Protective Immune Responses Generated in a Murine Model Following Immunization with Recombinant Schistosoma japonicum Insulin Receptor
Abstract
:1. Introduction
2. Results
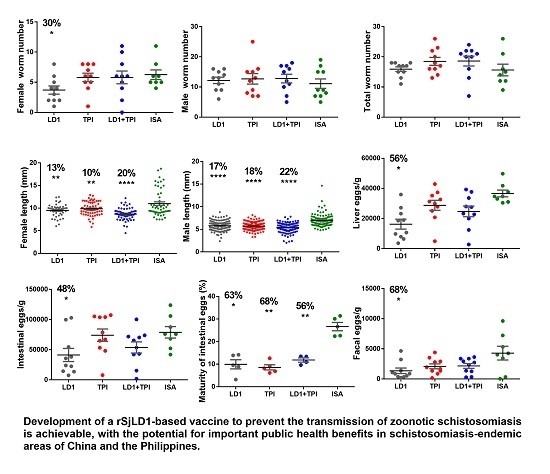
2.1. Protective Potential of SjLD1
2.1.1. Specific Antibody Titres
2.1.2. Cytokine Production
Cytokine Responses in Mice Vaccinated with rSjLD1, rSjTPI and the rSjLD1 + rSjTPI Combination Adjuvanted with ISA720
Cytokine Responses in Mice Vaccinated with rSjLD1, rSjTPI and rSjLD1 + rSjTPI Adjuvanted with QuilA
Ratio of Th1/Th2 in Splenic CD4+ T Cells Harvested from Mice Vaccinated with rSjLD1, rSjTPI and rSjLD1 + rSjTPI, when Stimulated with rSjLD1, rSjTPI, SWAP or SEA
2.2. The rSjLD1 Vaccine Had No Effect on Host Glucose Metabolism
2.3. No Immunological Cross Reactivity Occurs between Human TPI and SjTPI on the Surface of Human Cells
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Parasites
4.3. Protective Efficacy of the Recombinant SjLD1 and SjTPI Vaccine
4.3.1. Escherichia coli Protein Expression
4.3.2. Animal Immunization and Challenge Experiments
4.3.3. Worm and Egg Counting and Pathology
4.3.4. Specific Antibody Responses
4.3.5. Blood Glucose Levels of SjLD1-Vaccinated Mice and Controls
4.3.6. Flow Cytometry Analysis
4.4. Immunofluorescence-Staining of Mouse Anti-SjTPI Antibody in Hepatic Stellate Cells and TPI Activity Assays
4.4.1. Immunofluorescence-Staining
4.4.2. TPI Activity Assays
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ross, A.G.; Bartley, P.B.; Sleigh, A.C.; Olds, G.R.; Li, Y.; Williams, G.M.; McManus, D.P. Schistosomiasis. N. Engl. J. Med. 2002, 346, 1212–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagan, P.; Appleton, C.C.; Coles, G.C.; Kusel, J.R.; Tchuem-Tchuente, L.A. Schistosomiasis control: Keep taking the tablets. Trends Parasitol. 2004, 20, 92–97. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Loukas, A. Current status of vaccines for schistosomiasis. Clin. Microbiol. Rev. 2008, 21, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.A.; Acosta, L.P.; Gray, D.J.; Olveda, R.M.; Jarilla, B.; Gobert, G.N.; Ross, A.G.; McManus, D.P. High prevalence of Schistosoma japonicum infection in Carabao from Samar Province, the Philippines: Implications for transmission and control. PLoS Negl. Trop. Dis. 2012, 6, e1778. [Google Scholar] [CrossRef] [PubMed]
- Da’dara, A.A.; Li, Y.S.; Xiong, T.; Zhou, J.; Williams, G.M.; McManus, D.P.; Feng, Z.; Yu, X.L.; Gray, D.J.; Harn, D.A. DNA-based vaccines protect against zoonotic schistosomiasis in water buffalo. Vaccine 2008, 26, 3617–3625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bueding, E. Carbohydrate metabolism of schistosoma mansoni. J. Gen. Physiol. 1950, 33, 475–495. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Zhang, W.; Jones, M.K.; Gobert, G.N.; Mulvenna, J.; Rees, G.; Spanevello, M.; Blair, D.; Duke, M.; Brehm, K.; et al. Cloning and characterisation of Schistosoma japonicum insulin receptors. PLoS ONE 2010, 5, e9868. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Williams, B.G.; Koumanov, F.; Wolstenholme, A.J.; Holman, G.D. FGT-1 is the major glucose transporter in C. elegans and is central to aging pathways. Biochem. J. 2013, 456, 219–229. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Gobert, G.N.; Duke, M.G.; Zhang, W.; Li, Y.; Jones, M.K.; McManus, D.P. The insulin receptor is a transmission blocking veterinary vaccine target for zoonotic Schistosoma japonicum. Int. J. Parasitol. 2012, 42, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Romeih, M.H.; Hassan, H.M.; Shousha, T.S.; Saber, M.A. Immunization against Egyptian Schistosoma mansoni infection by multivalent DNA vaccine. Acta Biochim. Biophys. Sin. 2008, 40, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.S.; Ribeiro, A.P.; Cardoso, F.C.; Martins, V.P.; Figueiredo, B.C.; Assis, N.R.; Morais, S.B.; Caliari, M.V.; Loukas, A.; Oliveira, S.C. A multivalent chimeric vaccine composed of Schistosoma mansoni SmTSP-2 and Sm29 was able to induce protection against infection in mice. Parasite Immunol. 2014, 36, 303–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Si, J.; Ham, D.A.; Yu, C.; He, W.; Hua, W.; Yin, X.; Liang, Y.; Xu, M.; Xu, R. The protective immunity produced in infected C57BL/6 mice of a DNA vaccine encoding Schistosoma japonicum Chinese strain triose-phosphate isomerase. Southeast Asian J. Trop. Med. Public Health 2002, 33, 207–213. [Google Scholar] [PubMed]
- Zhu, Y.; Si, J.; Harn, D.A.; Xu, M.; Ren, J.; Yu, C.; Liang, Y.; Yin, X.; He, W.; Cao, G. Schistosoma japonicum triose-phosphate isomerase plasmid DNA vaccine protects pigs against challenge infection. Parasitology 2006, 132, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; He, Y.K.; Xiong, T.; Zhao, Y.Q.; Shi, M.Z.; Zhou, J.; Liu, Z.C.; Luo, X.S.; Fu, X.; He, H.B.; et al. Protective effects of co-immunization with SjCTPI-Hsp70 and interleukin-12 DNA vaccines against Schistosoma japonicum challenge infection in water buffalo. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2006, 24, 433–436. (In Chinese) [Google Scholar] [PubMed]
- Reis, E.A.; Mauadi Carmo, T.A.; Athanazio, R.; Reis, M.G.; Harn, D.A., Jr. Schistosoma mansoni triose phosphate isomerase peptide MAP4 is able to trigger naive donor immune response towards a type-1 cytokine profile. Scand. J. Immunol. 2008, 68, 169–176. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Gobert, G.N.; Cai, P.; Mou, R.; Nawaratna, S.; Fang, G.; Villinger, F.; McManus, D.P. Suppression of the insulin receptors in adult Schistosoma japonicum impacts on parasite growth and development: Further evidence of vaccine potential. PLoS Negl. Trop. Dis. 2015, 9, e0003730. [Google Scholar] [CrossRef] [PubMed]
- El Ridi, R.; Tallima, H. Why the radiation-attenuated cercarial immunization studies failed to guide the road for an effective schistosomiasis vaccine: A review. J. Adv. Res. 2015, 6, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Torben, W.; Ahmad, G.; Zhang, W.; Siddiqui, A.A. Role of antibodies in Sm-p80-mediated protection against Schistosoma mansoni challenge infection in murine and nonhuman primate models. Vaccine 2011, 29, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Langermans, J.A.; van Dam, G.J.; Vervenne, R.A.; Hall, S.L.; Borges, W.C.; Dillon, G.P.; Thomas, A.W.; Coulson, P.S. Elimination of Schistosoma mansoni adult worms by rhesus macaques: Basis for a therapeutic vaccine? PLoS Negl. Trop. Dis. 2008, 2, e290. [Google Scholar] [CrossRef] [PubMed]
- Farias, L.P.; Cardoso, F.C.; Miyasato, P.A.; Montoya, B.O.; Tararam, C.A.; Roffato, H.K.; Kawano, T.; Gazzinelli, A.; Correa-Oliveira, R.; Coulson, P.S.; et al. Schistosoma mansoni Stomatin like protein-2 is located in the tegument and induces partial protection against challenge infection. PLoS Negl. Trop. Dis. 2010, 4, e597. [Google Scholar] [CrossRef] [PubMed]
- Teixeira de Melo, T.; Araujo, J.M.; Campos de Sena, I.; Carvalho Alves, C.; Araujo, N.; Toscano Fonseca, C. Evaluation of the protective immune response induced in mice by immunization with Schistosoma mansoni schistosomula tegument (Smteg) in association with CpG-ODN. Microbes Infect. 2013, 15, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Hewitson, J.P.; Hamblin, P.A.; Mountford, A.P. Immunity induced by the radiation-attenuated schistosome vaccine. Parasite Immunol. 2005, 27, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Luo, X.; Zhang, F.; Zhu, Y.; Yang, B.; Hou, M.; Xu, Z.; Yu, C.; Chen, Y.; Chen, L.; et al. SjTat-TPI facilitates adaptive T-cell responses and reduces hepatic pathology during Schistosoma japonicum infection in BALB/c mice. Parasites Vectors 2015, 8, 664. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, S.; Tang, J.; Xing, Y.; Qu, G.; Dai, J.; Jin, X.; Wang, X. Evaluation of protective efficacy induced by different heterologous prime-boost strategies encoding triosephosphate isomerase against Schistosoma japonicum in mice. Parasites Vectors 2017, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, X.; Tang, J.; Zhao, S.; Xing, Y.; Dai, J.; Jin, X.; Zhu, Y. Enhancement of protective efficacy through adenoviral vectored vaccine priming and protein boosting strategy encoding triosephosphate isomerase (SjTPI) against Schistosoma japonicum in mice. PLoS ONE 2015, 10, e0120792. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, R.A.; Hassan, A.A. Murine schistosomiasis as a model for human schistosomiasis mansoni: Similarities and discrepancies. Parasitol. Res. 2010, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.J.; MacDonald, A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002, 2, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Mentink-Kane, M.M.; Pesce, J.T.; Ramalingam, T.R.; Thompson, R.; Wynn, T.A. Immunopathology of schistosomiasis. Immunol. Cell Biol. 2007, 85, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.H.; Pearson, M.S.; Bethony, J.M.; Smyth, D.J.; Jones, M.K.; Duke, M.; Don, T.A.; McManus, D.P.; Correa-Oliveira, R.; Loukas, A. Tetraspanins on the surface of Schistosoma mansoni are protective antigens against schistosomiasis. Nat. Med. 2006, 12, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, B.C.; Assis, N.R.; Morais, S.B.; Ricci, N.D.; Pinheiro, C.S.; Martins, V.P.; Bicalho, R.M.; Da’dara, A.A.; Skelly, P.J.; Oliveira, S.C. Schistosome syntenin partially protects vaccinated mice against Schistosoma mansoni infection. PLoS Negl. Trop. Dis. 2014, 8, e3107. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Visitsunthorn, K.; Dalton, J.P.; Ndao, M. A vaccine consisting of Schistosoma mansoni cathepsin B formulated in Montanide ISA 720 VG induces high level protection against murine schistosomiasis. BMC Infect. Dis. 2016, 16, 112. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.A.; Li, X.H.; Castro-Borges, W. Do schistosome vaccine trials in mice have an intrinsic flaw that generates spurious protection data? Parasites Vectors 2016, 9, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebens, M.; Sun, J.B.; Czerkinsky, C.; Holmgren, J. Current status and future prospects for a vaccine against schistosomiasis. Expert Rev. Vaccines 2004, 3, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fu, Z.; Feng, X.; Shi, Y.; Yuan, C.; Liu, J.; Hong, Y.; Li, H.; Lu, K.; Lin, J. Comparison of worm development and host immune responses in natural hosts of Schistosoma japonicum, yellow cattle and water buffalo. BMC Vet. Res. 2012, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Cai, P.; Tebeje, B.M.; Li, Y.; McManus, D.P. Schistosome vaccines for domestic animals. Trop. Med. Infect. Dis. 2018, 3, 68. [Google Scholar] [CrossRef] [PubMed]
- Gobert, G.N.; Moertel, L.; Brindley, P.J.; McManus, D.P. Developmental gene expression profiles of the human pathogen Schistosoma japonicum. BMC Genom. 2009, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, S.; Brindley, P.J.; McManus, D.P. Bacterial expression and characterization of functional recombinant triosephosphate isomerase from Schistosoma japonicum. Protein Exp. Purif. 1999, 17, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Weerakoon, K.G.; Mu, Y.; Olveda, D.U.; Piao, X.; Liu, S.; Olveda, R.M.; Chen, Q.; Ross, A.G.; McManus, D.P. A parallel comparison of antigen candidates for development of an optimized serological diagnosis of schistosomiasis japonica in the philippines. EBioMedicine 2017, 24, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, A.; Dalton, J.P.; Ndao, M. Evaluation of the immune response and protective efficacy of Schistosoma mansoni Cathepsin B in mice using CpG dinucleotides as adjuvant. Vaccine 2015, 33, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Reynaert, H.; Thompson, M.G.; Thomas, T.; Geerts, A. Hepatic stellate cells: Role in microcirculation and pathophysiology of portal hypertension. Gut 2002, 50, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pham, T.; McWhinney, B.C.; Ungerer, J.P.; Pretorius, C.J.; Richard, D.J.; Mortimer, R.H.; D’Emden, M.C.; Richard, K. Sex hormone binding globulin modifies testosterone action and metabolism in prostate cancer cells. Int. J. Endocrinol. 2016, 2016, 6437585. [Google Scholar] [CrossRef] [PubMed]






| Adjuvant | Group | Number Adult Worms Mean ± SE | Mean Length of Adult Worms (mm) Mean ± SE %Reduction (p Value) | Liver Eggs/g Mean ± SE %Reduction (p Value) | Intestinal Eggs/g Mean ± SE %Reduction (p Value) | Maturity of Intestinal Eggs in Stage V (%) Mean ± SE %Reduction (p Value) | Faecal Eggs/g/f Mean ± SE %Reduction (p Value) |
|---|---|---|---|---|---|---|---|
| QuilA. | Control n = 10 | (F) 5.9 ± 0.7 (M) 8.5 ± 0.8 | (F) 10.4 ± 0.2 (M) 6.6 ± 0.2 | 41886 ± 6001 | 64483 ± 9998 | 21.6 ± 2 | 1398 ± 474 |
| SjLD1 n = 10 | (F) 3.3 ± 0.6 44% ** (p = 0.008) (M) 4.8 ± 0.8 43.5% * (p = 0.03) | (F) 10.8 ± 0.3 (M) 7.7 ± 0.3 | 23285 ± 4834 44% * (p = 0.03) | 34533 ± 8038 46% * (p = 0.04) | 8.9 ± 1.5 58% * (p = 0.04) | 546 ± 276 61% * (p = 0.02) | |
| SjTPI n = 10 | (F) 5.7 ± 0.6 (M) 7.4 ± 1.0 | (F) 10.7 ± 0.3 (M) 8.3 ± 0.3 | 42894 ± 4548 | 55473 ± 7574 14% ns (p = 0.4) | 4.4 ± 0.3 79% *** (p = 0.0002) | 776 ± 354 44% ns (p = 0.36) | |
| SjLD1 + TPI n = 10 | (F) 5.7 ± 0.9 (M) 8.2 ± 1.1 | (F) 10.6 ± 0.2 (M) 7.1 ± 0.3 | 41649 ± 4391 | 61291 ± 12505 | 8.4 ± 2.3 67% * (p = 0.03) | 885 ± 320 36% ns (p = 0.32) | |
| ISA-720 | Control n = 10 | (F) 5.3 ± 0.9 (M) 11.1 ± 1.6 | (F) 11.1 ± 0.4 (M) 6.9 ± 0.1 | 36625 ± 2271 | 78969 ± 9486 | 26.6 ± 1.8 | 4278 ± 1019 |
| SjLD1 n = 10 | (F) 3.7 ± 0.7 30% * (p = 0.05) (M) 12.2 ± 1.1 | (F) 9.5 ± 0.2 13% ** (p = 0.003) (M) 5.8 ± 0.1 16% *** (p < 0.0001) | 16233 ± 3276 56% *** (p = 0.0003) | 41331 ± 10912 48% * (p = 0.013) | 9.9 ± 2.0 63% * (p = 0.01) | 1349 ± 459 68% * (p = 0.05) | |
| SjTPI n = 10 | (F) 5.8 ± 0.7 (M) 12.7 ± 1.7 | (F) 9.8 ± 0.2 10% ** (p = 0.009) (M) 5.7 ± 0.1 18% *** (p < 0.0001) | 28785 ± 3306 21% ns (p = 0.08) | 74104 ± 10278 | 8.6 ± 1.1 68% *** (p = 0.0007) | 2089 ± 410 51% ns (p = 0.06) | |
| SjLD1 + TPI n = 10 | (F) 5.8 ± 1.1 (M) 12.8 ± 1.4 | (F) 8.7 ± 0.2 20% *** (p < 0.0001) (M) 5.4 ± 0.1 22% *** (p < 0.0001) | 24735 ± 3558 33% * (p = 0.03) | 53788 ± 10381 32% * (p = 0.05) | 11.8 ± 0.9 56% * (p = 0.06) | 2158 ± 399 50% ns (p = 0.07) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, H.; Harvie, M.; Du, X.; Rivera, V.; Zhang, P.; McManus, D.P. Protective Immune Responses Generated in a Murine Model Following Immunization with Recombinant Schistosoma japonicum Insulin Receptor. Int. J. Mol. Sci. 2018, 19, 3088. https://doi.org/10.3390/ijms19103088
You H, Harvie M, Du X, Rivera V, Zhang P, McManus DP. Protective Immune Responses Generated in a Murine Model Following Immunization with Recombinant Schistosoma japonicum Insulin Receptor. International Journal of Molecular Sciences. 2018; 19(10):3088. https://doi.org/10.3390/ijms19103088
Chicago/Turabian StyleYou, Hong, Marina Harvie, Xiaofeng Du, Vanessa Rivera, Ping Zhang, and Donald P. McManus. 2018. "Protective Immune Responses Generated in a Murine Model Following Immunization with Recombinant Schistosoma japonicum Insulin Receptor" International Journal of Molecular Sciences 19, no. 10: 3088. https://doi.org/10.3390/ijms19103088