Systemic Lipopolysaccharide-Induced Pain Sensitivity and Spinal Inflammation Were Reduced by Minocycline in Neonatal Rats
Abstract
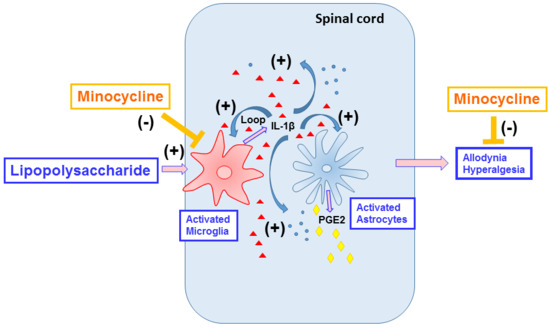
1. Introduction
2. Results
2.1. Minocycline Attenuated Systemic LPS-Induced Mechanical Allodynia and Thermal Hyperalgesia
2.2. Minocycline Attenuated Systemic LPS-Induced Increase in Microglia Activation and Inflammatory Responses in the Spinal Cord
2.3. Minocycline Attenuated Systemic LPS-Induced Increase in Astrocyte Activation, COX-2, and PGE2 Expression in the Spinal Cord
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Animals
4.3. Animal Treatment
4.4. Von Frey Filament Test
4.5. Tail-Flick Test
4.6. Immunohistochemistry
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Quantification of Data and Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blum, E.; Procacci, P.; Conte, V.; Hanani, M. Systemic inflammation alters satellite glial cell function and structure. A possible contribution to pain. Neuroscience 2014, 274, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Zouikr, I.; Tadros, M.A.; Barouei, J.; Beagley, K.W.; Clifton, V.L.; Callister, R.J.; Hodgson, D.M. Altered nociceptive, endocrine, and dorsal horn neuron responses in rats following a neonatal immune challenge. Psychoneuroendocrinology 2014, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.C.; Wang, S.J.; Fan, L.W.; Cai, Z.; Rhodes, P.G.; Tien, L.T. Interleukin-1 receptor antagonist ameliorates neonatal lipopolysaccharide-induced long-lasting hyperalgesia in the adult rats. Toxicology 2011, 279, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Boisse, L.; Spencer, S.J.; Mouihate, A.; Vergnolle, N.; Pittman, Q.J. Neonatal immune challenge alters nociception in the adult rat. Pain 2005, 119, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.J. Pain, plasticity, and premature birth: A prescription for permanent suffering? Nat. Med. 2000, 6, 971–973. [Google Scholar] [CrossRef] [PubMed]
- LaPrairie, J.L.; Murphy, A.Z. Long-term impact of neonatal injury in male and female rats: Sex differences, mechanisms and clinical implications. Front. Neuroendocrinol. 2010, 31, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J. Infant pain: Does it hurt? Nature 2006, 444, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Fan, L.W.; Kaizaki, A.; Tien, L.T.; Ma, T.; Pang, Y.; Lin, S.; Lin, R.C.; Simpson, K.L. Neonatal systemic exposure to lipopolysaccharide enhances susceptibility of nigrostriatal dopaminergic neurons to rotenone neurotoxicity in later life. Dev. Neurosci. 2013, 35, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Oka, T.; Hori, T.; Takahashi, S. Prostanoids in the preoptic hypothalamus mediate systemic lipopolysaccharide-induced hyperalgesia in rats. Brain Res. 2001, 916, 41–49. [Google Scholar] [CrossRef]
- Hori, T.; Oka, T.; Hosoi, M.; Abe, M.; Oka, K. Hypothalamic mechanisms of pain modulatory actions of cytokines and prostaglandin E2. Ann. N. Y. Acad. Sci. 2000, 917, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Yirmiya, R.; Goshen, I.; Iverfeldt, K.; Holmlund, L.; Takeda, K.; Shavit, Y. Impairment of interleukin-1 (IL-1) signaling reduces basal pain sensitivity in mice: Genetic, pharmacological and developmental aspects. Pain 2003, 104, 471–480. [Google Scholar] [CrossRef]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Wilkerson, J.L.; Milligan, E.D. The Central Role of Glia in Pathological Pain and the Potential of Targeting the Cannabinoid 2 Receptor for Pain Relief. ISRN Anesthesiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Wells, J.; Giuliani, F.; Casha, S.; Power, C.; Metz, L.M. The promise of minocycline in neurology. Lancet Neurol. 2004, 3, 744–751. [Google Scholar] [CrossRef]
- Henry, C.J.; Huang, Y.; Wynne, A.; Hanke, M.; Himler, J.; Bailey, M.T.; Sheridan, J.F.; Godbout, J.P. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J. Neuroinflamm. 2008, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Bastos, L.F.; Godin, A.M.; Zhang, Y.; Jarussophon, S.; Ferreira, B.C.; Machado, R.R.; Maier, S.F.; Konishi, Y.; de Freitas, R.P.; Fiebich, B.L.; et al. A minocycline derivative reduces nerve injury-induced allodynia, LPS-induced prostaglandin E2 microglial production and signaling via toll-like receptors 2 and 4. Neurosci. Lett. 2013, 543, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Pang, Y.; Lin, S.; Rhodes, P.G.; Cai, Z. Minocycline attenuates lipopolysaccharide-induced white matter injury in the neonatal rat brain. Neuroscience 2005, 133, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Pang, Y.; Lin, S.; Tien, L.T.; Ma, T.; Rhodes, P.G.; Cai, Z. Minocycline reduces lipopolysaccharide-induced neurological dysfunction and brain injury in the neonatal rat. J. Neurosci. Res. 2005, 82, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013, 4, e525. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Mesa, N.; Zarzuelo, A.; Galvez, J. Minocycline: Far beyond an antibiotic. Br. J. Pharmacol. 2013, 169, 337–352. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Patel, D.; Dougherty, P.M. Minocycline blocks lipopolysaccharide induced hyperalgesia by suppression of microglia but not astrocytes. Neuroscience 2012, 221, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J. Neuropathol. Exp. Neurol. 2004, 63, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Bartels, A.L.; Leenders, K.L. Cyclooxygenase and neuroinflammation in Parkinson’s disease neurodegeneration. Curr. Neuropharmacol. 2010, 8, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, A. Prostaglandin E2 and pain—An update. Biol. Pharm. Bull. 2011, 34, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M. The development of nociceptive circuits. Nat. Rev. Neurosci. 2005, 6, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M. What do we really know about newborn infant pain? Exp. Physiol. 2015, 100, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Leslie, A.T.; Akers, K.G.; Martinez-Canabal, A.; Mello, L.E.; Covolan, L.; Guinsburg, R. Neonatal inflammatory pain increases hippocampal neurogenesis in rat pups. Neurosci. Lett. 2011, 501, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Beggs, S.; Currie, G.; Salter, M.W.; Fitzgerald, M.; Walker, S.M. Priming of adult pain responses by neonatal pain experience: Maintenance by central neuroimmune activity. Brain 2012, 135, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, M.P.; Koerber, H.R. Neurotrophic Factors and Nociceptor Sensitization. In Translational Pain Research: From Mouse to Man; Kruger, L., Light, A.R., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2010. [Google Scholar]
- Woolf, C.J.; Salter, M.W. Neuronal plasticity: Increasing the gain in pain. Science 2000, 288, 1765–1769. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.D.; Chien, C.C.; Wen, Y.R.; Fu, W.M.; Sun, W.Z. The pattern and distribution of calcitonin gene-related peptide (CGRP) terminals in the rat dorsal following neonatal peripheral inflammation. Neuroreport 2003, 14, 1919–1921. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, Y.; Zhang, L.; Cheng, J.K.; Ji, R.R. Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008, 28, 5189–5194. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sandoval, E.A.; Horvath, R.J.; DeLeo, J.A. Neuroimmune interactions and pain: Focus on glial-modulating targets. Curr. Opin. Investig. Drugs 2008, 9, 726–734. [Google Scholar] [PubMed]
- Ren, K.; Torres, R. Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 2009, 60, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.; Woolf, C.J. The neuropathic pain triad: Neurons, immune cells and glia. Nat. Neurosci. 2007, 10, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia 2002, 40, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.J.; Moalem-Taylor, G. The neuro-immune balance in neuropathic pain: Involvement of inflammatory immune cells, immune-like glial cells and cytokines. J. Neuroimmunol. 2010, 229, 26–50. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, H.; Tabarki, B.; Verellen, G.; de Prez, C.; Rona, A.M.; Sebire, G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology 2001, 56, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.H.; Jun, J.K.; Romero, R.; Park, K.H.; Gomez, R.; Choi, J.H.; Kim, I.O. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am. J. Obstet. Gynecol. 1997, 177, 19–26. [Google Scholar] [CrossRef]
- Cunha, J.M.; Cunha, F.Q.; Poole, S.; Ferreira, S.H. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br. J. Pharmacol. 2000, 130, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Reeve, A.J.; Patel, S.; Fox, A.; Walker, K.; Urban, L. Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur. J. Pain 2000, 4, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H.; Lorenzetti, B.B.; Bristow, A.F.; Poole, S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature 1988, 334, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Biology of interleukin 1. FASEB J. 1988, 2, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Lee, S.Y.; Yang, K.Y.; Nam, S.H.; Kim, H.J.; Kim, Y.J.; Bae, Y.C.; Ahn, D.K. Differential regulation of peripheral IL-1beta-induced mechanical allodynia and thermal hyperalgesia in rats. Pain 2014, 155, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Porreca, E.; Reale, M.; di Febbo, C.; di Gioacchino, M.; Barbacane, R.C.; Castellani, M.L.; Baccante, G.; Conti, P.; Cuccurullo, F. Down-regulation of cyclooxygenase-2 (COX-2) by interleukin-1 receptor antagonist in human monocytes. Immunology 1996, 89, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Huxtable, A.G.; Smith, S.M.; Vinit, S.; Watters, J.J.; Mitchell, G.S. Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia. J. Appl. Physiol. 2013, 114, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.W.; Kaizaki, A.; Tien, L.T.; Pang, Y.; Tanaka, S.; Numazawa, S.; Bhatt, A.J.; Cai, Z. Celecoxib attenuates systemic lipopolysaccharide-induced brain inflammation and white matter injury in the neonatal rats. Neuroscience 2013, 240, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Cai, Z.; Rhodes, P.G. Disturbance of oligodendrocyte development, hypomyelination and white matter injury in the neonatal rat brain after intracerebral injection of lipopolysaccharide. Brain Res. Dev. Brain Res. 2003, 140, 205–214. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jiang, Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology 2004, 201, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Moreno, B.; Jukes, J.P.; Vergara-Irigaray, N.; Errea, O.; Villoslada, P.; Perry, V.H.; Newman, T.A. Systemic inflammation induces axon injury during brain inflammation. Ann. Neurol. 2011, 70, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Goehler, L.E.; Gaykema, R.P.; Nguyen, K.T.; Lee, J.E.; Tilders, F.J.; Maier, S.F.; Watkins, L.R. Interleukin-1beta in immune cells of the abdominal vagus nerve: A link between the immune and nervous systems? J. Neurosci. 1999, 19, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Rivest, S. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 2009, 9, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, N.; Lacroix, S.; Rivest, S. An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia. J. Neurosci. 1999, 19, 10923–10930. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Lin, S.; Fan, L.W.; Pang, Y.; Rhodes, P.G. Minocycline alleviates hypoxic-ischemic injury to developing oligodendrocytes in the neonatal rat brain. Neuroscience 2006, 137, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Dehoux, M.J.; van Beneden, R.P.; Fernandez-Celemin, L.; Lause, P.L.; Thissen, J.P. Induction of MafBx and Murf ubiquitin ligase mRNAs in rat skeletal muscle after LPS injection. FEBS Lett. 2003, 544, 214–217. [Google Scholar] [CrossRef]
- Fernandez-Celemin, L.; Pasko, N.; Blomart, V.; Thissen, J.P. Inhibition of muscle insulin-like growth factor I expression by tumor necrosis factor-alpha. Am. J. Physiol. Endocrinol. MeTable 2002, 283, E1279–E1290. [Google Scholar] [CrossRef] [PubMed]
- Premer, D.M.; Goertz, R.; Georgieff, M.K.; Mammel, M.C.; Schwarzenberg, S.J. Muscle proteolysis and weight loss in a neonatal rat model of sepsis syndrome. Inflammation 2002, 26, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ghefreh, A.A.; Masocha, W. Enhancement of antinociception by coadministration of minocycline and a non-steroidal anti-inflammatory drug indomethacin in naive mice and murine models of LPS-induced thermal hyperalgesia and monoarthritis. BMC Musculoskelet. Disord. 2010, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Hains, L.E.; Loram, L.C.; Weiseler, J.L.; Frank, M.G.; Bloss, E.B.; Sholar, P.; Taylor, F.R.; Harrison, J.A.; Martin, T.J.; Eisenach, J.C.; et al. Pain intensity and duration can be enhanced by prior challenge: Initial evidence suggestive of a role of microglial priming. J. Pain 2010, 11, 1004–1014. [Google Scholar] [CrossRef] [PubMed]
- Carniglia, L.; Ramirez, D.; Durand, D.; Saba, J.; Turati, J.; Caruso, C.; Scimonelli, T.N.; Lasaga, M. Neuropeptides and Microglial Activation in Inflammation, Pain, and Neurodegenerative Diseases. Mediators Inflamm. 2017, 2017, 5048616. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Berta, T.; Nedergaard, M. Glia and pain: Is chronic pain a gliopathy? Pain 2013, 154 (Suppl. 1), S10–S28. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Stavrovskaya, I.G.; Drozda, M.; Kim, B.Y.; Ona, V.; Li, M.; Sarang, S.; Liu, A.S.; Hartley, D.M.; Wu, D.C.; et al. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature 2002, 417, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, F.; Hader, W.; Yong, V.W. Minocycline attenuates T cell and microglia activity to impair cytokine production in T cell-microglia interaction. J. Leukoc. Biol. 2005, 78, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, C.A.; Schreiber, K.L.; Brewer, K.L.; Yu, C.G.; Stone, L.S.; Kitto, K.F.; Nguyen, H.O.; Grocholski, B.M.; Shoeman, D.W.; Kehl, L.J.; et al. Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury. Proc. Natl. Acad. Sci. USA 2000, 97, 10584–10589. [Google Scholar] [CrossRef] [PubMed]
- Vega-Avelaira, D.; Ballesteros, J.J.; Lopez-Garcia, J.A. Inflammation-induced hyperalgesia and spinal microglia reactivity in neonatal rats. Eur. J. Pain 2013, 17, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.F.; Jackson, G.D.; Martin, T.; Westbrook, R.F. Bacterial lipopolysaccharides induce peripheral nerve disturbances in rats that mimic human immune-mediated polyneuropathies. Lab. Anim. Sci. 1997, 47, 354–361. [Google Scholar] [PubMed]
- Wongchanapai, W.; Tsang, B.K.; He, Z.; Ho, I.K. Differential involvement of opioid receptors in intrathecal butorphanol-induced analgesia: Compared to morphine. Pharmacol. Biochem. Behav. 1998, 59, 723–727. [Google Scholar] [CrossRef]
- Kaizaki, A.; Tien, L.T.; Pang, Y.; Cai, Z.; Tanaka, S.; Numazawa, S.; Bhatt, A.J.; Fan, L.W. Celecoxib reduces brain dopaminergic neuronaldysfunction, and improves sensorimotor behavioral performance in neonatal rats exposed to systemic lipopolysaccharide. J. Neuroinflamm. 2013, 10, 45. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-T.; Lee, Y.-J.; Dai, X.; Ojeda, N.B.; Lee, H.J.; Tien, L.-T.; Fan, L.-W. Systemic Lipopolysaccharide-Induced Pain Sensitivity and Spinal Inflammation Were Reduced by Minocycline in Neonatal Rats. Int. J. Mol. Sci. 2018, 19, 2947. https://doi.org/10.3390/ijms19102947
Hsieh C-T, Lee Y-J, Dai X, Ojeda NB, Lee HJ, Tien L-T, Fan L-W. Systemic Lipopolysaccharide-Induced Pain Sensitivity and Spinal Inflammation Were Reduced by Minocycline in Neonatal Rats. International Journal of Molecular Sciences. 2018; 19(10):2947. https://doi.org/10.3390/ijms19102947
Chicago/Turabian StyleHsieh, Cheng-Ta, Yih-Jing Lee, Xiaoli Dai, Norma Beatriz Ojeda, Hyun Joon Lee, Lu-Tai Tien, and Lir-Wan Fan. 2018. "Systemic Lipopolysaccharide-Induced Pain Sensitivity and Spinal Inflammation Were Reduced by Minocycline in Neonatal Rats" International Journal of Molecular Sciences 19, no. 10: 2947. https://doi.org/10.3390/ijms19102947
APA StyleHsieh, C.-T., Lee, Y.-J., Dai, X., Ojeda, N. B., Lee, H. J., Tien, L.-T., & Fan, L.-W. (2018). Systemic Lipopolysaccharide-Induced Pain Sensitivity and Spinal Inflammation Were Reduced by Minocycline in Neonatal Rats. International Journal of Molecular Sciences, 19(10), 2947. https://doi.org/10.3390/ijms19102947