Apoptosis Induction of Agave lechuguilla Torrey Extract on Human Lung Adenocarcinoma Cells (SK-LU-1)
Abstract
:1. Introduction
2. Results & Discussion
3. Materials and Methods
3.1. Plant Material
3.1.1. Ethanolic Extract from A. lechuguilla
3.1.2. Hydrolysis Extract and Its Fractionated Hydrolyzed Extract of A. lechuguilla
3.2. Polyphenol and Flavonoid Total Quantification
3.3. Antioxidant Capacity Assays
3.4. Mass Spectrometry
3.5. Screening Cells Viability Assay by Sulforhodamine B
3.6. Viability Assay by 3-(4,5-DiMethyl-2-Thiazolyl)-2,5-Diphenyl-2H-Tetrazolium Bromide
3.7. Phosphatidylserine Translocation (Annexin V-APC)
3.8. In Silico Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 7-AAD | 7-amino-actinomycin D |
| ACN | Acetonitrile |
| ALK | Anaplastic lymphoma kinase |
| APC | Aloficocianina |
| BID | BH3 interacting-domain death agonist |
| Caspases | Cysteine aspartate-specific proteases |
| CML | Chronic myelogenous leukemia |
| DISC | Death inducing signaling complex |
| DMSO | Dimethyl sulfoxide |
| DR | Death receptor |
| EDTA | 2,2′,2″,2‴-(Ethane-1,2-diyldinitrilo) tetraacetic acid |
| EGFR | Epidermal growth factor receptor |
| FADD | Fas-associated death domain protein |
| FBS | Fetal bovine serum |
| FITC | Fluorescein |
| IC | Inhibitory concentration |
| K-Ras | Kirsten rat sarcoma virus |
| LGA | Lamarckian genetic algorithm |
| MAPK | Mitogen activated protein kinase |
| MEK | MARK/ERK kinase |
| MLKL | Mixed lineage kinase domain like protein |
| MTT | 3-(4,5-diMethyl-2-Thiazolyl)-2,5-diphenyl-2H-Tetrazolium bromide |
| NSCLC | Not-small cell lung cancer |
| OD | Optical density |
| ORAC | Oxygen radical absorbance capacity |
| PBS | Phosphate buffered saline |
| PM | Particulate matter |
| RIP | Receptor-interacting protein |
| RPM | Revolutions per minute |
| SCLC | Small cell lung cancer |
| SRB | Sulforhodamine B |
| tBID | Truncated BID |
| TGFβ receptor | Transforming growth factor beta receptor |
| TEAC | Trolox equivalent antioxidant capacity |
| TCA | Trichloroacetic acid |
| TFA | Trifluoroacetic acid |
| TNF | Tumor necrosis factor |
| Tris | Tris(hydroxymethyl)aminomethane |
| UNAM | Universidad Nacional Autónoma de México |
References
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B.; Zhang, Y.-J.; Gan, R.-Y.; Li, S.; et al. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Cancer Observatory. Available online: http://gco.iarc.fr/ (accessed on 26 September 2018).
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Gazdar, A.F.; Girard, L.; Lockwood, W.W.; Lam, W.L.; Minna, J.D. Lung cancer cell lines as tools for biomedical discovery and research. J. Natl. Cancer Inst. 2010, 102, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung cancer in never smokers—A different disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-H.; Loo, C.-Y.; Ghadiri, M.; Leong, C.-R.; Young, P.M.; Traini, D. The potential to treat lung cancer via inhalation of repurposed drugs. Adv. Drug Deliv. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Karapetis, C.S.; Khambata-Ford, S.; Jonker, D.J.; O’Callaghan, C.J.; Tu, D.; Tebbutt, N.C.; Simes, R.J.; Chalchal, H.; Shapiro, J.D.; Robitaille, S.; et al. K-ras Mutations and Benefit from Cetuximab in Advanced Colorectal Cancer. N. Engl. J. Med. 2008, 359, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Lissowska, J.; Foretova, L.; Dąbek, J.; Zaridze, D.; Szeszenia-Dabrowska, N.; Rudnai, P.; Fabianova, E.; Cassidy, A.; Mates, D.; Bencko, V.; et al. Family history and lung cancer risk: International multicentre case–control study in Eastern and Central Europe and meta-analyses. Cancer Causes Control 2010, 21, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Greiser, C.M.; Greiser, E.M.; Dören, M. Menopausal hormone therapy and risk of lung cancer—Systematic review and meta-analysis. Maturitas 2010, 65, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A.K.; Law, M.R.; Wald, N.J. The accumulated evidence on lung cancer and environmental tobacco smoke. BMJ 1997, 315, 980–988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.C.; Hayes, R.B.; Ahn, J.; Shao, Y.; Silverman, D.T.; Jones, R.R.; Garcia, C.; Thurston, G.D. Association between long-term exposure to ambient air pollution and diabetes mortality in the US. Environ. Res. 2018, 165, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, S.; Aunan, K.; Martin Seip, H.; Hao, J. Air pollution and lung cancer risks in China—A meta-analysis. Sci. Total Environ. 2006, 366, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Lissowska, J.; Bardin-Mikolajczak, A.; Fletcher, T.; Zaridze, D.; Szeszenia-Dabrowska, N.; Rudnai, P.; Fabianova, E.; Cassidy, A.; Mates, D.; Holcatova, I.; et al. Lung Cancer and Indoor Pollution from Heating and Cooking with Solid FuelsThe IARC International Multicentre Case-Control Study in Eastern/Central Europe and the United Kingdom. Am. J. Epidemiol. 2005, 162, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Brenner, D.R.; McLaughlin, J.R.; Hung, R.J. Previous Lung Diseases and Lung Cancer Risk: A Systematic Review and Meta-Analysis. PLoS ONE 2011, 6, e17479. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Altenburg, H.-P.; Bueno-de-Mesquita, B.; Boshuizen, H.C.; Agudo, A.; Berrino, F.; Gram, I.T.; Janson, L.; Linseisen, J.; Overvad, K.; et al. Fruits and vegetables and lung cancer: Findings from the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2004, 108, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Sidana, J.; Singh, B.; Sharma, O.P. Saponins of Agave: Chemistry and bioactivity. Phytochemistry 2016, 130, 22–46. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-R.; Zhang, Y.; Jacob, M.R.; Khan, S.I.; Zhang, Y.-J.; Li, X.-C. Antifungal Activity of C-27 Steroidal Saponins. Antimicrob. Agents Chemother. 2006, 50, 1710–1714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.; Vieira, I.; Braz-Filho, R.; Branco, A.; Santos, J.D.G.; Vieira, I.J.C.; Braz-Filho, R.; Branco, A. Chemicals from Agave sisalana Biomass: Isolation and Identification. Int. J. Mol. Sci. 2015, 16, 8761–8771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizwan, K.; Zubair, M.; Rasool, N.; Riaz, M.; Zia-Ul-Haq, M.; de Feo, V.; Rizwan, K.; Zubair, M.; Rasool, N.; Riaz, M.; et al. Phytochemical and Biological Studies of Agave attenuata. Int. J. Mol. Sci. 2012, 13, 6440–6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona, J.E.; Morales-Martínez, T.K.; Mussatto, S.I.; Castillo-Quiroz, D.; Ríos-González, L.J. Propiedades químicas, estructurales y funcionales de la lechuguilla (Agave lechuguilla Torr.). Rev. Mex. Cienc. For. 2017, 8, 100–122. [Google Scholar]
- Blando-Navarrete, J.L.; Baca-Marin, S. Determinación del potencial productivo de la lechuguilla (Agave lechuguilla Torr) en el municipio de San Juan de Guadalupe Dgo. Rev. Chapingo Ser. Zonas Áridas 2001, II, 100–105. [Google Scholar]
- Reyes-Agüero, J.A.; Aguirre-Rivera, J.R.; Peña-Valdivía, C.B. Biología y aprovechamiento de Agave lechuguilla Torrey. Bol. Soc. Botánica México 2000, 67, 75–88. [Google Scholar]
- Nava-Cruz, N.Y.; Medina-Morales, M.A.; Martinez, J.L.; Rodriguez, R.; Aguilar, C.N. Agave biotechnology: An overview. Crit. Rev. Biotechnol. 2015, 35, 546–559. [Google Scholar] [CrossRef] [PubMed]
- March, R.E.; Miao, X.-S. A fragmentation study of kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Spec. Issue Honour Jean-Claude Tabet 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Justino, G.C.; Borges, C.M.; Florêncio, M.H. Electrospray ionization tandem mass spectrometry fragmentation of protonated flavone and flavonol aglycones: A re-examination. Rapid Commun. Mass Spectrom. 2009, 23, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Florita Ramos Casillas, Cytotoxic activity of Agave lechuguilla Torr. Afr. J. Biotechnol. 2012, 11. [CrossRef]
- Sak, K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn. Rev. 2014, 8, 122–146. [Google Scholar] [CrossRef] [PubMed]
- Bikadi, Z.; Hazai, E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J. Cheminform. 2009, 1, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- L Rosas-Trigueros, J.; Ilizaliturri-Flores, I.; G Benitez-Cardoza, C.; Correa-Basurto, J.; Zamorano-Carrillo, A. Computational Modeling and Simulation of the Bcl-2 Family: Paving the Way for Rational Drug Design. Curr. Med. Chem. 2012, 19, 6081–6094. [Google Scholar] [CrossRef]
- Li, J.; Yin, Q.; Wu, H. Chapter Five—Structural Basis of Signal Transduction in the TNF Receptor Superfamily. In Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 119, pp. 135–153. ISBN 0065-2776. [Google Scholar]
- Kantari, C.; Walczak, H. Caspase-8 and Bid: Caught in the act between death receptors and mitochondria. Mitochondria Deadly Organelle 2011, 1813, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Spetz, J.; Presser, A.G.; Sarosiek, K.A. T Cells and Regulated Cell Death: Kill or Be Killed. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2018; ISBN 1937-6448. [Google Scholar]
- Sathishkumar, N.; Sathiyamoorthy, S.; Ramya, M.; Yang, D.-U.; Lee, H.N.; Yang, D.-C. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J. Enzyme Inhib. Med. Chem. 2012, 27, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McQuade, T.; Siemer, A.B.; Napetschnig, J.; Moriwaki, K.; Hsiao, Y.-S.; Damko, E.; Moquin, D.; Walz, T.; McDermott, A.; et al. The RIP1/RIP3 Necrosome Forms a Functional Amyloid Signaling Complex Required for Programmed Necrosis. Cell 2012, 150, 339–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Román, M.; Baraibar, I.; López, I.; Nadal, E.; Rolfo, C.; Vicent, S.; Gil-Bazo, I. KRAS oncogene in non-small cell lung cancer: Clinical perspectives on the treatment of an old target. Mol. Cancer 2018, 17, 33. [Google Scholar] [CrossRef] [PubMed]
- Vander Ark, A.; Cao, J.; Li, X. TGF-β receptors: In and beyond TGF-β signaling. Cell. Signal. 2018, 52, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.P.C. Multidrug resistance protein 1 (MRP1, ABCC1), a “multitasking” ATP-binding cassette (ABC) transporter. J. Biol. Chem. 2014, 289, 30880–30888. [Google Scholar] [CrossRef] [PubMed]
- Troy, A. Baudino Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr. Drug Discov. Technol. 2015, 12, 3–20. [Google Scholar] [CrossRef]
- Li, Y.H.; Yu, C.Y.; Li, X.X.; Zhang, P.; Tang, J.; Yang, Q.; Fu, T.; Zhang, X.; Cui, X.; Tu, G.; et al. Therapeutic target database update 2018: Enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Res. 2018, 46, D1121–D1127. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, K.M.; Hall, M.D.; Goldsborough, A.S.; Callaghan, R.; Gottesman, M.M. Collateral sensitivity as a strategy against cancer multidrug resistance. Drug Resist. Updat. 2012, 15, 98–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vicente-Vicente, L.; Prieto, M.; Morales, A.I. Eficacia y seguridad de la quercetina como complemento alimenticio. Rev. Toxicol. 2013, 30, 171–181. [Google Scholar]
- Dunnick, J.K.; Hailey, J.R. Toxicity and carcinogenicity studies of quercetin, a natural component of foods. Fundam. Appl. Toxicol. Off. J. Soc. Toxicol. 1992, 19, 423–431. [Google Scholar]
- Haghi, G.; Hatami, A. Simultaneous quantification of flavonoids and phenolic acids in plant materials by a newly developed isocratic high-performance liquid chromatography approach. J. Agric. Food Chem. 2010, 58, 10812–10816. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, C.V.; Rojas, M.G.V.; Ramírez, C.A.; Chávez-Servín, J.L.; García-Gasca, T.; Ferriz Martínez, R.A.; García, O.P.; Rosado, J.L.; López-Sabater, C.M.; Castellote, A.I.; et al. Total phenolic compounds in milk from different species. Design of an extraction technique for quantification using the Folin–Ciocalteu method. Food Chem. 2015, 176, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, Y.; Binh, N.T.; Asakura, H.W.; Hibino, Y.; Hitomi, Y.; Nakamura, H.; Ogino, K. Efficient Assay for Total Antioxidant Capacity in Human Plasma Using a 96-Well Microplate. J. Clin. Biochem. Nutr. 2009, 44, 46–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Parrilla, E.; de la Rosa, L.A.; Amarowicz, R.; Shahidi, F. Antioxidant Activity of Fresh and Processed Jalapeño and Serrano Peppers. J. Agric. Food Chem. 2011, 59, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.D.A.; Baird, S.K. Honey is cytotoxic towards prostate cancer cells but interacts with the MTT reagent: Considerations for the choice of cell viability assay. Food Chem. 2018, 241, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.L.; Lawson, L.B. Determination of permeation pathways of hydrophilic or hydrophobic dyes through the mammary papilla. Int. J. Pharm. 2018, 545, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Eisenbrand, G.; Pool-Zobel, B.; Baker, V.; Balls, M.; Blaauboer, B.; Boobis, A.; Carere, A.; Kevekordes, S.; Lhuguenot, J.-C.; Pieters, R.; et al. Methods of in vitro toxicology. Food Chem. Toxicol. 2002, 40, 193–236. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Virtua Drug Ltd. Molecular Docking Server. Available online: https://www.dockingserver.com/web (accessed on 5 September 2018).

| Extract of A. lechuguilla | Total Polyphenols 1 | Total Flavonoids 2 | TEAC 3 | ORAC 3 |
|---|---|---|---|---|
| Ethanolic | 23.44 ± 1.47 | 19.62 ± 1.23 | 87.36 ± 3.57 | 1962.99 ± 211.86 |
| Hydrolyzed | 37.45 ± 3.57 | 3.08 ± 0.00 | 46.53 ± 0.72 | 1216.83 ± 4.88 |
| Fraction | 2.69 ± 0.12 | 1.47 ± 0.00 | 6.12 ± 0.91 | 49.20 ± 0.80 |
| Possible Molecule | Rt 1 | m/z | Ions Assignation | CF 2 |
|---|---|---|---|---|
| Ethanolic extract of A. lechuguilla | ||||
| Tigogenin-glycoside or Neotigogenin-glycoside or Smilagenin-glycoside or Sarsasapogenin-glycoside | 0.42 | 417.339 579.396 741.450 903.506 1035.549 1197.605 | [M + H] [M + Hex + H] [M + Hex + Hex + H] [M + Hex + Hex + Hex + H] [M + Hex + Hex + Hex + Pent + H] [M + Hex + Hex + Hex + Pent + Hex + H] | C56H92O27 |
| Hecogenin-glycoside or Sisalagenin-glycoside or Gloriogenin-glycoside or Yuccagenin-glycoside | 0.56 | 431.336 593.375 755.429 917.484 1049.528 1211.584 | [M + H] [M + Hex +H] [M + Hex + Hex + H] [M + Hex + Hex + Hex + H] [M + Hex + Hex + Hex + Pent + H] [M + Hex + Hex + Hex + Pent + Hex + H] | C56H90O28 |
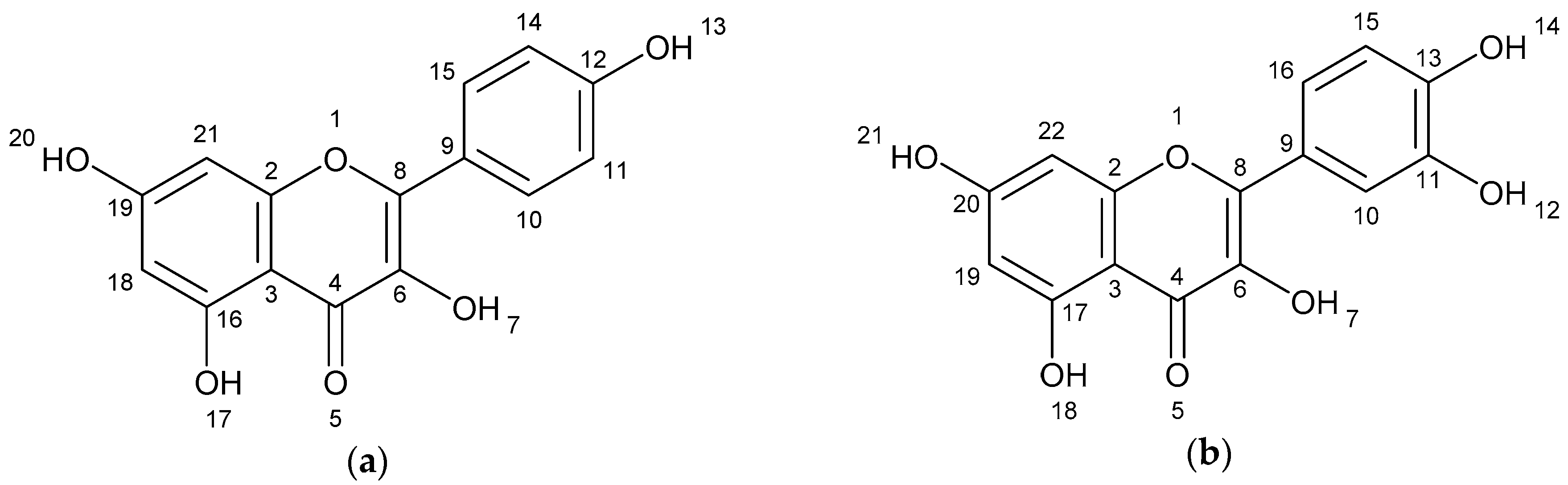
| Kaempferol | 5.06 | 245.082 269.085 287.096 | [M − C2H2O + H] [M − H2O + H] [M + H] | C15H10O6 |
| Quercetin | 5.44 | 257.082 285.080 303.091 | [M − H2O − CO +H] [M − H2O + H] [M + H] | C15H10O7 |
| Diosgenin-glycoside or Yamogenin-glycoside | 6.09 | 415.325 577.381 709.424 871.479 1003.523 | [M + H] [M + Hex + H] [M + Hex + Pent + H] [M + Hex + Pent + Hex + H] [M + Hex + Pent + Hex + Hex + H] | C50H80O22 |
| Tigogenin-glycoside or Neotigogenin-glycoside or Smilagenin-glycoside or Sarsasapogenin-glycoside | 6.34 | 417.342 579.397 741.451 903.506 1065.561 1197.606 | [M + H] [M + Hex + H] [M + Hex + Hex + H] [M + Hex + Hex + Hex + H] [M + Hex + Hex + Hex + Hex + H] [M + Hex + Hex + Hex + Hex + Pent + H] | C56H92O27 |
| Tigogenin-glycoside or Neotigogenin-glycoside or Smilagenin-glycoside or Sarsasapogenin-glycoside | 6.57 | 417.341 579.396 741.451 903.506 1035.550 | [M + H] [M + Hex + H] [M + Hex + Hex + H] [M + Hex + Hex + Hex + H] [M + Hex + Hex + Hex + Pent + H] | C50H82O22 |
| Hydrolyzed extract of A. lechuguilla | ||||
| Unknow | 0.54 | 701.503 475.332 340.264 | ND | ND |
| Unknow | 0.68 | 701.503 475.332 | ND | ND |
| Kaempferol | 4.97 | 245.082 269.085 287.096 | [M − C2H2O + H] [M − H2O + H] [M + H] | C15H10O6 |
| Quercetin | 5.34 | 245.082 257.085 261.135 285.080 303.091 | [M − C2H2O2 + H] [M − H2O − CO + H] [M − C2H2O + H][M − H2O + H] [M + H] | C15H10O7 |
| Diosgenin or Yamogenin | 5.36 | 415.155 | [M + H] [M + Na + H] | C27H42O3 |
| Tigogenin or Neotigogenin or Smilagenin or Sarsasapogenin | 6.92 | 417.342 | [M + H] | C27H44O3 |
| Unknow | 7.12 | 449.326 | ND | ND |
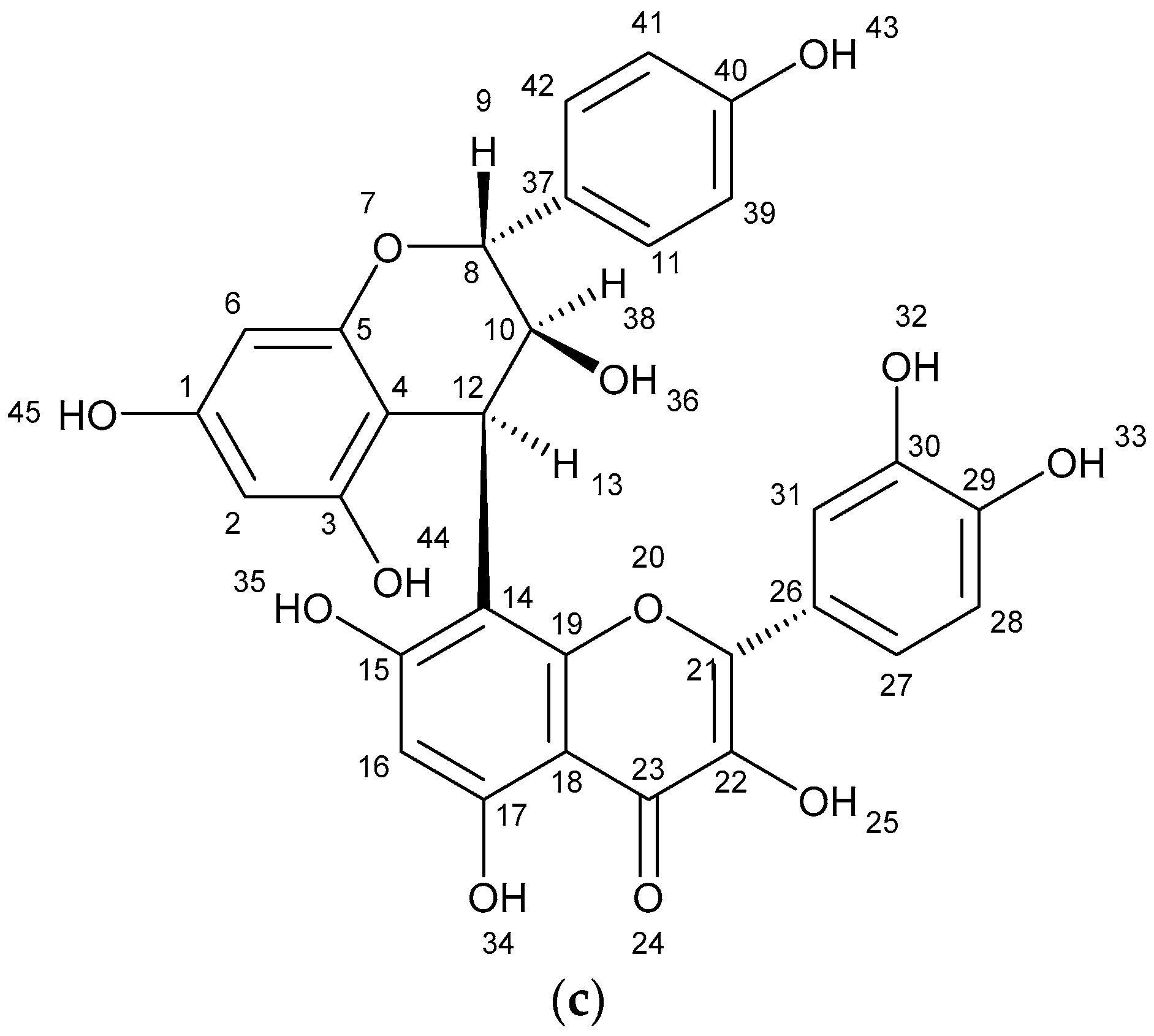
| Biflavonoid (isomer) | 8.37 | 230.251 245.083 261.135 304.304 579.397 | [Quercetin − 2CO − H2O + H] [Quercetin − C2H2O2 + H] [Quercetin − C2H2O + H] [Quercetin + 2H] [Quercetin + Afzelequin + 3H] | C30H22O12 |
| Biflavonoid (isomer) | 8.56 | 230.251 245.083 261.135 304.304 579.397 | [Quercetin − 2CO − H2O + H] [Quercetin − C2H2O2 + H] [Quercetin − C2H2O + H] [Quercetin + 2H] [Quercetin + Afzelequin + 3H] | C30H22O12 |
| Hecogenin or Sisalagenin or Gloriogenin or Yuccagenin. | 10.01 | 431.296 453.216 | [M + H] [M + Na + H] | C27H42O4 |
| Extract fraction of A. lechuguilla | ||||
| Kaempferol | 4.99 | 245.082 269.085 287.096 | [M − C2H2O + H] [M − H2O + H] [M + H] | C15H10O6 |
| Quercetin | 5.33 | 257.085 285.080 303.091 | [M − H2O − CO + H] [M − H2O + H] [M + H] | C15H10O7 |
| Unknow | 6.48 | 269.086 365.111 | ND | ND |
| Biflavonoid (isomer) | 8.54 | 245.083 304.306 579.399 601.381 | [Quercetin − C2H2O2 + H] [Quercetin + 2H] [Quercetin + Afzelequin + 3H] [Quercetin + Afzelequin + Na + 3H] | C30H22O12 |
| Biflavonoid (isomer) | 8.88 | 245.083 304.306 579.399 601.381 | [Quercetin − C2H2O2 + H] [Quercetin + 2H] [Quercetin + Afzelequin + 3H] [Quercetin + Afzelequin + Na + 3H] | C30H22O12 |
| Grow Inhibition 1 [%] | ||||||
|---|---|---|---|---|---|---|
| Control | HCT-15 | MCF-7 | PC-3 | U-251 | SK-LU-1 | K-562 |
| 1.2 ± 0.5 | 33.4 ± 3.6 | 10.5 ± 4.0 | 11.5 ± 1.8 | 24.0 ± 2.6 | 75.7 ± 2.3 | 17.1 ± 1.0 |
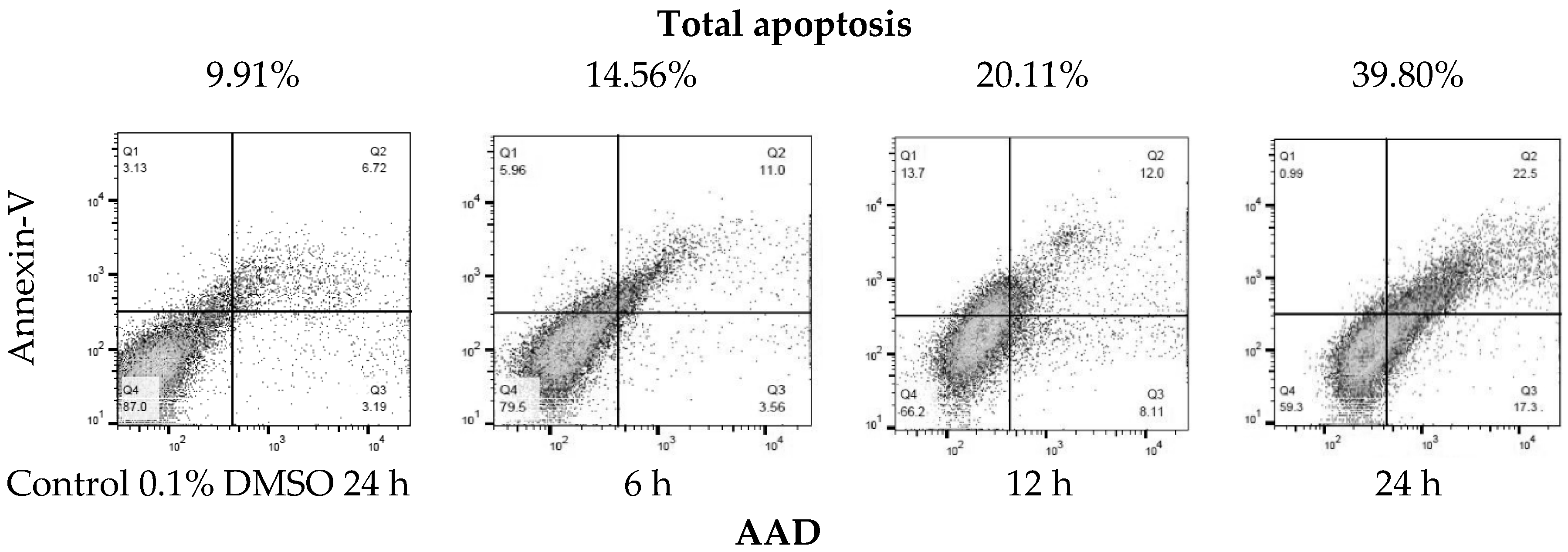
| Treatment [h] | Live Cells | Early Apoptotic Cells | Late Apoptotic Cells | Necrosis Cells | Total Apoptosis |
|---|---|---|---|---|---|
| [%] | |||||
| Control | 87.00 | 3.19 | 6.72 | 3.13 | 9.91 |
| 6 | 79.50 | 3.56 | 11.00 | 5.96 | 14.56 |
| 12 | 66.20 | 8.11 | 12.00 | 13.70 | 20.11 |
| 24 | 59.30 | 17.30 | 22.5 | 0.99 | 39.80 |
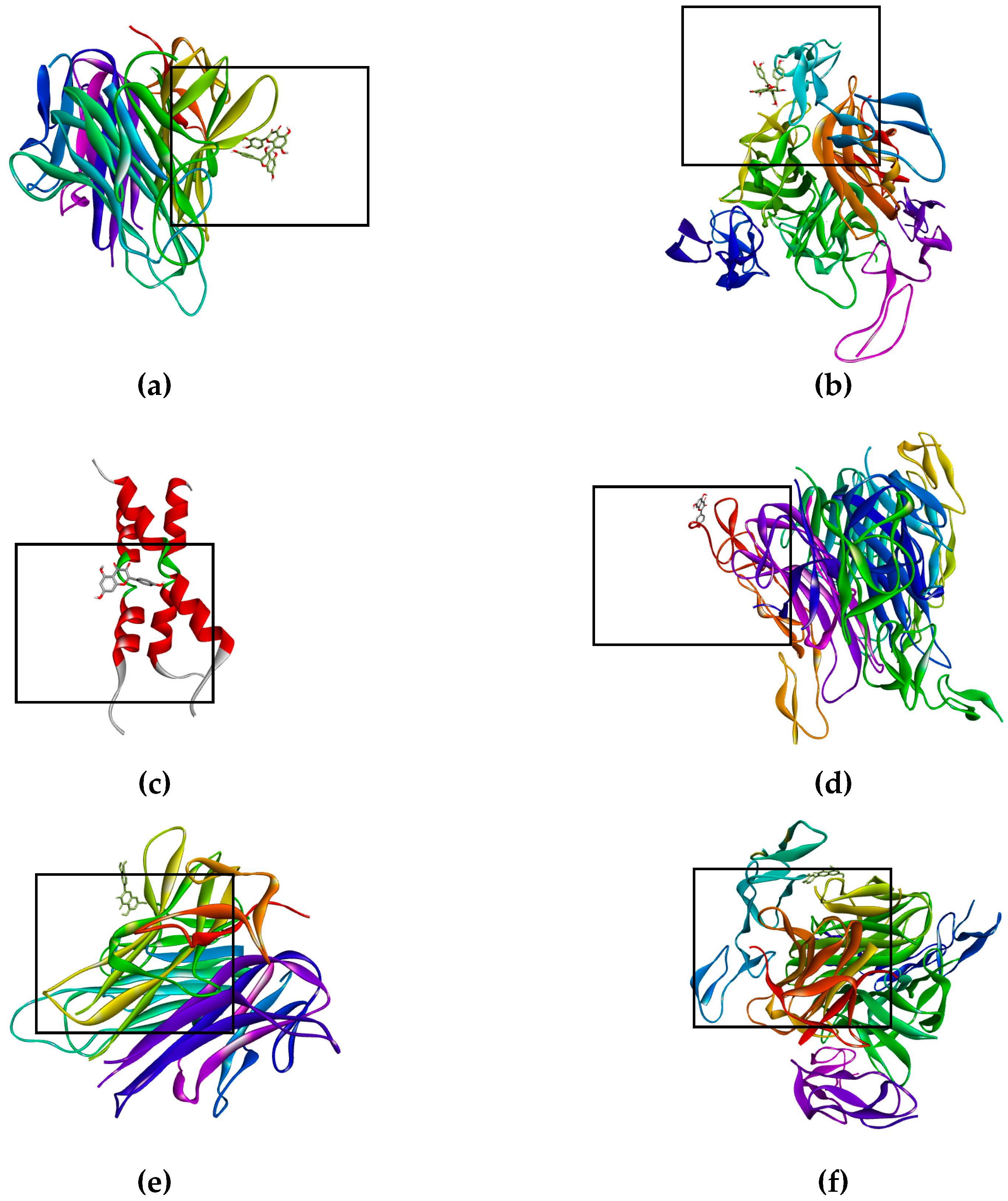
| Molecular Docking | Hydrogen Bonds | Polar Interactions | Hydrophobic Interactions | |||||
|---|---|---|---|---|---|---|---|---|
| Protein/Enzyme | Free Energy a/Kib | Ligand | Atom/aa | DST c | Atom/aa | DST c | Atom/aa | DST c |
| Death Receptor Signaling | ||||||||
| Fas/CD95 | +0.38/--- +0.13/--- +0.13/--- +0.48/--- | Docetaxel Kaempferol Quercetin Biflavonoid | --- O1/W189 --- --- | --- 3.18 --- --- | --- --- --- --- | --- --- --- --- | --- --- --- --- | --- --- --- --- |
| TNF-R1 | −6.35/245.01 +21.13/--- −5.03/206.98 −4.63/405.84 | Docetaxel Kaempferol Quercetin Biflavonoid | O8/T89 O11/T89 --- --- --- O6/T89 O7/T89 O5/T147 --- --- --- O3/T89 --- --- --- --- --- --- --- --- | 3.11 3.44 --- --- --- 2.89 3.01 3.09 --- --- --- 2.81 --- --- --- --- --- --- --- --- | O12/T89 H4/T89 O2/T89 O9/T89 --- H3/T89 H4/T89 O4/N92 O3/N92 H2/N34 H2/T147 O9/S81 H18/S81 H14/T89 O9/N92 H18/N92 H17/E135 O7/E135 H16/E135 O8/E135 | 3.71 3.35 3.84 3.50 --- 2.23 2.05 3.70 3.81 3.76 3.63 3.37 3.49 2.04 3.00 2.07 1.99 3.03 2.13 2.90 | --- --- --- --- --- C14/P90 C15/P90 C8/V91 --- --- --- C13/P90 C30/P90 C21/P90 C24/P90 --- --- --- --- --- | --- --- --- --- --- 3.34 3.52 3.48 --- --- --- 3.75 3.64 3.61 3.80 --- --- --- --- --- |
| DR4 | −4.29/712.86 −4.39/607.34 −4.78/315.05 −5.12/176.35 | Docetaxel Kaempferol Quercetin Biflavonoid | O6/R158 --- --- --- --- --- --- --- --- --- --- --- --- --- --- O1/T129 O5/T129 --- --- --- --- | 3.33 --- --- --- --- --- --- --- --- --- --- --- --- --- --- 3.41 3.02 --- --- --- --- | H4/T129 O7/E155 H3/E155 O7/R158 H3/R158 O5/R158 H2/R158 H2/T149 H15/T129 O1/T129 O1/E155 O2/E155 O4/E155 O2/R158 O5/H270 O1/E155 O2/E155 O4/E155 O2/R158 H15/T129 O5/H270 --- --- --- | 3.51 2.90 2.00 3.49 3.41 3.80 3.68 3.67 2.26 3.75 3.36 3.31 3.86 3.31 3.38 3.87 3.64 3.46 3.17 2.26 3.38 --- --- --- | --- --- --- --- --- --- --- --- --- C13/V152 --- --- --- --- --- --- --- --- --- C23/V152 C24/V152 C30/V152 C13/C180 C14/C180 | --- --- --- --- --- --- --- --- --- 3.26 --- --- --- --- --- --- --- --- --- 3.35 3.61 3.39 3.72 3.29 |
| DR5 | −1.73/53.64 × 103 −5.85/6.76 × 103 +6.28/--- +204.31/--- | Docetaxel Kaempferol Quercetin Biflavonoid | O7/S22 O5/S22 H2/S22 H2/P23 --- --- --- --- --- | 3.15 3.17 3.50 3.72 --- --- --- --- --- | --- O3/R39 H1/R39 O1/D40 O2/D40 O4/D40 H1/D40 --- --- | --- 3.55 3.16 3.08 3.77 3.64 3.85 --- --- | --- --- --- --- --- --- --- --- --- | --- --- --- --- --- --- --- --- --- |
| Mitochondrial Control of Apoptosis | ||||||||
| tBID | −3.24/4.21 × 103 −2.40/17.55 × 103 −2.69/10.58 × 103 −2.77/9.29 × 103 | Docetaxel Kaempferol Quercetin Biflavonoid | O10/R84 O3/S78 O6/R63 O7/R63 O7/R71 O6/R71 O3/S78 O11/R71 O9/S78 | 2.57 2.19 2.68 1.95 1.76 1.88 1.88 2.95 2.17 | --- --- --- --- --- --- --- --- --- | --- --- --- --- --- --- --- --- --- | --- --- --- --- --- --- --- --- --- | --- --- --- --- --- --- --- --- --- |
| Bax | +0.02/--- −5.33/122.89 +0.13/--- +0.46/--- | Docetaxel Kaempferol Quercetin Biflavonoid | --- --- --- --- --- --- --- --- --- --- --- --- | --- --- --- --- --- --- --- --- --- --- --- --- | --- H1/D31 O2/D31 O4/D31 O5/N31 O5/S98 H2/S98 O2/T151 O4/S154 O4/L203 --- --- | --- 3.33 3.36 3.29 3.70 3.52 3.65 3.58 3.57 3.76 --- --- | --- --- --- --- --- --- --- --- --- --- --- --- | --- --- --- --- --- --- --- --- --- --- --- --- |
| Bak | +0.14/--- +0.51/--- +0.13/--- +0.42/--- | Docetaxel Kaempferol Quercetin Biflavonoid | --- --- --- --- | --- --- --- --- | --- --- --- --- | --- --- --- --- | --- --- --- --- | --- --- --- --- |
| Bcl-2 | +0.04/--- +0.51/--- +0.13/--- +0.42/--- | Docetaxel Kaempferol Quercetin Biflavonoid | --- --- --- --- | --- --- --- --- | --- --- --- --- | --- --- --- --- | --- --- --- --- | --- --- --- --- |
| Caspases | ||||||||
| Casp-3 | −6.79/10.47 −6.15/30.95 +0.13/--- +0.43/--- | Docetaxel Kaempferol Quercetin Biflavonoid | O6/Y204 O11/Y204 --- --- --- --- --- --- --- --- O1/H121 O5/Q161 O1/C163 H2/R64 H2/Q161 H2/A162 --- --- | 3.40 3.17 --- --- --- --- --- --- --- --- 3.12 3.09 3.44 3.80 3.73 3.57 --- --- | O4/T62 H1/T62 O12/H121 N1/H121 H5/H121 O8/Y204 O2/Y204 --- --- --- O5/R64 O6/E123 H3/E123 O4/Y204 H1/Y204 O3/R207 --- --- | 3.83 3.52 3.53 3.71 2.90 3.07 3.57 --- --- --- 3.38 2.76 1.82 3.26 3.59 3.62 --- --- | C26/H121 C43/C163 C35/C163 C29/C163 C33/C163 C38/C163 C8/F256 C4/F256 C23/F256 C21/F256 C1/C163 C2/C163 C3/C163 C4/C163 --- --- --- --- | 3.71 3.17 3.25 3.83 3.41 3.75 3.76 3.76 3.25 3.58 3.45 3.35 3.76 3.66 --- --- --- --- |
| Casp-8 | −1.94/38.07 × 103 −1.05/170.68 × 103 −1.13/147.65 × 103 −0.41/502.84 | Docetaxel Kaempferol Quercetin Biflavonoid | --- --- --- --- --- --- --- --- --- | --- --- --- --- --- --- --- --- --- | --- O1/L224 --- --- --- --- --- --- --- | --- 3.20 --- --- --- --- --- --- --- | C39/K224 --- C7/K224 C11/K224 O3/K224 C1/K224 C6/L224 C27/K224 O8/K224 | 3.55 --- 3.68 3.56 3.75 3.67 3.75 3.53 3.88 |
| Necroptosis | ||||||||
| RIP1 | ND −7.66/2.44 −7.64/2.53 +63.84/--- | Docetaxel Kaempferol Quercetin Biflavonoid | ND --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- | ND --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- --- | ND O5/N68 H2/R71 --- --- --- --- --- --- --- --- O5/H136 H2/H136 --- --- --- --- --- --- --- --- | ND 3.68 3.65 --- --- --- --- --- --- --- --- 3.72 2.91 --- --- --- --- --- --- --- --- | ND C10/L70 C8/L78 C11/L78 C13/L78 C13/L90 C15/L90 C11/M92 C13/M92 C15/M92 C14/M92 C7/V75 C11/V75 C2/V76 C3/V76 C14/L78 C14/M92 C12M92 C15/M92 C11/L129 --- | ND 3.42 3.38 3.34 3.62 3.41 3.65 3.48 3.03 3.11 3.32 3.66 3.55 3.66 3.59 3.89 3.41 3.87 3.88 3.44 --- |
| MLKL | ND −4.98/225.37 −5.28/135.30 +0.58/--- | Docetaxel Kaempferol Quercetin Biflavonoid | ND --- --- --- --- --- --- --- --- --- --- O1/L230 --- --- --- --- --- --- --- --- --- --- | ND --- --- --- --- --- --- --- --- --- --- 3.00 --- --- --- --- --- --- --- --- --- --- | ND O3/R210 H1/R210 O1/K230 H2/K230 O5/E250 H2/E250 O2/S335 O1/N336 O2/N336 H3/E351 O3/R210 O7/Q236 H4/Q236 O5/E250 H2/E250 O6/K331 H3/L331 O1/N336 O2/N336 O4/N336 --- | ND 3.46 3.85 3.39 3.77 3.06 2.09 3.85 3.59 3.67 2.16 3.81 3.38 3.74 3.00 2.06 3.21 3.30 3.88 3.79 3.42 --- | ND C10/L338 C9/A348 C10/A348 --- --- --- --- --- --- --- C11/L338 C9/A348 C11/A348 --- --- --- --- --- --- --- --- | ND 3.59 3.56 3.65 --- --- --- --- --- --- --- 3.66 3.42 3.46 --- --- --- --- --- --- -- --- |
| FADD | ND +0.51/--- +0.13/--- +0.45/--- | Docetaxel Kaempferol Quercetin Biflavonoid | ND --- --- --- | ND --- --- --- | ND --- --- --- | ND --- --- --- | ND --- --- --- | ND --- --- --- |
| Epidermal Growth Factor Receptor (MAPK/ERK Pathway), Transforming Growth Factor B Receptor & Multidrug Resistance Protein 1 | ||||||||
| EGFR | ND −6.16/30.34 −6.82/10.10 +7.77/--- | Docetaxel Kaempferol Quercetin Biflavonoid | ND O2/S262 O4/S262 --- --- --- --- O2/S262 O4/S262 --- --- --- --- --- --- | ND 3.20 2.88 --- --- --- --- 2.96 2.82 --- --- --- --- --- --- | ND H2/H280 O6/D238 H3/D238 O3/H280 O4/S282 --- O5/H280 H2/H280 O6/D238 H3/D238 H4/D238 --- --- --- | ND 3.16 3.07 2.27 3.61 3.77 --- 3.87 3.05 2.85 1.89 3.72 --- --- --- | ND C8/P242 C15/L245 C14/L245 C12/L245 C9/P242 C10/P242 C8/P242 C13/L245 C15/L245 C14/L245 C10/L245 C11/P242 C9/P242 --- | ND 3.11 3.51 3.01 3.28 3.40 3.34 3.08 3.10 3.39 3.80 3.51 3.20 3.55 --- |
| TGFβ receptor | ND +0.51/--- +0.13/--- +0.44/--- | Docetaxel Kaempferol Quercetin Biflavonoid | ND --- --- ---- | ND --- --- --- | ND --- --- --- | ND --- --- --- | ND --- --- --- | ND --- --- --- |
| K-Ras | ND −6.72/11.93 −7.45/3.45 −6.59/14.71 | Docetaxel Kaempferol Quercetin Biflavonoid | ND --- --- --- --- --- --- --- --- O5/S145 H2/D119 H2/S145 H2/K147 O12/K117 H21/L120 --- --- --- --- --- | ND --- --- --- --- --- --- --- --- 3.43 3.07 3.60 3.81 3.36 3.86 --- --- --- --- --- | ND O6/S17 H3/S17 H1/D30 H3/T35 O5/D119 H2/D119 O5/S145 H2/S145 O7/S17 H4/S17 O5/D119 --- H18/S17 O6/D30 H16/D30 H15/D119 O5/D119 O4/K147 O5/K147 | ND 3.41 2.50 3.88 3.89 3.10 2.18 3.66 3.14 3.63 3.47 2.92 --- 3.57 3.46 3.83 2.00 2.76 3.55 3.54 | ND C11/A18 --- --- --- --- --- --- --- C4/A18 --- --- --- C9/Y32 --- --- --- --- --- --- | ND 3.32 --- --- --- --- --- --- --- 3.88 --- --- --- 3.81 --- --- --- --- --- --- |
| ALK | ND −3.01/6.25 × 103 −2.77/9.29 × 103 +180.68/--- | Docetaxel Kaempferol Quercetin Biflavonoid | ND --- --- --- --- --- --- --- --- --- --- --- --- | ND --- --- --- --- --- --- --- --- --- --- --- --- | ND O5/E1167 H2/E1167 O6/D1203 H3/D1203 H2/D1270 O5/D1270 O7/N1254 H4/N1254 O6/D1270 H3/D1270 O7/D1270 --- | ND 3.53 3.74 3.01 2.19 2.74 2.71 3.54 3.13 3.13 2.48 3.72 --- | ND C3/L1256 C5/L1256 O6/L1256 C11/L1256 C13/L1256 --- C1/L1256 C2/L1256 C6/L1256 C7/L1256 C8/L1256 --- | ND 3.74 3.37 3.46 3.17 3.44 --- 3.31 3.42 3.53 3.59 3.42 --- |
| MEK | ND +0.51/--- +0.14/--- +0.90/--- | Docetaxel Kaempferol Quercetin Biflavonoid | ND --- --- --- | ND --- --- --- | ND --- ---- --- | ND --- --- --- | ND --- --- --- | ND --- --- --- |
| MAPK | ND −3.34/3.58 × 103 −3.43/3.08 × 103 −2.64/11.70 × 103 | Docetaxel Kaempferol Quercetin Biflavonoid | ND O4/S51 --- --- --- --- --- O7/S51 H4/N49 H4/S51 O12/S51 H21/S51 --- | ND 3.03 --- --- --- --- --- 3.32 3.55 2.93 3.22 3.31 --- | ND O3/N49 H1/N49 O2/S51 O3/S51 H1/S51 H3/R56 O2/S51 H1/R56 O7/N49 O3/N49 H14/N49 --- | ND 3.47 3.57 3.67 3.76 3.88 3.12 3.78 3.13 2.91 3.90 3.54 --- | ND C12/L53 --- --- --- --- --- --- --- --- C22/V35 C23/V35 C19/P33 | ND 3.54 --- --- --- --- --- --- --- --- 3.81 3.80 3.89 |
| MRP1 | ND +0.50/--- +0.13/--- +0.41/--- | Docetaxel Kaempferol Quercetin Biflavonoid | ND --- --- --- | ND --- --- --- | ND --- --- --- | ND --- --- --- | ND --- --- --- | ND --- --- --- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anguiano-Sevilla, L.A.; Lugo-Cervantes, E.; Ordaz-Pichardo, C.; Rosas-Trigueros, J.L.; Jaramillo-Flores, M.E. Apoptosis Induction of Agave lechuguilla Torrey Extract on Human Lung Adenocarcinoma Cells (SK-LU-1). Int. J. Mol. Sci. 2018, 19, 3765. https://doi.org/10.3390/ijms19123765
Anguiano-Sevilla LA, Lugo-Cervantes E, Ordaz-Pichardo C, Rosas-Trigueros JL, Jaramillo-Flores ME. Apoptosis Induction of Agave lechuguilla Torrey Extract on Human Lung Adenocarcinoma Cells (SK-LU-1). International Journal of Molecular Sciences. 2018; 19(12):3765. https://doi.org/10.3390/ijms19123765
Chicago/Turabian StyleAnguiano-Sevilla, Luis Alberto, Eugenia Lugo-Cervantes, Cynthia Ordaz-Pichardo, Jorge Luis Rosas-Trigueros, and María Eugenia Jaramillo-Flores. 2018. "Apoptosis Induction of Agave lechuguilla Torrey Extract on Human Lung Adenocarcinoma Cells (SK-LU-1)" International Journal of Molecular Sciences 19, no. 12: 3765. https://doi.org/10.3390/ijms19123765






