauer, L.L.; Gourineni, V.; Pelkman, C.L.; Fahey, G.C., Jr.; Swanson, K.S. Agave Inulin Supplementation Affects the Fecal Microbiota of Healthy Adults Participating in a Randomized, Double-Blind, Placebo-Controlled, Crossover Trial. J. Nutr. 2015, 145, 2025–2032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limburg, P.J.; Mahoney, M.R.; Ziegler, K.L.; Sontag, S.J.; Schoen, R.E.; Benya, R.; Lawson, M.J.; Weinberg, D.S.; Stoffel, E.; Chiorean, M.; et al. Randomized phase II trial of sulindac, atorvastatin, and prebiotic dietary fiber for colorectal cancer chemoprevention. Cancer Prev. Res. 2011, 4, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Nishihara, R.; Cao, Y.; Song, M.; Mima, K.; Qian, Z.R.; Nowak, J.A.; Kosumi, K.; Hamada, T.; Masugi, Y.; et al. Association of Dietary Patterns With Risk of Colorectal Cancer Subtypes Classified by Fusobacterium nucleatum in Tumor Tissue. JAMA Oncol. 2017, 3, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Ben, Q.; Sun, Y.; Chai, R.; Qian, A.; Xu, B.; Yuan, Y. Dietary fiber intake reduces risk for colorectal adenoma: A meta-analysis. Gastroenterology 2014, 146, 689–699.e6. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Skeie, G.; Landberg, R.; Lund, E.; Palmqvist, R.; Johansson, I.; Dragsted, L.O.; Egeberg, R.; Johnsen, N.F.; Christensen, J.; et al. Intake of dietary fiber, especially from cereal foods, is associated with lower incidence of colon cancer in the HELGA cohort. Int. J. Cancer 2012, 131, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.Y.; Kitahara, C.M.; Cantwell, M.M.; Berndt, S.I. Dietary fiber intake and risk of colorectal cancer and incident and recurrent adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am. J. Clin. Nutr. 2015, 102, 881–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, N.; Norat, T.; Ferrari, P.; Jenab, M.; Bueno-de-Mesquita, B.; Skeie, G.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjonneland, A.; et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European prospective investigation into cancer and nutrition (EPIC). PLoS ONE 2012, 7, e39361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathers, J.C.; Movahedi, M.; Macrae, F.; Mecklin, J.-P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, G.; Maher, E.R.; Bertario, L.; et al. Long-term effect of resistant starch on cancer risk in carriers of hereditary colorectal cancer: An analysis from the CAPP2 randomised controlled trial. Lancet Oncol. 2012, 13, 1242–1249. [Google Scholar] [CrossRef]
- Wu, Q.J.; Yang, Y.; Vogtmann, E.; Wang, J.; Han, L.H.; Li, H.L.; Xiang, Y.B. Cruciferous vegetables intake and the risk of colorectal cancer: A meta-analysis of observational studies. Ann. Oncol. 2013, 24, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.L.; Neuhouser, M.L.; Cheng, T.D.; Tinker, L.F.; Shikany, J.M.; Snetselaar, L.; Martinez, J.A.; Kato, I.; Beresford, S.A.; Chapkin, R.S.; et al. The Interaction between Dietary Fiber and Fat and Risk of Colorectal Cancer in the Women’s Health Initiative. Nutrients 2016, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef] [PubMed]
- Wesolowska, O.; Wisniewski, J.; Sroda-Pomianek, K.; Bielawska-Pohl, A.; Paprocka, M.; Dus, D.; Duarte, N.; Ferreira, M.J.; Michalak, K. Multidrug resistance reversal and apoptosis induction in human colon cancer cells by some flavonoids present in citrus plants. J. Nat. Prod. 2012, 75, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230s–242s. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243s–255s. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S. Bioavailability of phenolic compounds. Crit. Rev. Food Sci. Nutr. 2004, 44, 453–464. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A. Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2, 309–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeram, N.P.; Henning, S.M.; Zhang, Y.; Suchard, M.; Li, Z.; Heber, D. Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours. J. Nutr. 2006, 136, 2481–2485. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Ho, C.T.; Huang, Q. Extraction, bioavailability, and bioefficacy of capsaicinoids. J. Food Drug Anal. 2017, 25, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.N.; Zock, P.L.; Katan, M.B. Bioavailability and antioxidant effects of olive oil phenols in humans: A review. Eur. J. Clin. Nutr. 2004, 58, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for clinical trials: More pitfalls than promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Schiborr, C.; David, F.; Ehrt, H.; Voggel, J.; Sus, N.; Behnam, D.; Bosy-Westphal, A.; Frank, J. The Oral Bioavailability of Trans-Resveratrol from a Grapevine-Shoot Extract in Healthy Humans is Significantly Increased by Micellar Solubilization. Mol. Nutr. Food Res. 2018, 62, e1701057. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anticancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef] [PubMed]
- Mueller, D.; Jung, K.; Winter, M.; Rogoll, D.; Melcher, R.; Richling, E. Human intervention study to investigate the intestinal accessibility and bioavailability of anthocyanins from bilberries. Food Chem. 2017, 231, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Choy, Y.Y.; Jaggers, G.K.; Oteiza, P.I.; Waterhouse, A.L. Bioavailability of intact proanthocyanidins in the rat colon after ingestion of grape seed extract. J. Agric. Food Chem. 2013, 61, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Rollyson, W.D.; Stover, C.A.; Brown, K.C.; Perry, H.E.; Stevenson, C.D.; McNees, C.A.; Ball, J.G.; Valentovic, M.A.; Dasgupta, P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release 2014, 196, 96–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Sharma, R.; Sarangal, V.; Kaur, N.; Prashar, P. Evaluation of anti-inflammatory effects of systemically administered curcumin, lycopene and piperine as an adjunct to scaling and root planing: A clinical study. Ayu 2017, 38, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of garlic and its bioactive components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Goel, A. The Holy Grail of Curcumin and Its Efficacy in Various Diseases: Is Bioavailability Truly a Big Concern? J. Restor. Med. 2017, 6, 27–36. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Niaz, K.; Maqbool, F.; Ismail Hassan, F.; Abdollahi, M.; Nagulapalli Venkata, K.C.; Nabavi, S.M.; Bishayee, A. Molecular Targets Underlying the Anticancer Effects of Quercetin: An Update. Nutrients 2016, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Shanmukhan, N.K.; Radhakishnan, A.; Maniarasan, V.; Kuppusamy, G.; Gideon, S. Bioformulative concepts on intracellular organ specific bioavailability. Ther. Deliv. 2018, 9, 775–796. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Bioavailability of anthocyanins. Drug Metab. Rev. 2014, 46, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Grimes, K.L.; Stuart, C.M.; McCarthy, J.J.; Kaur, B.; Cantu, E.J.; Forester, S.C. Enhancing the Cancer Cell Growth Inhibitory Effects of Table Grape Anthocyanins. J. Food Sci. 2018, 83, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T. Chapter 26—Chemical Properties, Bioavailability, and Metabolomics of Fruit Proanthocyanidins. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 339–351. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Zheng, Q.; Wang, M.; Deng, W.; Li, Q.; Firempong, C.K.; Wang, S.; Tong, S.; Xu, X.; et al. In vitro and in vivo evaluation of capsaicin-loaded microemulsion for enhanced oral bioavailability. J. Sci. Food Agric. 2015, 95, 2678–2685. [Google Scholar] [CrossRef] [PubMed]
- Rather, R.A.; Bhagat, M. Cancer Chemoprevention and Piperine: Molecular Mechanisms and Therapeutic Opportunities. Front. Cell Dev. Biol. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khayata, N.; Abdelwahed, W.; Chehna, M.F.; Charcosset, C.; Fessi, H. Preparation of vitamin E loaded nanocapsules by the nanoprecipitation method: From laboratory scale to large scale using a membrane contactor. Int. J. Pharm. 2012, 423, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Nakamura, H.; Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug Deliv. Rev. 2013, 65, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Tang, B.C.; Whitehead, K.A.; Anderson, D.G.; Langer, R. Managing diabetes with nanomedicine: Challenges and opportunities. Nat. Rev. Drug Discov. 2015, 14, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Gupta, B.K.; Jaggi, M.; Chauhan, S.C. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010, 351, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Radu, I.C.; Hudita, A.; Zaharia, C.; Stanescu, P.O.; Vasile, E.; Iovu, H.; Stan, M.; Ginghina, O.; Galateanu, B.; Costache, M.; et al. Poly(HydroxyButyrate-co-HydroxyValerate) (PHBHV) Nanocarriers for Silymarin Release as Adjuvant Therapy in Colo-rectal Cancer. Front. Pharmacol. 2017, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Cisterna, B.A.; Kamaly, N.; Choi, W.I.; Tavakkoli, A.; Farokhzad, O.C.; Vilos, C. Targeted nanoparticles for colorectal cancer. Nanomedicine 2016, 11, 2443–2456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathi, M.; Martín, Á.; McClements, D.J. Nanoencapsulation of food ingredients using carbohydrate based delivery systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Alvani, K.; Qi, X.; Tester, R. Used of carbohydrates, including dextrin for oral delivery. Starch-Stärke 2011, 63, 424–431. [Google Scholar] [CrossRef]
- Yamashita, C.; Chung, M.M.S.; dos Santos, C.; Mayer, C.R.M.; Moraes, I.C.F.; Branco, I.G. Microencapsulation of an anthocyanin-rich blackberry (Rubus spp.) by product extract by freeze-drying. LWT Food Sci. Technol. 2017, 84, 256–262. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Liang, H.; Chen, Y.; Shi, M.; Wu, J.; Liu, X.; Li, Z.; Liu, B.; Yuan, Q.; et al. Intestine-Specific Delivery of Hydrophobic Bioactives from Oxidized Starch Microspheres with an Enhanced Stability. J. Agric. Food Chem. 2015, 63, 8669–8675. [Google Scholar] [CrossRef] [PubMed]
- Rokhade, A.P.; Agnihotri, S.A.; Patil, S.A.; Mallikarjuna, N.N.; Kulkarni, P.V.; Aminabhavi, T.M. Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. Carbohydr. Polym. 2006, 65, 243–252. [Google Scholar] [CrossRef]
- Nienaltowska, K.; Perfetti, G.; Meesters, G.M.H.; Ronsse, F.; Pieters, J.G.; Dewettinck, K. Attrition strength of water-soluble cellulose derivatives coatings. Adv. Powder Technol. 2010, 198, 298–309. [Google Scholar] [CrossRef]
- Chomto, P.; Nunthanid, J. Physicochemical and powder characteristics of various citrus pectins and their application for oral pharmaceutical tablets. Carbohydr. Polym. 2017, 174, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Akolade, J.O.; Oloyede, H.O.B.; Onyenekwe, P.C. Encapsulation in chitosan-based polyelectrolyte complexes enhances antidiabetic activity of curcumin. J. Funct. Foods 2017, 35, 584–594. [Google Scholar] [CrossRef]


| Dietary Compounds | Chemical Structure | Representative Compounds | Sources |
|---|---|---|---|
| Flavonoids | |||
| Flavones |  | Apigenin, luteolin | Celery (Apium graveolens L.), onions (Allium cepa L.), broccoli (Brassica oleracea italic Plenck.), red orange (Citrus x sinensis L. Osbeck), green pepper (Capsicum sp.) |
| Flavonols |  | Quercetin, kaempferol, myricetin | Broccoli (Brassica oleracea italic Plenck.), lettuce (Lactiva sativa L.), onions (Allium cepa L.), kale (Brassica oleracea L.), leek (Allium ampeloprasum L.), apricot (Prunus armeniaca L.), apple (Malus domestica Borkh.), blueberry (Vaccinium corymbosum Rydb.), black currant (Ribes nigrum L.) |
| Flavonones |  | Naringenin, hesperitin, diosmetin | Orange (Citrus x aurantium sp. L.), grepfruit (Citrus paradise Macfad.), lemon (Citrus limon L.) |
| Isoflavones |  | Genistein, daidzein, glycitein | Soybeans (Glycine max L.) boiled, miso, tofu, soy milk, soy flour |
| Flavan-3-Ol Monomeric catechins |  | Catechin, epicatechin, epigallocatechin, epigallocatechin-3-O-gallate | Apple (Malus domestica Borkh.), peach (Prunus persica L.), green tea, black tea (Camelia sinensis L.), red wine, grapes (Vitis vinifera L.), beans (Phaseolus vulgaris L.) |
| Protoanthocyanidins or condensed tannins |  Protoanthocyanidin B | Proanthocyanidin A, B | American cranberry (Vaccinium macrocarpon Aiton.), cranberry (Vaccinium oxycoccus L.), chokeberries (Aronia melanocarpa Elliott.), plum (Prunus domestica L.), blueberry (Vaccinium myrtillus L.), red currant (Ribes rubrum L.), lingonberries (Vaccinium vitis-idaea L.), pear (Pyrus communis L.), apple (Malus domestica Borkh.), almonds (Prunus dulcis Mill.), hazelnuts (Corylus spp.), pecans (Carya illinoinensis Wangenh.), pistachio nuts (Pistacia vera L.), walnuts (Juglans regia L.), grape seeds (Vitis vinifera L.), beans (Phaseolus vulgaris L.), cowpea (Vigna unguiculata Walp.), lentils (Lens culinaris Medik.), barley (Hordeum vulgare L.), rice (Oryza sativa L.), sorghum (Sorghum bicolor L. Moench.) |
| Anthocyanidins/Anthocianins |  | Delphinidin (R1, OH; R2, OH; R3, OH); cyanidin (R1, OH; R2, OH; R3, H) malvidin (R1, OH; R2, OCH3; R3, OCH3); pelargonidin (R1, OH; R2, H; R3, H) | Blackberry (Rubus fruticosus L.), raspberry (Rubus idaeus L.), bilberry (Vaccinium myrtillus L.), plum (Prunus sp), sour cherry (Prunus cerasus L.), black grapes (Vitis vinifera L.), elderberry (Sambucus nigra L.), red cabbage (Brassica oleracea L.) |
| Non-Flavonoids | |||
| Tannins A. Gallotannins derivatives |  | Ellagic acid | Raspberry (Rubus idaeus L.), strawberry (Fragaria x ananassa Duchesne.), pomegranate (Punica granatum L.), black currants (Ribes nigrum L.), blackberry (Rubus fruticosus L.), guava (Psidium guajava L.) |
| B. Elagotannins derivatives |  | Gallic acid | |
| Phenol-Carboxilic Acids A. Hydroxybenzoic acids |  | Gallic acid (R1, OH; R2, OH; R3, OH), syringic acid (R1, OCH3, R2, OCH3, R3, OH) | Broccoli (Brassica oleracea italica Plenck.), cowpea (Vigna unguiculata Walp.), spinach (Spinacia oleracea L.), black currant (Ribes nigrum L.), acai palm (Euterpe oleracea Mart.), sugar apple (Ananona squamosa L.) |
| B. Hydroxy-cinnamic acids |  | Cinnamic acid (R1, H; R2, H; R3, H; R4, OH), Caffeic acid (R1, OH; R2, OH; R3, H; R4, OH), chlorogenic acid, ferulic acid acid (R1, H; R2, OH; R3, OCH3; R4, OH), sinapic acid (R1, OCH3; R2, OH; R3, OCH3; R4, OH), p-coumaric acid (R1, H; R2, OH; R3, H; R4, OH), rosmarinic acid | Pear (Pyrus communis L.), kiwi (Actinidia deliciosa C.F.Liang and A.R.Ferguson), plum (Prunus domestica L.), apple (Malus domestica Borkh.), artichoke (Cynara scolymus L.), potato (Solanum tuberosum L.), coffee (Coffea arabica L.), rosemary (Rosmarinus officinalis L.), basil (Ocimum basilicum L.), oregano (Origanum vulgare L.), sage (Salvia officinalis L.) |
| Lignans |  | Secoisolariciresinol, sesaminol, sesamol, sesamin | Flaxseed/ linseed (Linum usitassimum L.), sesameseed (Sesamum indicum L.) |
| Stilbens |  | Resveratrol (R1, OH; R2, OH; R3, OH) | Red wine, red grapes (Vitis vinifera L.), plum (Prunus domestica L.) |
| Other Compounds |  | Curcumin | Turmeric (Curcuma longa L.) |
 | Gingerol | Ginger (Zingiberis officinale Roscoe.) | |
| Dietary Compounds | Chemical Structure | Representative Compounds | Sources |
|---|---|---|---|
| Carotenoids |  | Lycopene | Tomatoes (Solanum lycopersicum L.), guava (Psidium guajava L.), pumpkin (Cucurbita pepo L.), carrot (Daucus carota subsp. sativus), rose hip (Rosa rugosa Thunb.), watermelon (Citrullus lanatus Thunb.), saffron (Crocus sativa L.) |
 | α-carotene | ||
 | β-Carotene | ||
 | Crocetin | ||
| Iridoids |  | Oleuropein | Unripe olive fruits, table olives, virgin olive oil (Olea europaea L.) |
| Nitrogen Compounds A. Alkaloids |  | Piperine | Black pepper (Piper nigrum L.); long pepper (Piper longum L.) |
| B. Non-alkaloidic compounds |  | Capsaicin | Chilli pepper (Capsicum sp.) |
| Organosulfur Compounds |  | Allicin | Garlic (Allium sativum L.), onion (Allium cepa L.), broccoli (Brassica oleracea italica Plenck.) |
 | Allin | ||
 | Sulforaphane | ||
| Phytosterols |  | β-sitosterol | Zucchini (Cucurbita pepo L.), lettuce (Lactuca sativa L.), mangold (Betta vulgaris L.), white cabbage (Brassica oleracea L.), pumpkin seed (Cucurbita pepo L.), oat (Avena sativa L.), peanut (Arachys hypogaea L.), mushrooms (Agaricus bisporus, Lentinula edodes, Grifolia frondosa, Boletus edulis, Pleurotus osteatrus, Armillaria mellea) |
 | Stigmasterol | ||
 | Ergosterol | ||
 | Campesterol | ||
| Essential Oil Compounds |  | Thymol | Essential oils of thyme (Thymus vulgaris L.), wild thyme (Thymus serphyllum L.), oregano (Origanum vulgare L.), lemon balm (Melissa officinalis L.), cinnamon (Cinnamomum sp.), aniseed (Pimpinella anisum L.), star anise (Illicium verum Hook.), fennel (Foeniculum vulgare Mill.), black cumin (Nigella sativa L.) |
 | Carvacrol | ||
 | Citronellal | ||
 | Cinnamaldehyde | ||
 | Anethole | ||
 | Thymoquinone | ||
| Polyunsaturated Fatty Acids A. Omega-3 fatty acids |  | α-linolenic acid (ALA) | Catfish (Silurus glanis), anchovy (Engraulis encrasicolus), bluefish (Pomatomus saltatrix), sardine (Sardina pilchardus), tuna (Thunnus thynnus), spiny lobster (Panulirus argus), mussels (Mytilus edulis), oyster (Ostrea lurida), herring (Clupea harengus membras, C. h. harengus), mullet (Mugil cephalus), shrimps (Palaemon serratus), seaweeds (Rhodophyta phylum, Phaeophyceae class, Chlorophyta phylum); seed oil from: Hemp (Cannabis sativa L.), chia (Salvia hispanica L.), echium (Echium plantagineum L.), flax (Linum usitassinum L.); walnut oil (Juglans regia L.) |
 | Docosahexaenoic acid (DHA) | ||
 | Eicosapentaenoic acid (EPA) | ||
| B. Omega-6 fatty acids |  | Linoleic acid (LA) | Black currant (Ribes nigrum L.) seed oil, gooseberries (Ribes grossularia L.) seed oil, hemp (Cannabis sativa L.) seed oil; meet from beef (Bos taurus), lamb (Ovis aries), pork (Sus scrofa domesticus) |
 | γ–linolenic acid (GLA) | ||
| Dietary Fiber |  | Inulin | Sweet potato (Ipomoea batatas L.), leek (Allium ampeloprasum L.), garlic (Allium sativum L.), onion (Allium cepa L.) |
 | Galactooligosaccharides | Apple (Malus domestica Borkh.), pear (Pyrus communis L.), potato (Solanum tuberosum L.), beans (Phaseolus vulgaris L.), brusselsprouts (Brassica oleracea var. gemmifera L.), whole wheat (Triticum L.), whole corn (Zea mays L.), chickpea (Cicer arientinum L.), apricot (Prunus armeaniaca L.) | |
 | Fructooligosaccharides | ||
 | β-glucans | Mushrooms (Agaricus bisporus, Lentinula edodes, Grifolia frondosa, Boletus edulis, Pleurotus osteatrus, Armillaria mellea) | |
 | Glucomannans | Konjac (Amorphophallus konjac Koch.) |
| Author, Year | Animal Models | Doses and Duration of Administration | Results |
|---|---|---|---|
| Genistein | |||
| Song S. et al., 2018 [157] | Mice with AOM/DSS CRC | 225 mg/kg diet; 450 mg/kg diet and 900 mg/kg diet—six month | Significant improvement of colon architectural repair, anti-inflammatory activity ↓ COX-2, TNF-α; ↓ expression of PI3K/AKT pathway; ↑expression of FOXO3, Bax proteins |
| Zhang Y. et al., 2013 [101] | Sprague–Dawley rats with AOM induced CRC | Pre-treatment diet supplementation with 140 mg/kg—six weeks, before induction of cancer | Inhibition of aberrant crypt foci, prevention of nuclear β-catenin accumulation, suppression of cyclin D1, c-myc expression and Wnt signaling genes (Wnt1, Wnt5a, Sfrp1, Sfrp5) |
| Son T.G. et al., 2013 [158] | BALB/mice subcutaneously injected with CT26 mouse colon cancer cells | Tumor bearing mice are treated with genistein 200 mg/kg 1 day before radiation (5, 10 Gy); evaluation of tumors after 12 h, 3.5 days | genistein increased progenitor cell survival and cell death after radiation, recovery of intestinal damage after radiation (↑ Ki-67), significant tumor regression for combined treatment |
| Cyanidin/Pelargonidin/Malvidin | |||
| Kang S.Y. et al., 2003 [159] | Apcmin mice-mutant mouse lineage predisposed to multiple intestinal neoplasia due to mutations in adeno-matous polyposis coli (APC) gene | 800 mg/L anthocyanidins/200 mg/L (rich in cyanidin glucosides) in the drinking water and modified diet with 200 g/kg freeze dried cherries | Fewer and smaller adenomas in the cecum compared to control Colon tumor volume was not significantly reduced |
| Shi N. et al., 2015 [160] | Male CRJ:CD-1 (ICR) mice with CRC induced with AOM/DSS | 2.5%; 5%; 10% freeze-dried strawberries—cyanidine glucoside (1.67%) and pelargonidin glucoside (41.1%)—20 weeks | Inhibition of tumor development from 100% (control) to 74–44%, ↓ of nitrotyrosine production, ↓ Nf-kb, PI3K/AKT phosphorylation, ↓ COX-2, iNOS expression |
| Silva R.M. et al., 2015 [161] | Wistar rats with AOM induced CRC | Administration of 1% (222 mg/zi) or 2% red grape juice (444 mg/zi), two weeks before AOM or 4 weeks after the last administration of AOM | ↓ COX-2 mRNA with 1% grape juice before AOM and with 2% juice after the last AOM administration |
| Fernandez J. et al., 2018 [162] | Male Fischer 344 rats with AOM/DSS induced CRC | 20 g/day/rat of functional sausage with 0.11 anthocyanins (mixture of 1:1 dehydrated strawberries and blackberries powder with 59% cyanidin-3-glucosides and 41% pelargonidin-3-glucosides) | Significant reduction of Peyer patches, caecum weight, number of polyps; Significant reduction of Bilophila wadsworthia—a bacteria that generated high level of H2S and has pro-inflammatory effects |
| Chlorogenic Acid | |||
| Matsunaga K. et al., 2002 [163] | Male F344 rats with CRC induced with AOM | 250 ppm chlorogenic acid was administered one week before and a one week after tumor induction with AOM; study duration 36 weeks | Significant decrease of colon tumors for pre- treatment with chlorogenic acid |
| Banerjee N. et al., 2016 [164] | Sprague Dawly rats with AOM induced CRC | Plum (Prunus salicina L.) beverage rich in chlorogenic and neochlorogenic acids, 10 weeks | Significant decrease of dysplastic polyps, ↓ expression of COX-2, Nf-kB, AKT/mTOR signaling pathway, ↑ miR-143 |
| Cinnamic Acid | |||
| Zhu B. et al., 2016 [165] | Female BALB/c nude mice inoculated with HT29 colon carcinoma cells | 1 and 1.5 mmol/kg x3/week for two weeks | Significant inhibition of tumor growth, ↑ expression of Bax and caspase 3 |
| p-Coumaric Acid | |||
| Sharma S.H. et al., 2017 [166] | Male albino rats with DMH induced CRC | 50 mg/kg, 100 mg/kg, 200 mg/kg; 15 weeks | Significant dose-dependent reduction of polyps incidence and formation of pre-neoplasic lesions, reduction of oxidative stress; significant decrease in gut microbial enzymes (mucinases and β-dehydrogenases) |
| Rosmarinic Acid (RA) | |||
| Venkatachalam K. et al., 2013 [167] | Male wistar rats with DMH induced CRC | 5 mg/kg received during administration of DMH (15 weeks) or one week after the last DMH dose (until 30 weeks) or through the whole period (30 weeks) | Supplementation with RA for the whole period showed the highest tumor reduction, ↓ stress oxidative markers, ↓mucosal bacterial enzymes activity, regulation of xenobiotic metabolizing enzymes, up-regulation of apoptotic factors |
| Gallic Acid | |||
| Giftson J.S. et al., 2011 [168] | Male albino Wistar rats with DMH induced CRC | 50 mg/kg received one week before DMH and continued 30 weeks (group 1), after cessation of DMH until 30 weeks (group 2), along the whole period (group 3) | Supplementation with gallic acid for the whole period showed the highest tumor reduction, regulation xenobiotic metabolizing enzymes, decreased tumor incidence |
| Secoisoscilaresinol | |||
| Shah N.R. and Patel B.M., 2016 [169] | Diabetic male Sprague Dawley rats with DMH induced CRC | 500 mg/kg p.o secoisolariciresinol rich extract of L. ussitatissimum—18 weeks | ↓ pro-inflammatory markers, ↓ PCNA, ↓ CEA, ↓ mRNA level of CDK4, reduction in hyperplastic cells |
| Gomides A.F. et al., 2016 [170] | C57 BL6 mice with DMH induced CRC | 10% defatted flaxseed meal—15 weeks | Reduction of precancerous lesions in the distal colon |
| Sesamol | |||
| Shimizu S. et al., 2014 [171] | C57/BL6-Apc Min/+ mice | 500 pp/8 weeks | ↓ pro-inflammatory factors, suppression of intestinal polyps formation |
| Author, Year | Animal Models | Doses and Duration of Administration | Results |
|---|---|---|---|
| Lycopene | |||
| Tang F.Y. et al., 2011 [173] | BALB/cAnN-Foxn1 nude mice with CRC induced by inoculation of HT-29 cells | 3/6 mg/kg—5 weeks | Significant inhibition of tumor growth |
| Dias M.C. et al., 2010 [174] | Male Wistar rats with DMH induced CRC | 300 mg/kg lycopen + symbiotic (60 mg oligofructose + 50 mg inulin + 109 CFU Bifidobacteria lactis)—eight weeks (before/during or after initiation with DMH) | ↓ PCNA, ↓ p-53 colonic cells, ↓ AFC, ↓colonic Paneth cells |
| Crocin | |||
| Kawabata K. et al., 2012 [175] | CD1 (ICR) mice with AOM/DSS induced CRC | 50, 100, 200 ppm for 15 weeks after initiation of colon cancer | Significant reduction of inflammation and mucosal ulcers, multiplicity of adenocarcinoma |
| Oleuropein | |||
| Giner E. et al., 2016 [176] | C57BL6 mice with AOM/DSS colorectal induced cancer | 50 mg/kg or 100 mg/kg—63 weeks | Inhibition of tumor formation, decreased cell proliferation, anti-inflammatory activity |
| Capsaicin | |||
| Caetano B.F.R. et al., 2018 [177] | Male WISTAR rats with DMH induced colorectal cancer | 5 mg/kg or 50 mg/kg—four weeks | ↓Ki-67, significant ↓ of tumor volume and number of AFC |
| Sulforaphane/Organosulfur Compounds | |||
| Rajendran P. et al., 2015 [178] | Male WT or Nrf2−/+ mice with DMH induced CRC | Mice received alternating or daily 400 ppm sulforaphane included in the diet for 25 weeks | Significant reduction in tumor multiplicity only after continuous treatment |
| β-Sitosterol | |||
| Baskar A.A. et al., 2012 [179] | Male albino Wistar rats with DMH induced CRC | 5 mg/kg; 10 mg/kg; 20 mg/kg —16 weeks | Significant increase of antioxidant defense system, ↑ GSH, ↓ hyperplasic lesions |
| Thymoquinone (TQ) | |||
| Asfour W. et al., 2013 [180] | Male albino rats with DMH induced CRC | Administration of 10 mg/kg for 10 weeks (in the initiation phase + DMH) and 11 weeks (in the post initiation phase, after induction of cancer) | Chemopreventive effect, significant inhibition of tumor growth (for simultaneously administration in the initiation phase), ↓ PCNA, inhibition of VEGF production |
| Kortum B. et al., 2015 [181] | Male and female Msh2 loxP/loxP Villin-Cre mice—transgenic mice that simulate intestinal carcinogenesis | Mice were divided in 5 groups: Group 1—regular chow; group 2—500 mg mesalazine/kg chow, group 3—2500 mg mesalazine/kg chow; group 4—37.5 mg TQ /kg chow; group 5—375 mg TQ/kg chow; treatment for 43 weeks | ↓ incidence of tumors dose dependent for TQ; no significant differences between TQ and mesalazine |
| Cinnamaldehyde | |||
| Long M. et al., 2015 [182] | Experimental Nrf2+/+ and Nrf2−/− C57BL/6 mice with AOM/DSS induced CRC | Supplementation of diet with 0.5% cinnamaldehyde—11 weeks | Supplementation significantly attenuated colon carcinogenesis only for Nrf2+/+ mice; anti-inflammatory and antioxidant effects |
| Carvacrol | |||
| Sivaranjani A. et al., 2016 [183] | Male Albino WISTAR rats with DMH induced CRC | Administration of 20, 40, 80 mg/kg for 16 weeks | Reduced tumor incidence, inhibition of aberrant crypts formation, ↑ in antioxidant defense system, ↓ activity of colonic bacterial enzymes |
| Omega-3/Omega-6 Fatty Acids | |||
| Wang W. et al., 2017 [184] | C57 BL6 mice with CRC induced by inoculation of MC38 CRC cells | Pre-treatment (3 weeks) with DHA diet (omega-6/omega-3 ratio = 1.26:1) and DHA high diet (omega-6/omega-3 ratio = 0.56:1) before tumor initiation (3 weeks) DHA diet—22 g/kg LA, 0.31 g/kg ALA, 17.2 g/kg DHA; DHA high diet—12.5 g/kg—LA, 0.17 g/kg—ALA, 21.9 g/kg DHA | Inhibition of colon growth, modulation of fatty acids profile in colon tumors (↓ARA, ↑EPA, DHA), ↓ EETS |
| Hao G.W. et al., 2015 [185] | BALB/c nude mice with CRC induced by inoculation of HCT116 colon cancer cells | Ketogenic diet with or without omega-3 fatty acids; supplementation received until tumor volume was 600–700 mm3 (45 days) | Delayed tumor growth ↓ tumor vascularity for ketogenic diet supplemented with omega-3 fatty acids |
| Piazzi G. et al., 2014 [186] | C57BLJ/6J mice with AOM/DSS induced CRC | Effect of 1% eicosapentaenoic free fatty acid on both initiation and progression of carcinogenesis, 105 days | Suppression of tumor development, increase of apoptosis, anti-inflammatory effects, modulation of gut microbiota |
| Barone M. et al., 2014 [187] | C57BLJ/6J mice with mutation for the Apc gene (Apc Min/+) | Supplementation of diet with olive oil and omega-3 fatty acids, 10 weeks | Decrease in polyps number, pro-apoptotic effects |
| Dietary Fibers | |||
| Wang J. et al., 2017 [188] | Athymic male nude mice BALB/c-nu with CRCobtained by inoculation of HT-29 cancer cells | Administration of a polysaccharide from Lentinus edodes (0.2 mg/kg; 1 mg/kg; 5 mg/kg) or 20 mg/kg 5-fluorouracil for 21 days after cancer induction | ↓ tumor growth, pro-apoptotic effects |
| Masuda Y. et al., 2013 [189] | Female BALB/c, BALB/c-nude, C3H/HeJ mice inoculated with colon-26 cancer cells | Administration of D fraction (β-glucan) from the maitake mushroom (Grifola frondolosa) for 19 days after cancer induction | Significant decrease of tumor growth through systemic immune responses |
| Pattananandecha T. et al., 2016 [190] | Male Sprague-Dawley rats with AOM induced CRC | Supplementation of diet with 10% inulin for 17 weeks | Reduction of colonic AFC, reduction in bacterial colon enzymes, increase in Lactobacillus sp, Bifidobacteria |
| Qamar T.R. et al., 2016 [191] | Male Wistar rats with DMH induced CRC | Administration of galacto-oligosaccharides (76–151 mg), inulin (114 mg) separately or co-administration for 16 weeks | For co-administration significant ↓ in AFC formation and fecal enzyme activities |
| Hijova E. et al., 2013 [192] | Male and female Sprague-Dawly rats with DMH induced CRC | Supplementation of diet with 80 g inulin/kg food for 28 weeks | Significant ↓ coliform counts and ↑ lactobacilli counts, ↓ fecal enzyme activities, anti-inflammatory effects |
| Verma A. and Shukla G., 2013 [193] | Male Sprague Dawly rats with DMH induced colorectal cancer | Administration of inulin 10 mg/0.1 mL for a week before initiation of CRC and 6 weeks after initiation | ↓ of AFC and nitroreductase/β-glucosidase activity |
| Stofilova J. et al., 2015 [194] | Male and female Sprague Dawly rats with DMH induced colorectal cancer | Co-administration of oligofructose-enriched inulin preparation (95% fructan chains and 5% monosaccharide and disaccharide) with 109 CFU/mL for 28 weeks | ↓ inflammatory process in the jejuna and colon mucosa |
| Wu W.T. et al., 2014 [195] | Male C57/BL/6J with AOM induced colorectal cancer | Administration of high-fat low fibre diet (1% cellulose) or high 5% fibre diet with konjac glucomannan, inulin, cellulose for 3 weeks before cancer initiation | Konjac glucomannan and inulin have anti-genotoxic effects, increase cecal short chain fatty-acids, up-regulate antioxidant enzymes genes |
| Author, Year | Date/Type of Study | Cases | Control Cases | Dose | OR/HR/RR/IRR/SRR (95%CI)/Observation | Conclusions |
|---|---|---|---|---|---|---|
| Isoflavones (IF) | ||||||
| Akhter M. et al. 2009 [202] | 2004–2005/control-study | 721; men and women 40–79 years old | 697 | 24.77–62.41 mg IF/day | OR 0.49 (0.27–0.90) to 0.53 (0.28–0.98) p < 0.05 | Significant inverse association between high intake of isoflavone consumption and CRC in women |
| Shin A. et al. 2015 [210] | Case control study/2010–2013 | 901 men and women | 2669 | Daidzein 3.20–9.89 mg/day Genistein 3.3–9.7 mg/day Glycitein 0.85–2.44 mg/day | OR Daidzein 1.25 (0.96–1.61) to 0.71 (0.54–0.95) Genistein 1.18 (0.91–1.53) to 0.75 (0.57–1) Glycitein 1.32 (1.03–1.70) to 0.39 (0.25–0.61) | A high intake of isoflavones is significantly associated with decreased risk of CRC in both men and women |
| Yang G. et al. 2009 [211] | Prospective cohort study /1996–2005 | 68,412 women 40–70 years old | NA | 12.8–21 g soy food intake/day equivalent to 15.1–48.9 mg IF/day | RR Soy food 0.88 (0.67–1.15) to 0.71 (0.53–0.95) Soy isoflavones 0.91 (0.69–1.19) to 0.80 (0.60–1.07) | High intake of soy food products and isoflavones is correlated with reduced incidence in CRC, especially for menopausal women |
| Ko K. et al. 2018 [205] | Case control study in Korean (1993–2004) and Vietnamese population (2003–2007) | 101 (Korean study) 222 cases (Vietnamese study) | 391 (Korean study) 226 (Vietnamese study) | Evaluation of plasma IF levels for patients with CRC | OR for genistein 0.67 (0.34–1.31) to 0.50 (0.25–0.98)—Korean patients OR for genistein 0.97 (0.54–1.74) to 0.43 (0.25–0.73)—Vietnamese patients OR for daidzein (Vietnamese patients) 0.84 (0.47–1.49) to 0.48 (0.28–0.82) | Significant inverse correlation between high isoflavones plasma concentrations and reduced colorectal incidence |
| Lignans | ||||||
| Principi M, et al. 2013 [208] | Randomized double blind placebo-controlled study | 30 patients | 30 placebo | Supplementation of diet with 750 mg insoluble oat fiber, 50 mg flaxseed dry extract with 20% secoisolariciresinol diglycoside +175 mg milk thistle extract (70% silymarin and 30% silibinin)—60 days prior to colonoscopy | Significant increase in ERβ/ERα ratio and activation of caspases | Modulation of ERβ receptor is important for a chemo-preventive effect |
| Calabrese C. et al. 2013 [209] | Open study /2012–2013 | 11 patients with familial adenomatous polyposis with ileal pouch anal anastomosis | NA | 5 mg Eviendep® (30% silibinin + 40% secoisolariciresinol diglucoside + indigestible fibers 5% lignin) × 2/day for 3 month | Significant reduction of number and size of polyps with 32% and 51% respectively | Chemo-preventive effect |
| Zamora-Ros R. et al. 2013 [204] | Case control study /1996–1998 | 426 | 401 | 0.27–0.50 mg lignans/1000 kcal day | RR for Lignans 0.72 (0.47–1.10) to 0.59 (0.34–0.99) | Significant inverse correlation between high intake of lignans and colorectal incidence |
| Anthocyanidins | ||||||
| Thomasset S. et al. 2009 [212] | Pilot study 2006–2008 | 15 patients with histological confirmed; 10 patients with colorectal liver metastasis | NA | 1.4/2.8/5.6 g of Mirtocyan (a standardized extract rich in anthocyanidins) for 7 days before surgery | Mild decrease of tumor tissue only for 1.4 g | Possible chemo-preventive effects in humans |
| Wang L. S. et al. 2014 [213] | Randomized double blind placebo control study | 14 patients with familial adenomatous polyposis | NA | Group I—7 patients placebo powder (60 g/day) + 2 rectal suppositories (each 720 mg freeze-dried black raspberry extract) Group 2—60 g/day black raspberry freeze dried extract + 2 rectal suppositories—9 month treatment | Reduction of polyps mainly for suppositories Significantly de-methylated regions in adenomas | Regressing of rectal polyps in patients with familial adenomatous polyposis |
| Organosulfur Compounds | ||||||
| Tanaka S. et al. 2006 [214] | Preliminary double blind randomized clinical trial | 37 patients with colorectal adenomas which are removed if the size was > 5 mm | NA | Group I—6 capsules of aged garlic extract (AGE) equivalent to 2.4 mL AGE/day Group II—control (low dose) 6 capsules of AGE equivalent to 0.16 mL AGE/day. Patients are evaluated after 6, 12 months | AGE suppressed colorectal adenomas after 6, 12 months | Chemo-preventive effect in humans |
| McCullough M. et al. 2012 [215] | CPSII Nutrition cohort 1999–2007 | 42,824 men 56,876 women | NA | Supplementation of diet with garlic cloves < 1 clove/month; 1–3 cloves/month; 1 clove/week; 2–4 cloves/week; 5–6 cloves/week, 1 clove daily | Protective effect - women HR for 1–3 cloves/week 1.08 (0.86–1.35); 0.95 (0.72–1.26) for 1 clove/week; 0.77(0.58–1.02) for 2–4 cloves/week; 0.74 (0.48–1.13) to 5–6 cloves/week and 0.87 (0.58–1.32) for 1 clove/day | Weak chemo-preventive effect of garlic consumption for women; but not for men |
| Meng S. et al. 2013 [216] | Cohort study 1984–2008 | 76,208 women 45,592 men | NA | Administration of garlic cloves < 1 clove/month; 1–3 cloves/month; 1 clove/week; 2–4 cloves/week; 5–6 cloves/week, 1 clove daily | Women HR 1–3 cloves/month 1.11 (0.94–1.31) compared to HR 1.21 (0.94–1.57) for 1 clove/day (p = 0.14) Men HR 1–3 cloves/month 0.99 (0.84–1.16) compared to HR 1.03 (0.73–1.45) for 1 clove/day (p = 0.99) | No association was found between garlic intake and CRC risk |
| Satia J. A. et al. 2009 [217] | Cohort study 2000–2002 | 428 | 76,084 | Administration of garlic pills at least once a week for > 1 year during previous 10 years | HR 1.35 (0.59–1.17) compared to 1.00 (Reference) p = 0.04 | Significant increase of CRC incidence with garlic administration |
| Carotenoids | ||||||
| Lu M. S. et al. 2015 [218] | On-going case control study 2010–2013 | 845 | 845 | Food frequency questionnaire regarding intake of fruits and vegetables rich in carotenoids | α-carotene OR 0.54 (0.42–0.70) to 0.41 (0.31–0.54) β-carotene OR 0.79 (0.61–1.03) to 0.62 (0.48–0.82) lycopene OR 0.66 (0.51–0.85) to 0.45 (0.35–0.60) p < 0.001 | Significant inverse correlation between carotenoids intake and CRC incidence |
| Leenders M. et al. 2014 [219] | Cohort study 1992–2000 | Colon cancer 898 Rectum cancer 501 | 898/501 | Food frequency questionnaire regarding intake of fruits and vegetables rich in carotenoids and vitamins | For colon cancer β-carotene OR 0.89 (0.67–1.18) to 0.69 (0.52–0.94) Vitamin C OR 0.98 (0.74–1.29) to 0.76 (0.57–1.01) Vitamin E OR 0.88 (0.67–1.16) to 0.99 (0.74–1.33) | Significant inverse correlation between CRC incidence and mainly dietary β-carotene, vitamin C intake |
| Kabat G. C. et al. 2012 [220] | Large, prospective, multicenter study | 88 CRC in post-menopausal women | 5389 | Analysis of antioxidants from fasting blood samples at baseline, 1/3/6 years follow-up | For CRC β-carotene HR 0.65 (0.39–1.09) to 0.54 (0.31–0.96) For colon cancer β-carotene HR 0.57 (0.32–1.00) to 0.47 (0.25–0.88) | Significant inverse correlation between β-carotene plasma levels and CRC incidence |
| Polyunsaturated Fatty Acids | ||||||
| Cockbain A. J. et al., 2014 [221] | Phase II double blind randomized placebo control trial 2010–2011 | 203 patients with CRC liver metastasis | NA | 1.Placebo—43 patients 2. Patients receiving 2 g/day of EPA for 30 days prior to surgery, follow-up 18 months after surgery | Significant higher content of EPA in tumor tissues 1.82% compared to 1.30% (for placebo) p = 0.0008, decreased PGE2 in tumor tissues, anti-angiogenic activity (p = 0.075) | Pre-operative treatment has shown provide post-operative benefit |
| Song M. et al. 2014 [222] | Study cohort 1984–2008 | 76,386 women 47,143 men | NA | Administration of fish 15–40 g/day (women) and 16–46 g/day (men), marine fish (0.15–0.30 g/day) | Significant risk of distal colon cancer for both fish intake HR 1.12 (0.85–1.48) to 1.36 (1–1.85) and marine fish HR 1.19 (0.89–1.58) to 1.36 (1.03–1.80) p ≤ 0.05 | Associated risk between marine fish intake and CRC risk |
| Sasazuki S. at al. 2011 [223] | Prospective study 1995–2006 | 827,833 subjects | NA | Food frequency questioners regarding fish intake; marine fish 0.49–2.18 g/day for men and women | Significant Decrease associated with marine fish intake only for men RR 0.97 (0.51–1.83) to 0.35 (0.14–0.88) p = 0.05 | Chemo-preventive effect of marine fish rich in omega-3 fatty acids |
| Mocellin M. C. et al. 2013 [224] | Prospective randomized controlled trial 2011–2012 | 57 patients with CRC undergoing, chemotherapy, only 11 are randomized | NA | 1. Control group (n = 5). 2. Supplemented group (n = 6) with 2g fish oil/day—9 weeks 2 g fish oil = 360 mg/day EPA, 240 mg/day DHA | Significant decrease of C-reactive protein from 18.14 mg/L to 1.14 mg/L (p = 0.04) Significant increase of EPA, DHA compared to control group (p = 0.014, p = 0.019), significant decrease of AA between baseline and 9 weeks follow-up for the supplemented group (p = 0.028) | Significant anti-inflammatory effects for patients undergoing chemotheraphy and increase for plasma fatty acid profile |
| Mocellin M. C. et al. 2016 [225] | Meta-analysis of Nine trials | 475 patients with CRC | NA | Supplementation of diet with omega-3 fatty acids or administration of 0.2 g/kg fish oil parenterally at post-operative period Patients undergoing chemotherapy supplementation with 0.6 g/day EPA+DHA -9 weeks | Significant decrease of IL-6 (p = 0.024) and increase of albumin (p = 0.014) Supplementation of EPA+ DHA during chemotherapy significantly reduced CRP concentration (p = 0.017) and CRP/albumin ratio (p = 0.016) | Use of omega-3 fatty acids have benefits, especially for inflammatory markers in CRC patients |
| Sorensen L. S. et al. 2014 [226] | Randomized double blind placebo controlled trial | 148 patients awaiting for CRC surgery | NA | 1. Control group 2. Supplemen-tation group with 2 g EPA + 2 g DHA for 7 days before and 7 days after surgery | Pre-operative treatment with omega-3 fatty acids determined a significant increase of EPA, DHA levels in granulocytes and a significant decrease of AA (p < 0.001) compared to control group | Potential immune-stimulatory effects and prevention of post-operative infections |
| Ma C. J. et al. 2015 [227] | Prospective randomized double-blind study 2009–2010 | 99 patients with gastric and CRC | NA | 1. Control group 2. Supplementation group with a lipid emulsion containing soybean oil (80–100 g/L), medium chain triglycerides (100 g/L) and PUFA (linoleic acid—38–58 g/L, α-linolenic acid—4–11 g/L) for 7 days after surgery | There are no significant differences regarding inflammatory markers between the control and the supplementation group. Significant positive effect on lipid markers (p < 0.05) | Improvements only in lipid metabolism |
| Dietary Fiber | ||||||
| Holscher H. D. et al. 2015 [228] | Prospective Randomized double-blind placebo 3-period controlled study | 30 heathy patients | NA | 1. Placebo group 2. Administration of 5 g or 7.5 g inulin for 21 days with 7 days wash-out between periods | Significant increase of Bifidobacterium sp. (p < 0.001), significant decrease of Ruminococcus sp. and Desulfovibrio sp. (p < 0.01); dietary intake was positively associated with fecal butyrate (p = 0.005) | Beneficial changes in gastro-intestinal microbiota are correlated with decreased CRC incidence |
| Limburg P et al, 2011 [229] | Randomized phase II clinical trial 2006–2008 | 85 patients with aberrant crypt foci ≥ 5 at baseline | NA | 1. Control group 2. Atorvastatin 20 mg/day 3. Sulindac 150 mg x 2/day 4. Oligo-fructose enriched inulin 6 g powder x 2/day for 6 months | All treatment didn’t provide a significant decrease in AFC number and size | No association was found between inulin intake and CRC |
| Mehta RS et al, 2018 [230] | Prospective cohort study 1980–2012 | 121,700 females 51,529 males | NA | Food frequency questionnaires regarding dietary fiber intake | High intake of fiber was associated with a low risk of Fusobacterium nucleatum positive CRCs HR 0.54 (0.33–0.89) to 0.40 (0.24–0.67) | Intestinal microbiota plays an important role in mediating the association between consumption of high amount of dietary fiber/whole grains and CRC incidence |
| Ben Q et al, 2014 [231] | Meta-analysis of 20 studies (case-control, cohort) | 10,984 patients with colorectal adenoma | NA | Administration of 10 g/day fibers | SRR for dietary fiber are 0.72 (0.63–0.83) in a high vs low intake, inverse association between total fiber intake and CRC risk SSR 0.66 (0.56–0.77) | Chemopreventive effect of dietary fiber |
| Hansen L et al, 2012 [232] | Cohort study, 1997–2008 | 108,081 patients | NA | Administration of fiber 16–28 g/day for men and 15–24 g for women | Significant inverse correlation between CRC incidence and dietary fiber intake for men IRR 0.93 (0.68–1.26) to 0.55 (0.38–0.79) | Chemopreventive effect of dietary fiber |
| Kunzman A et al, 2015 [233] | Cohort study 1993–2009 | 2036 patients | 15,976 | Administration of fiber 9.9–12.8 g/1000 kcal/day from fruits/vegetables | Significant inverse correlation between dietary fiber intake and distal colon or rectal adenoma in men OR 0.88 (0.75–1.04) to 0.76 (0.63–0.91) p = 0.003 | Chemopreventive effect of dietary fiber against CRC |
| Murphy N et al, 2012 [234] | On-going multicentre prospective cohort study 1992–2000 | 477,312 patients | NA | Administration of dietary fiber 16.4–28.5 g/day | Significant inverse correlation between dietary intake and colon-distal cancer HR 0.90(0.75–1.07) to 0.70 (0.53–0.92) p = 0.021 colon proximal cancer HR 0.93 (0.78–1.1) to 0.86 (0.69–1.07) p = 0.16 rectum cancer HR 1 (0.87–1.17) to 0.79 (0.65–0.96) p = 0.012 | Chemopreventive effect of dietary fiber against CRC |
| Mathers JC et al, 2012 [235] | Randomized control trial | 937 eligible patients with Lynch syndrome | NA | 1. 463 patients received 30 g resistant starch/day 2. 455 patients—resistant starch placebo 3. 19 patients 600 mg aspirin/day—29 months | No significant effect of resistant-starch administration on cancer development IRR resistant starch vs resistant starch placebo 1.15 (0.66–2.00) p = 0.61 | No detectable effect on cancer development |
| Compound of Interest | Source | Bioavailability | In Vivo Studies | Ref. |
|---|---|---|---|---|
| Curcumin | Turmeric | - poor absorption, rapid metabolism, rapid elimination - enhanced by piperine with 2000% - better results on animal experiments | - 2g/kg of curcumin in rats → Cmax = 1.35 ± 0.23 μg/mL in 0.83 h - 2 g/kg of curcumin in human subjects → Cmax = 0.006 ± 0.005 μg/mL | [120,248] |
| EGCG | Green tea | - poor absorption - alkaloids, vitamins, proteins and fish oil improve absorption - air contact oxidation, metal ions like Ca2+ and Mg2+ and milk reduce absorption | - one oral dose of EGCG half-time = 3.4 ± 0.3 h | [249] |
| Resveratrol | Grapes | - poor absorption - bioavailability increased by a liquid micellar formulation | - 500 mg of Vineatrol → Cmax of trans-resveratrol = 10.6 fold higher - no detection of trans-ε-viniferin in plasma or urine | [250] |
| Quercetin | Onion Apples Beans Broccoli | - better bioavailability of quercetin glucoside | - 100 mg quercetin → absorption of quercetin glucoside = 3–17% | [251] |
| Genistein | Soy | - lower bioavailability in vivo - total genistein—a better bioavailability than genistein aglycone | - 20 mg/kg of genistein in FVB mice → genistein aglycone bioavailability = 23.4% - soy protein feeding Balb/c mice females → bioavailability of total genistein = 90% → bioavailability of genistein aglycone = < 15% | [252] |
| Anthocyanins | Bilberries | - poor bioavailability - 30% of amthocyanins are stable in the upper intestine for 8 h. | - bioavailability of anthocyanins from bilberries → ↑ amount of anthocyanins and degradants in the heathy compared to ileostomists group | [253] |
| Proantho-cyanidin | Apples Grapes Green tea | - oligomeric flavonoids with limited bioavailability | - ad libitum diet of grape seed extract in lab rats → the presence of PAC in the colonic contents - 11% of PAC—present in the feces | [254] |
| Capsaicin | Chilli | - low bioavailability - capsaicin was absorbed into intestinal tissues, jejunum and serosal fluid | - 1 mM of capsaicin in rats → absorption = 50% in the stomach, 80% in the jejunum and 70% in the ileum. | [255] |
| Piperine | Black pepper | - insoluble in water with a low bioavailability - used in clinical assays single or as an enhancer for other dietary agents - improved the bioavailability of resveratrol, curcumin and lycopene | - resveratrol + piperine in mice → piperine enhanced the bioavailability of resveratrol with 229% - curcumin + piperine in rats and human subjects → piperine enhanced bioavailability of curcumin with 2000% | [256,257] |
| Aliicin | Garlic | - poor bioavailability | - administration of garlic/ pure allicin → no detection in urine or blood | [258] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costea, T.; Hudiță, A.; Ciolac, O.-A.; Gălățeanu, B.; Ginghină, O.; Costache, M.; Ganea, C.; Mocanu, M.-M. Chemoprevention of Colorectal Cancer by Dietary Compounds. Int. J. Mol. Sci. 2018, 19, 3787. https://doi.org/10.3390/ijms19123787
Costea T, Hudiță A, Ciolac O-A, Gălățeanu B, Ginghină O, Costache M, Ganea C, Mocanu M-M. Chemoprevention of Colorectal Cancer by Dietary Compounds. International Journal of Molecular Sciences. 2018; 19(12):3787. https://doi.org/10.3390/ijms19123787
Chicago/Turabian StyleCostea, Teodora, Ariana Hudiță, Oana-Alina Ciolac, Bianca Gălățeanu, Octav Ginghină, Marieta Costache, Constanța Ganea, and Maria-Magdalena Mocanu. 2018. "Chemoprevention of Colorectal Cancer by Dietary Compounds" International Journal of Molecular Sciences 19, no. 12: 3787. https://doi.org/10.3390/ijms19123787
APA StyleCostea, T., Hudiță, A., Ciolac, O.-A., Gălățeanu, B., Ginghină, O., Costache, M., Ganea, C., & Mocanu, M.-M. (2018). Chemoprevention of Colorectal Cancer by Dietary Compounds. International Journal of Molecular Sciences, 19(12), 3787. https://doi.org/10.3390/ijms19123787