Spatial Distribution of Glucan Type and Content between Caps and Stalks in Pleurotus eryngii: Impact on the Anti-inflammatory Functionality
Abstract
:1. Introduction
2. Results
2.1. Glucan Content Mushroom Stalk and Cap
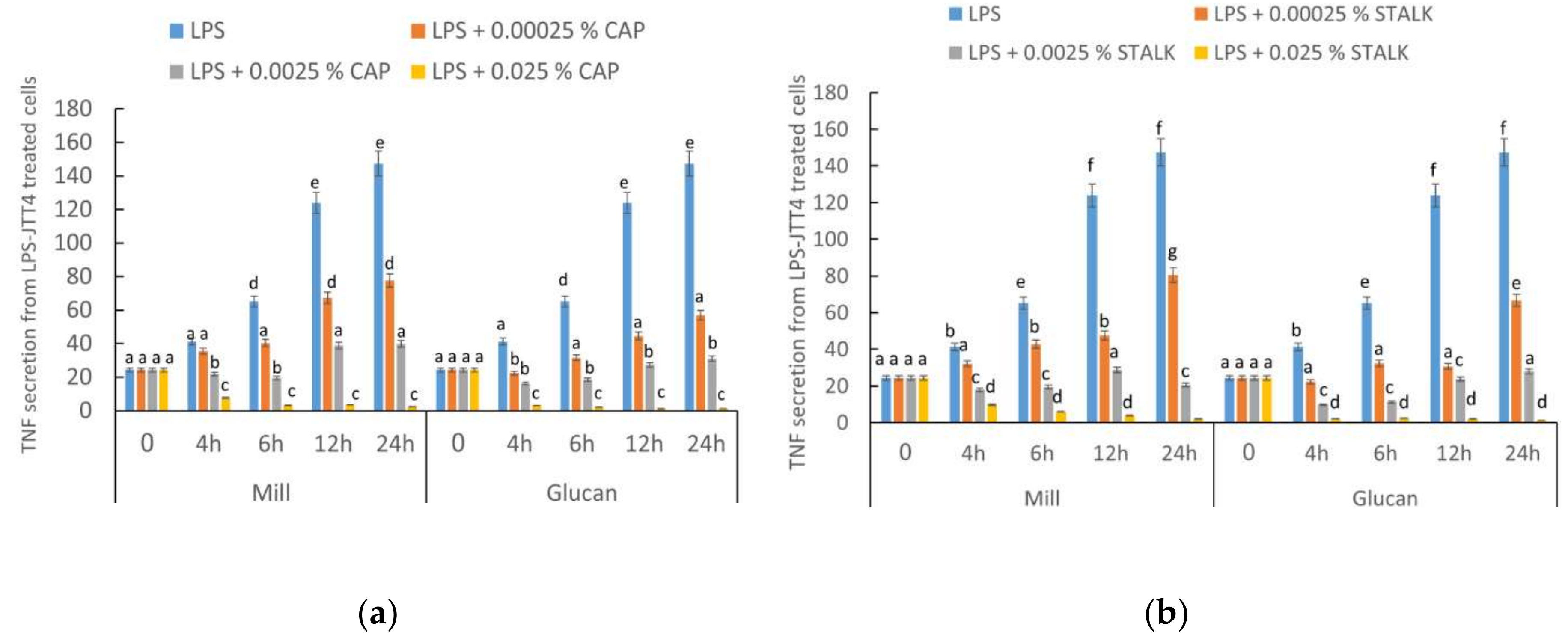
2.2. P. eryngii-Derived Glucans Suppressed TNF-α Secretion from Lipopolysaccharide-Treated J774.2 Cells
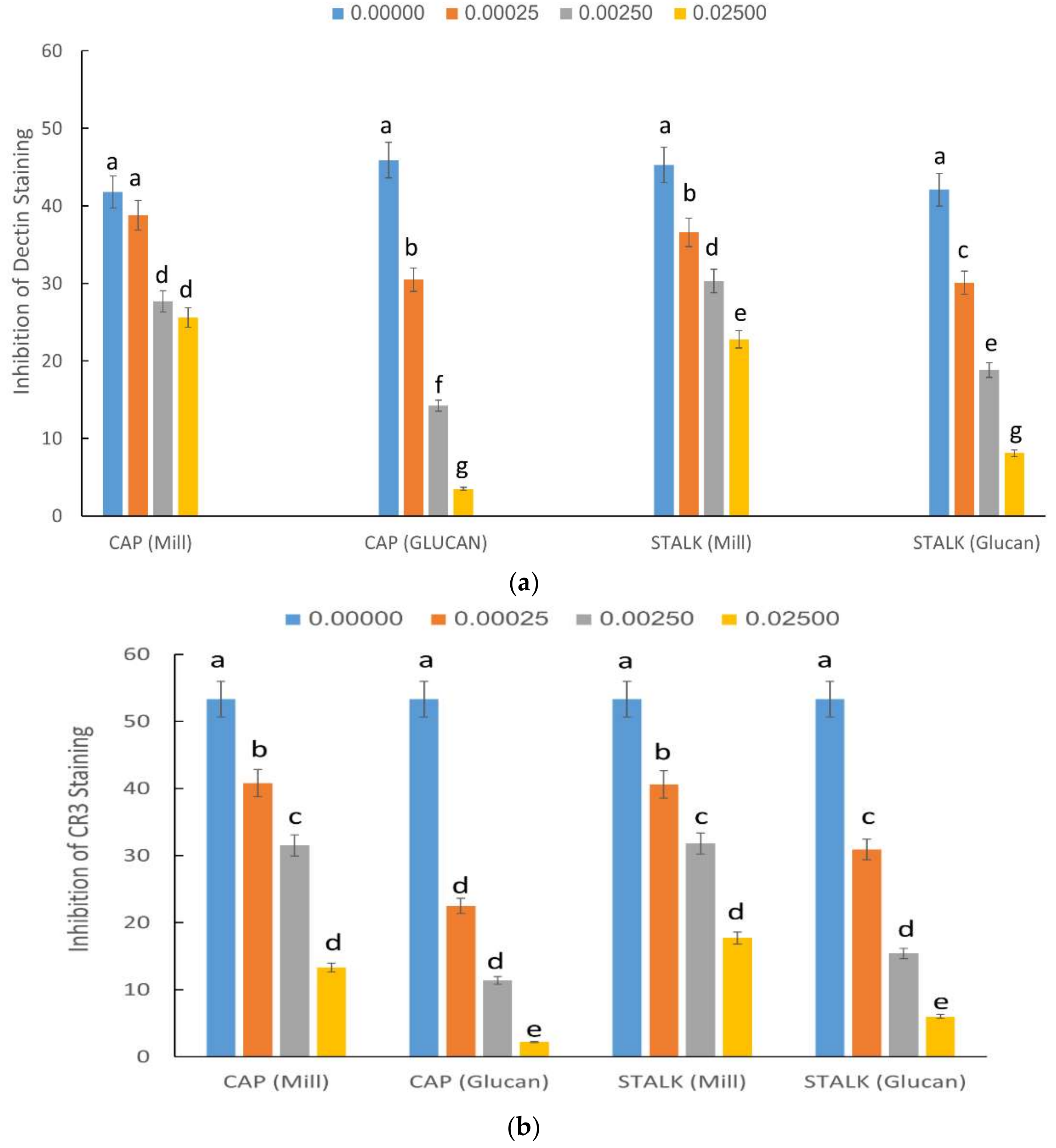
2.3. Inhibition of Staining of CR3 and Dectin-1 Receptors by P. eryngii Glucans
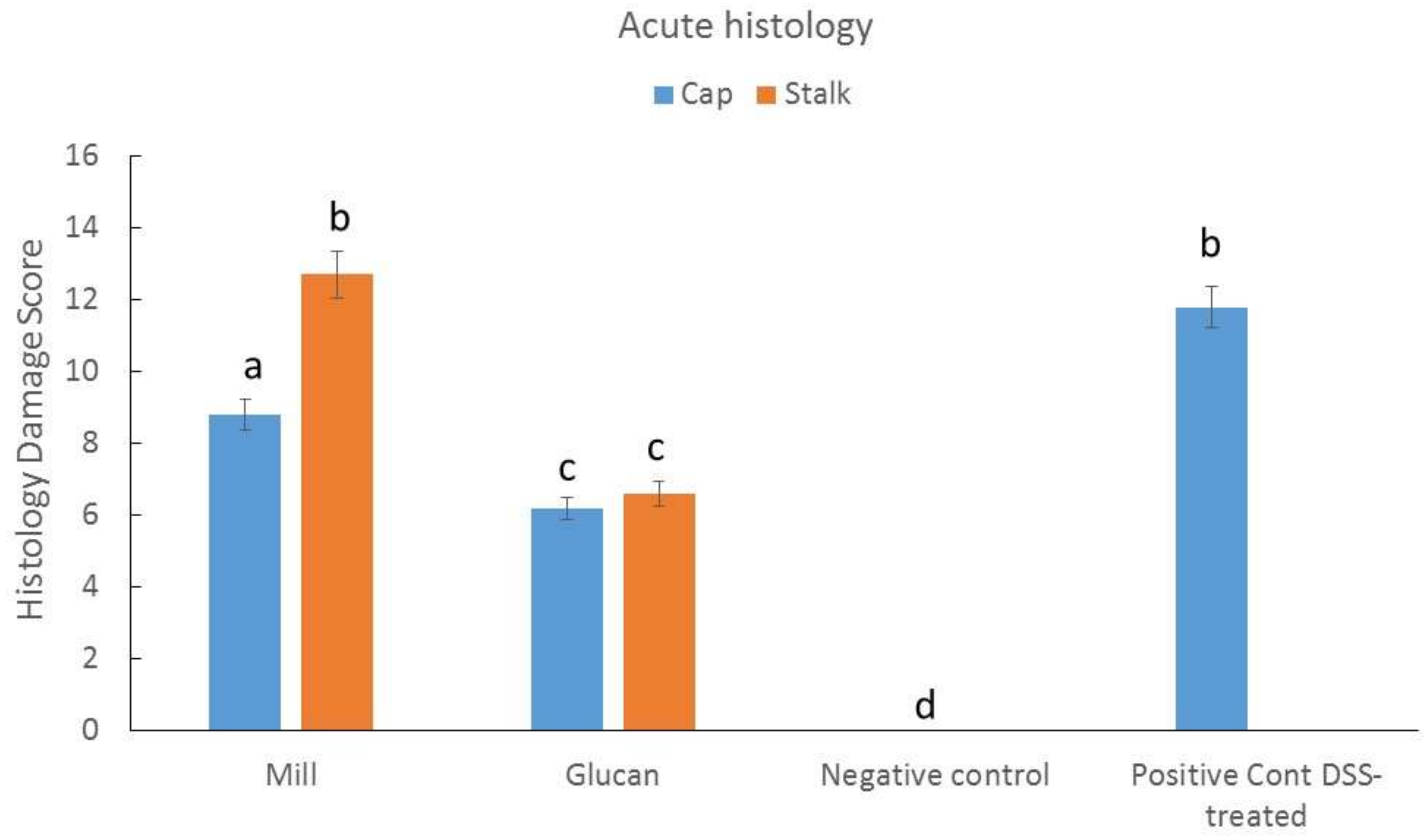
2.4. Effect of Isolated Glucans from Stalk and Cap on Acute Colitis Induction in Mice
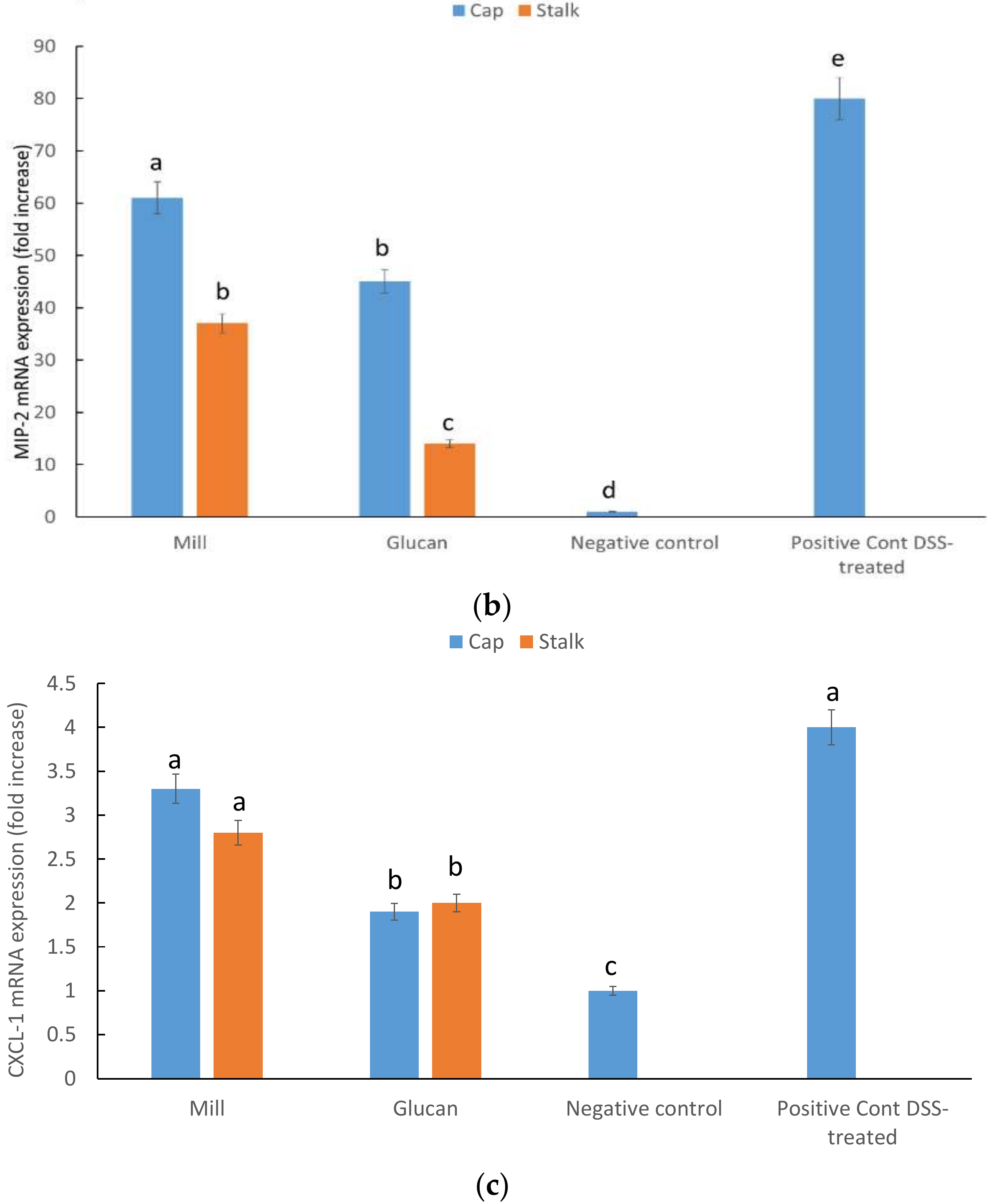
2.5. CXCL1, MIP-2, and INF-γ mRNA Expression in Large Intestine
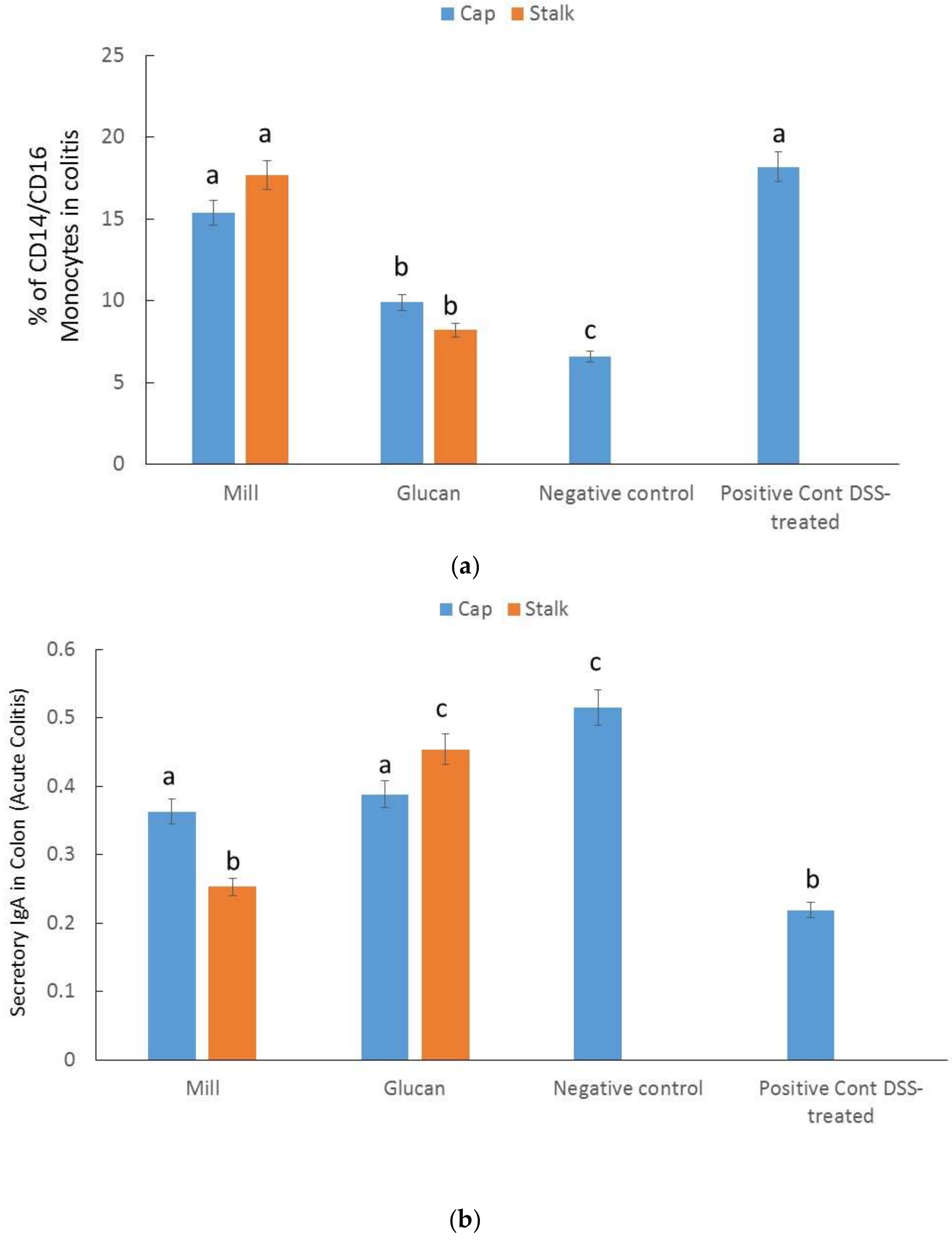
2.6. Percentages of CD14/CD16 Monocytes in Colitis
2.7. Secretory IgA in the Colon
3. Discussion
4. Materials and Methods
4.1. Preparation of Whole Mill and Glucan Extractions from Caps and Stalks from P. eryngii
4.2. Glucans Analysis
4.3. TNF-α Secretion from LPS- and Glucan-treated Mouse Macrophage Cell Line J774.2
4.4. Inhibition of CR3 and Dectin-1 Staining by Mill and Glucan Extracts from P. eryngii Caps and Stalks
4.5. Animals
4.6. Acute Colitis Induction in Mice
4.7. Monocytes Staining
4.8. RNA Preparation and Real-time PCR
4.9. Secretory Intestinal IgA
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, G.; Kimatu, B.M.; Zhao, L.; Yang, W.; Pei, F.; Hu, Q. Impacts of Dietary Pleurotus eryngii Polysaccharide on Nutrient Digestion, Metabolism, and Immune Response of the Small Intestine and Colon-An iTRAQ-Based Proteomic Analysis. Proteomics 2018, 18, e1700443. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jung, E.G.; Han, K.I.; Patnaik, B.B.; Kwon, H.J.; Lee, H.S.; Kim, W.J.; Han, M.D. Immunomodulatory Effects of Extracellular beta-Glucan Isolated from the King Oyster Mushroom Pleurotus eryngii (Agaricomycetes) and Its Sulfated Form on Signaling Molecules Involved in Innate Immunity. Int. J. Med. Mushrooms 2017, 19, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, H.; Zheng, W.; Gao, Y.; Wang, M.; Zhang, Y.; Gao, Q. Charaterization and immunomodulatory activities of polysaccharide isolated from Pleurotus eryngii. Int. J. Biol. Macromol. 2016, 92, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of β-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Lavi, I.; Friesem, D.; Geresh, S.; Hadar, Y.; Schwartz, B. An aqueous polysaccharide extract from the edible mushroom Pleurotus ostreatus induces anti-proliferative and pro-apoptotic effects on HT-29 colon cancer cells. Cancer Lett. 2006, 244, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Lavi, I.; Levinson, D.; Peri, I.; Nimri, L.; Hadar, Y.; Schwartz, B. Orally administered glucans from the edible mushroom Pleurotus pulmonarius reduce acute inflammation in dextran sulfate sodium-induced experimental colitis. Br. J. Nutr. 2010, 103, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Lavi, I.; Nimri, L.; Levinson, D.; Peri, I.; Hadar, Y.; Schwartz, B. Glucans from the edible mushroom Pleurotus pulmonarius inhibit colitis-associated colon carcinogenesis in mice. J. Gastroenterol. 2012, 47, 504–518. [Google Scholar] [CrossRef] [PubMed]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrieres, V. Molecular Interactions of β-(1-->3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto-Furusho, J.K. Inflammatory bowel disease therapy: Blockade of cytokines and cytokine signaling pathways. Curr. Opin. Gastroenterol. 2018, 34, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Avni, S.; Ezove, N.; Hanani, H.; Yadid, I.; Karpovsky, M.; Hayby, H.; Gover, O.; Hadar, Y.; Schwartz, B.; Danay, O. Olive Mill Waste Enhances α-Glucan Content in the Edible Mushroom Pleurotus eryngii. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Earnshaw, S.R.; McDade, C.L.; Chu, Y.; Fleige, L.E.; Sievenpiper, J.L. Cost-effectiveness of Maintaining Daily Intake of Oat β-Glucan for Coronary Heart Disease Primary Prevention. Clin. Ther. 2017, 39, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.C.; Chan, W.K.; Sze, D.M. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra Dore, C.M.; Azevedo, T.C.; De Souza, M.C.; Rego, L.A.; De Dantas, J.C.; Silva, F.R.; Rocha, H.A.; Baseia, I.G.; Leite, E.L. Antiinflammatory, antioxidant and cytotoxic actions of beta-glucan-rich extract from Geastrum saccatum mushroom. Int. Immunopharmacol. 2007, 7, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Nosal’ova, V.; Bobek, P.; Cerna, S.; Galbavy, S.; Stvrtina, S. Effects of pleuran (β-glucan isolated from Pleurotus ostreatus) on experimental colitis in rats. Physiol. Res. 2001, 50, 575–581. [Google Scholar] [PubMed]
- Soltanian, S.; Stuyven, E.; Cox, E.; Sorgeloos, P.; Bossier, P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit. Rev. Microbiol. 2009, 35, 109–138. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Glucans and Cancer: Comparison of Commercially Available β-glucans—Part IV. Anticancer Res. 2018, 38, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Helsper, J.P.; Wei, S.; Baars, J.J.; Van Griensven, L.J.; Sonnenberg, A.S.; Mensink, R.P.; Plat, J. Effects of mushroom-derived β-glucan-rich polysaccharide extracts on nitric oxide production by bone marrow-derived macrophages and nuclear factor-κB transactivation in Caco-2 reporter cells: Can effects be explained by structure? Mol. Nutr. Food Res. 2010, 54, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sheng, X.; Shi, A.; Hu, H.; Yang, Y.; Liu, L.; Fei, L.; Liu, H. β-Glucans: Relationships between Modification, Conformation and Functional Activities. Molecules 2017, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Willment, J.A.; Marshall, A.S.; Reid, D.M.; Williams, D.L.; Wong, S.Y.; Gordon, S.; Brown, G.D. The human β-glucan receptor is widely expressed and functionally equivalent to murine Dectin-1 on primary cells. Eur. J. Immunol. 2005, 35, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirtz, S.; Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007, 59, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Shin-Ya, M.; Kishida, T.; Urano, A.; Takada, R.; Sakagami, J.; Imanishi, J.; Kita, M.; Ueda, Y.; Iwakura, Y.; et al. Interferon-γ is causatively involved in experimental inflammatory bowel disease in mice. Clin. Exp. Immunol. 2006, 146, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Yamada, M.; Kikuyama, M.; Maruyama, Y.; Iwaoka, Y.; Hirayama, K.; Nagata, S.; et al. Adsorptive depletion of elevated proinflammatory CD14+CD16+DR++ monocytes in patients with inflammatory bowel disease. Am. J. Gastroenterol. 2008, 103, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.; Kucharzik, T.; Heidemann, J.; Nusrat, A.; Luegering, A. Investigating the role of proinflammatory CD16+ monocytes in the pathogenesis of inflammatory bowel disease. Clin. Exp. Immunol. 2010, 161, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Dieleman, L.A.; Palmen, M.J.; Akol, H.; Bloemena, E.; Pena, A.S.; Meuwissen, S.G.; Van Rees, E.P. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin. Exp. Immunol. 1998, 114, 385–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetvicka, V.; Volny, T.; Saraswat-Ohri, S.; Vashishta, A.; Vancikova, Z.; Vetvickova, J. Glucan and resveratrol complex—Possible synergistic effects on immune system. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech. 2007, 151, 41–46. [Google Scholar] [CrossRef]
- Cao, A.T.; Yao, S.; Gong, B.; Elson, C.O.; Cong, Y. Th17 cells upregulate polymeric Ig receptor and intestinal IgA and contribute to intestinal homeostasis. J. Immunol. 2012, 189, 4666–4673. [Google Scholar] [CrossRef] [PubMed]

| P. eryngii Parts and Preparations | α-Glucan (g/100 g) | β-Glucan (g/100 g) | Total Glucans (g/100 g) |
|---|---|---|---|
| Cap whole mill | 0.804 ± 0.006 | 29.519 ± 0.98 | 30.32 ± 0.5 |
| Stalk whole mill | 4.505 ± 0.35 * | 38.412 ± 1.2 * | 42.92 ± 0.77 * |
| Cap glucan extract | 6.545 ± 1.38 | 21.804 ± 1.27 | 28.5 ± 1.32 |
| Stalk glucan extract | 16.9851 ± 1.6 ** | 29.807 ± 2.6 * | 46.79 ± 2.12 * |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| MIP-2 | 5′-TGGGTGGGATGTAGCTAGTTCC | 5′-AGTTTGCCTTGACCCTGAAGCC |
| Cxcl1 | 5’- GCCACACTCAAGAATGGTCG | 5’-TGGGGACACCCTTTAGCATC |
| INF-γ | 5’-GTCTCTTCTTGGATATCTGGAGGAACT | 5’-GTAGTAATCAGGTGTGATTCAATGACGC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetvicka, V.; Gover, O.; Hayby, H.; Danay, O.; Ezov, N.; Hadar, Y.; Schwartz, B. Spatial Distribution of Glucan Type and Content between Caps and Stalks in Pleurotus eryngii: Impact on the Anti-inflammatory Functionality. Int. J. Mol. Sci. 2018, 19, 3371. https://doi.org/10.3390/ijms19113371
Vetvicka V, Gover O, Hayby H, Danay O, Ezov N, Hadar Y, Schwartz B. Spatial Distribution of Glucan Type and Content between Caps and Stalks in Pleurotus eryngii: Impact on the Anti-inflammatory Functionality. International Journal of Molecular Sciences. 2018; 19(11):3371. https://doi.org/10.3390/ijms19113371
Chicago/Turabian StyleVetvicka, Vaclav, Ofer Gover, Hilla Hayby, Ofer Danay, Nirit Ezov, Yitzhak Hadar, and Betty Schwartz. 2018. "Spatial Distribution of Glucan Type and Content between Caps and Stalks in Pleurotus eryngii: Impact on the Anti-inflammatory Functionality" International Journal of Molecular Sciences 19, no. 11: 3371. https://doi.org/10.3390/ijms19113371




