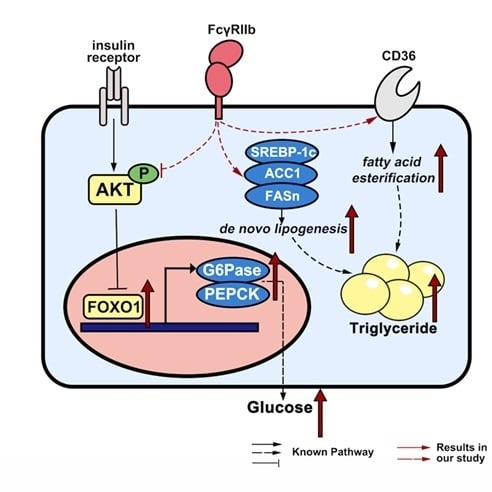
Fc Gamma Receptor IIb Expressed in Hepatocytes Promotes Lipid Accumulation and Gluconeogenesis
Abstract
:1. Introduction
2. Results
2.1. FcγRIIb Expressed in Hepatocytes
2.2. Increase of FcγRIIb Expression after Oleic Acid Treatment
2.3. Increase of FcγRIIb Expression after Oleic Acid Treatment
2.4. FcγRIIb-Deficiency Attenuated HFD-Induced Hepatic Steatosis in Mice
2.5. FcγRIIb Decreased Hepatic Insulin Sensitivity In Vitro and In Vivo
3. Discussion
4. Materials and Methods
4.1. Primary Hepatocyte Isolation and Culture
4.2. Cell Culture
4.3. Animal
4.4. RNA Interference
4.5. Quantitative Real-Time PCR
4.6. Western Blot Analysis
4.7. Histology and Immunohistochemistry
4.8. Triglyceride Content Assay
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NAFLD | Non-alcoholic fatty liver disease |
| OA | Oleic Acid |
| CRP | C Reactive Protein |
| NCD | Normal Chow Diet |
| HFD | High Fat Diet |
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. Nonalcoholic Fatty Liver Disease as a Nexus of Metabolic and Hepatic Diseases. Cell Metab. 2018, 27, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Samuel, V.T.; Shulman, G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Investig. 2016, 126, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhang, D.; Charney, N.; Jin, E.; Vandommelon, K.; Stamper, K.; Gupta, N.; Saldate, J.; Yin, L. DDB1-Mediated CRY1 Degradation Promotes FOXO1-Driven Gluconeogenesis in Liver. Diabetes 2017, 66, 2571. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human Fatty Liver Disease: Old Questions and New Insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakhshandehroo, M.; Knoch, B.; Müller, M.; Kersten, S. Peroxisome proliferator-activated receptor α target genes. PPAR Res. 2010, 2010. [Google Scholar] [CrossRef] [PubMed]
- Espéli, M.; Smith, K.G.; Clatworthy, M.R. FcγRIIB and autoimmunity. Immunol. Rev. 2016, 269, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Tanigaki, K.; Sundgren, N.; Khera, A.; Vongpatanasin, W.; Mineo, C; Shaul, P.W. Fcγ Receptors and Ligands and Cardiovascular Disease. Circ. Res. 2015, 116, 368–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanigaki, K.; Vongpatanasin, W.; Barrera, J.A.; Atochin, D.N.; Huang, P.L.; Bonvini, E.; Shaul, P.W.; Mineo, C. C-Reactive Protein Causes Insulin Resistance in Mice Through Fcγ Receptor IIB–Mediated Inhibition of Skeletal Muscle Glucose Delivery. Diabetes 2013, 62, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Tanigaki, K.; Chambliss, K.L.; Yuhanna, I.S.; Sacharidou, A.; Ahmed, M.; Atochin, D.N.; Huang, P.L.; Shaul, P.W.; Mineo, C. Endothelial Fcγ Receptor IIB Activation Blunts Insulin Delivery to Skeletal Muscle to Cause Insulin Resistance in Mice. Diabetes 2016, 65, 1996–2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanigaki, K.; Sacharidou, A.; Peng, J.; Chambliss, K.L.; Yuhanna, I.S.; Ghosh, D.; Ahmed, M.; Szalai, A.J.; Vongpatanasin, W.; Mattrey, R.F.; et al. Hyposialylated IgG activates endothelial IgG receptor Fcγ RIIB to promote obesity-induced insulin resistance. J. Clin. Investig. 2018, 128, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kang, S.I.; Shin, H.S.; Yoon, S.A.; Kang, S.W.; Ko, H.C.; Kim, S.J. Sasa quelpaertensis and p-Coumaric Acid Attenuate Oleic Acid-Induced Lipid Accumulation in HepG2 Cells. J. Agric. Chem. Soc. Jpn. 2013, 77, 1595–1598. [Google Scholar]
- Li, Z.; Zhang, H.; Liu, J.; Liang, C.P.; Li, Y.; Li, Y.; Teitelman, G.; Beyer, T.; Bui, H.H.; Peake, D.A.; et al. Reducing Plasma Membrane Sphingomyelin Increases Insulin Sensitivity. Mol. Cell. Biol. 2011, 31, 4205–4218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of nonalcoholic fatty liver disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.Y.; Jiao, P.; Feng, B.; Li, Y.; He, Q.; Xu, H. Hepatic ERK activity plays a role in energy metabolism. Mol. Cell. Endocrinol. 2013, 375, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.Y.; Qiu, L.W.; Chen, X.F.; Lü, L.; Mei, Z.C. Oleic Acid-Induced Hepatic Steatosis is Coupled with Downregulation of Aquaporin 3 and Upregulation of Aquaporin 9 via Activation of p38 Signaling. Horm. Metab. Res. 2014, 47, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Bieghs, V.; Trautwein, C. The innate immune response during liver inflammation and metabolic disease. Trend. Immunol. 2013, 34, 446–452. [Google Scholar] [CrossRef]
- Mendez-Fernandez, Y.V.; Stevenson, B.G.; Diehl, C.J.; Braun, N.A.; Wade, N.S.; Covarrubias, R.; van Leuven, S.; Witztum, J.L.; Major, A.S. The inhibitory Fcγ RIIb modulates the inflammatory response and influences atherosclerosis in male apoE−/− mice. Atherosclerosis 2011, 214, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Cutler, A.J.; Brownlie, R.J.; Fairfax, K.; Lawlor, K.E.; Severinson, E.; Walker, E.U.; Manz, R.A.; Tarlinton, D.M.; Smith, K.G.C. FcγRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 2007, 8, 419–429. [Google Scholar]
- Huang, H.; Tindall, D.J. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim. Biophys. Acta 2011, 1813, 1961–1964. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Q.; Yang, M.; Zhu, B.; Cui, Y.; Xue, Y.; Gong, N.; Cui, A.; Wang, M.; Shen, L.; et al. Mouse KLF11 regulates hepatic lipid metabolism. J. Hepatol. 2013, 58, 763–770. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, T.; Song, X.; Cui, X.; Ge, W.; Gao, R.; Wang, J. Fc Gamma Receptor IIb Expressed in Hepatocytes Promotes Lipid Accumulation and Gluconeogenesis. Int. J. Mol. Sci. 2018, 19, 2932. https://doi.org/10.3390/ijms19102932
Shu T, Song X, Cui X, Ge W, Gao R, Wang J. Fc Gamma Receptor IIb Expressed in Hepatocytes Promotes Lipid Accumulation and Gluconeogenesis. International Journal of Molecular Sciences. 2018; 19(10):2932. https://doi.org/10.3390/ijms19102932
Chicago/Turabian StyleShu, Ting, Xiaomin Song, Xingxing Cui, Weipeng Ge, Ran Gao, and Jing Wang. 2018. "Fc Gamma Receptor IIb Expressed in Hepatocytes Promotes Lipid Accumulation and Gluconeogenesis" International Journal of Molecular Sciences 19, no. 10: 2932. https://doi.org/10.3390/ijms19102932